-
减数分裂是植物生活史中的一个重要环节,是有性生殖的前提,是保持物种稳定性的基础[1]。本研究的实验材料‘无籽’瓯柑Citrus suavissima ‘Seedless’是1996 年发现的一个瓯柑Citrus suavissima的无核突变体,具有果实完全无核的特性[2]。张迟等[3]采用半薄树脂切片、扫描电镜、透射电镜等方法对其花药发育过程和花粉形态进行观察,发现在四分体时期出现异常,在单核期出现明显异常,认为花粉败育开始于小孢子母细胞减数分裂期至四分体时期。近年来,模式植物减数分裂行为中的染色体配对和联会方面的研究取得了较大进展。PANOLI等[4]研究了拟南芥Arabidopsis thaliana的直系同源基因Atspo11-1,染色体重组起始于Spoll调控的DNA双链解开,Atspo11-1突变体中Atspo11-1的缺失会抑制染色体破碎化。LI等[5]利用荧光原位杂交技术观察了拟南芥突变体rad51c-1的早期减数分裂行为,发现该突变体的同源染色体无法正常配对并出现严重的破碎化现象。敲除RAD51C基因后得到的不育植株伴随有大孢子和花粉母细胞粗线期染色体异常[6]。ITO等[7]研究了拟南芥中的MALE STERILITY1(MS1)基因,它对减数分裂后的花粉发育很重要,与花粉壁、花粉细胞质内容物和绒毡层发育有关。在果树方面,目前的研究多采用分子标记法来发掘育性相关基因。如王跃进等[8]通过分离、提取和纯化经随机扩增多态DNA(RAPD)标记获得的特异片段,根据其序列人工合成一条寡聚核苷酸作引物(5′CCAGT TCGCC CGTAA ATG 3′),在多个葡萄Vitis vinifera品系中进行验证,凡出现约590 bp的DNA片段者即为葡萄无核基因携带者和无核性状表现者。杨英军等[9]又进一步根据此特异片段设计合成了2对引物,开发出鉴别无核品种的SCAR(特定序列扩增标记)。姚春潮等[10]利用 RAPD 技术对美味猕猴桃Actinidia deliciosa var. deliciosa海瓦德×秦雄 201 杂交 F1代雌雄分离群体进行分析,通过对 300 个随机引物的筛选和研究,得到了与猕猴桃雄性基因链锁的 RAPD 标记S1032-850。NWAFOR等[11]采集了野生型葡萄和一个无核突变体材料的果实进行转录组测序技术(RNA-Seq)分析,对得到的差异表达基因进行表达模式和功能富集分析,发现花粉和胚囊两者的发育途径具有密切关联,但无核性状具体由哪些基因控制仍不明了。本研究根据‘无籽’瓯柑及其野生型小孢子母细胞时期花药转录组的测序结果,选择了RAD51和MS1这2个减数分裂特性基因,研究它们在‘无籽’瓯柑和瓯柑花粉发育不同时期的相对表达量差异,来分析‘无籽’瓯柑小孢子母细胞减数分裂异常的分子机制。此外,鉴于减数分裂只在特定时期和特定部位发生,本研究选取了花蕾和花药2种材料进行基因相对表达量的测定,进而分析不同材料所得结果的差异。
HTML
-
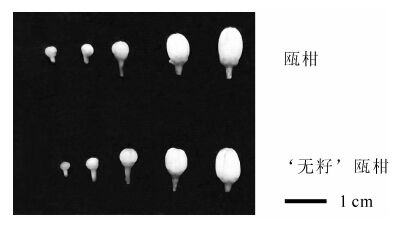
供试材料瓯柑和‘无籽’瓯柑采自丽水市林业科学研究院百果园。当花蕾刚露白时开始取样,根据预备试验的结果,采集‘无籽’瓯柑和瓯柑花粉母细胞形成期(Ⅰ),四分体时期(Ⅱ),单核花粉粒时期(Ⅲ),双核花粉粒时期(Ⅳ) 和花粉粒成熟期(Ⅴ)共5个时期的花蕾和对应花药,5个时期的花蕾直径分别为2.0~2.4 mm,2.8~3.1 mm,3.5~4.5 mm,4.5~6.5 mm和即将开放的花蕾(6.5~7.1 mm,图 1),保存于-70 ℃超低温冰箱。
-
采用改良的Trizol法提取瓯柑和‘无籽’瓯柑各5个时期花蕾的RNA,用Aidlab公司的‘EASYspin PLUS植物RNA快速提取试剂盒’提取花药RNA,10.0 g·kg-1的琼脂糖凝胶对各样品的 RNA进行电泳检测,再经NanoDrop-1000测吸光度值,检测样品RNA的质量。
-
cDNA的合成参照TaKaRa反转录试剂盒PrimeScrip® RT reagent kit with gDNA Eraser (perfect real time)的说明指南。根据转录组测序结果中的基因序列(已提交GenBank)设计荧光定量特异引物(表 1),以甜橙Citrus sinensis的Actin基因(GU911361)为内参,采用定量仪器CFX96 real-time system实时荧光定量PCR仪(Bio-Rad)和荧光染料SYBR®Premix Ex TaqTM Ⅱ(Tli RNaseH Plus)进行实时表达量测定。反应程序为95 ℃,30 s;95 ℃,5 s;57 ℃,30 s,39个循环;65~95 ℃,隔5 s上升0.5 ℃做融解曲线。设立重复3个·样品-1,根据2-△△Ct法[12]进行基因相对表达量计算,利用SPSS 16.0的最小显著差(LSD)法在0.01水平上比较2个基因在不同组织和不同时期中的表达量差异。
基因 登录号 正向引物(5'→3') 反向引物(5'→3') RAD51C KU204895 AGAAGCTGAAAGATGCGGGT GATGGAGTTGGGTAGCACTGG MS1 KU204897 AGTGCTTGCATTGATGATCC TGAGGCATTTAGGCATAACG 内参 Actin GU911361 ATCTGCTGGAAGGTGCTGAG CCAAGCAGCATGAAGATCAA Table 1. Specific primers for qRT-PCR
1.1. 材料
1.2. 方法
1.2.1. RNA提取
1.2.2. 荧光定量PCR
-
以瓯柑第Ⅰ时期花蕾的表达量为基准计算瓯柑和‘无籽’瓯柑各时期花蕾的RAD51的相对表达量。‘无籽’瓯柑和瓯柑花蕾中RAD51的表达主要集中在第Ⅰ时期;‘无籽’瓯柑中RAD51基因的相对表达量逐渐下降,瓯柑先下降,在第Ⅳ时期和第Ⅴ时期略有上升(图 2)。
-
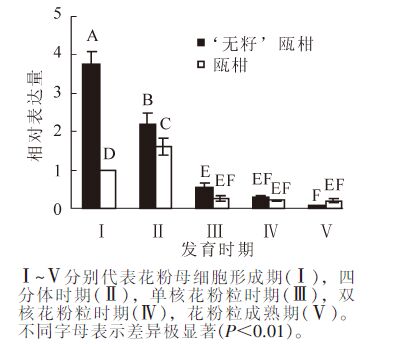
以瓯柑第Ⅰ时期花药的表达量为基准计算瓯柑和‘无籽’瓯柑各时期花药的RAD51相对表达量。‘无籽’瓯柑和瓯柑花药中RAD51的表达主要集中在第Ⅰ时期和第Ⅱ时期,且此时‘无籽’瓯柑中的相对表达量均极显著(P<0.01)高于瓯柑,第Ⅰ时期达3.7倍左右;‘无籽’瓯柑中RAD51基因的相对表达量逐渐下降,瓯柑在第Ⅱ时期最高,随后逐渐下降(图 3)。
-
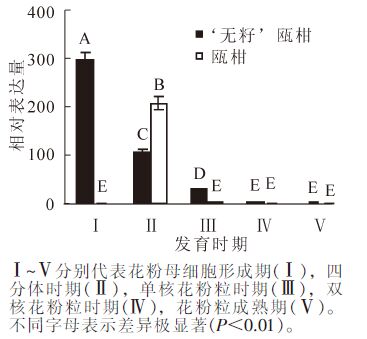
以瓯柑第Ⅰ时期花蕾的表达量为基准计算瓯柑和‘无籽’瓯柑各时期花蕾的MS1相对表达量。‘无籽’瓯柑和瓯柑花蕾中MS1的表达主要集中在第Ⅰ时期和第Ⅱ时期,且此时期存在极显著(P<0.01)差异;第Ⅰ时期‘无籽’瓯柑是瓯柑的140.0倍左右,但第Ⅱ时期瓯柑是‘无籽’瓯柑的2.5倍;‘无籽’瓯柑中MS1基因的相对表达量逐渐下降,瓯柑在第Ⅱ时期相对表达量最高,随后逐渐下降(图 4)。
-
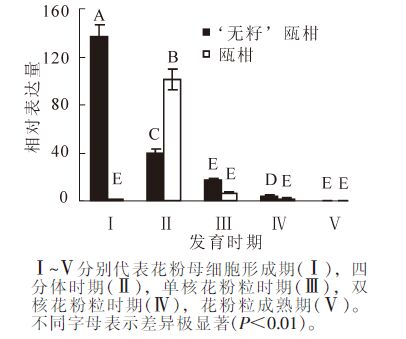
以瓯柑第Ⅰ时期花药的表达量为基准计算瓯柑和‘无籽’瓯柑各时期花药的MS1相对表达量。‘无籽’瓯柑和瓯柑花药中MS1的表达也主要集中在第Ⅰ时期和第Ⅱ时期,且存在极显著(P<0.01)差异;第Ⅰ时期‘无籽’瓯柑是瓯柑的300.0倍左右,但第Ⅱ时期瓯柑是‘无籽’瓯柑的2.0倍;‘无籽’瓯柑中MS1基因的相对表达量逐渐下降,瓯柑在第Ⅱ时期相对表达量最高,随后逐渐下降(图 5),此趋势与花蕾中相似(图 4)。
-
分别以‘无籽’瓯柑和瓯柑各自花蕾第Ⅰ时期的表达量为基准计算同种(品种)花蕾和花药间的相对表达量,比较同一基因在不同组织材料中的表达差异。除个别时期无极显著(P<0.01)差异外,2个种(品种)的其他时期均是花药中的相对表达量极显著(P<0.01)高于各自时期的花蕾(图 6)。
2.1. RAD51基因的时空定量表达
2.1.1. 花蕾中RAD51基因的表达
2.1.2. 花药中RAD51基因的表达
2.2. MS1基因的时空定量表达
2.2.1. 花蕾中MS1基因的表达
2.2.2. 花药中MS1基因的表达
2.3. 同种(品种)花蕾和花药之间基因表达量的比较分析
-
RAD51的功能是修复SPO11引发产生的双链DNA分子断裂(double-strand breaks,DSBs)。RAD51会附着到由蛋白复合体 MRX 产生的3′单链末端上,以促进单链 DNA 入侵到同源染色体双链中形成重组中间体,在复合体解开的过程中形成染色体的交换(crossover,CO)和非交换(noncrossover,NCO)[13]。AtRAD51 基因单突变体雌、雄减数分裂过程均不正常,单突变体植株不育,减数分裂细胞中无法正常形成联会复合体且存在大量染色体碎片,而Atspo11-1Atrad51 双突变体中不存在染色体碎片,说明 Atrad51 中的染色体碎片来自于 AtSPO11 引发的 DSBs[14]。动物RAD51基因是大肠埃希菌Escherichia coli RecA的同源基因,与植物RAD51也具有一定同源性[15],在动物生命科学研究中发现RAD51蛋白在多种受损组织中表达增高,认为这是DNA被损伤后细胞的一种反应[16-17]。
OSAKABE等[18]对拟南芥进行不同强度的γ射线照射,诱导产生DSBs,对不同器官内RAD51基因进行半定量PCR分析,结果显示:花芽中RAD51表达量最高,且随照射强度增加而增高,说明细胞内产生的DSBs越多,RAD51基因越活跃。本研究中,‘无籽’瓯柑第Ⅰ时期和第Ⅱ时期的花药中RAD51的相对表达量极显著(P<0.01)高于瓯柑(图 3),表明‘无籽’瓯柑小孢子母细胞时期和四分体时期细胞内可能存在某种机制引起的DNA受损现象。
-
MS1基因编码一个含有植物同源域(plant homeo domain,PHD)指基序的转录因子,参与绒毡层的发育和花粉壁的形成[19]。拟南芥ms1突变体花粉的透射电镜观察[20]显示,前期突变体与野生型均能正常发育,但从四分体解体开始,突变体中的小孢子和绒毡层细胞的细胞质中出现颗粒状物质和非正常液泡,小孢子内孢粉素分布及外壁柱状基粒棒的发育均异常,并在随后的发育中被液泡充斥而萎缩退化。而‘无籽’瓯柑花药的绒毡层和花粉壁结构与瓯柑无明显差异,但最终花粉粒形态出现明显差异,瓯柑花粉粒饱满,‘无籽’瓯柑的花粉粒大多干瘪、畸形且无内含物,说明MS1在不同物种中的功能存在一定差异[3]。正常情况下,MS1在小孢子母细胞减数分裂完成、单个小孢子从四分体分离时在绒毡层细胞中表达量较高[19]。本研究中,瓯柑MS1的表达趋势符合此规律。拟南芥ms1-1突变体[21]MS1的转录本在小孢子母细胞时期(PMC)到减数分裂期间表达量是野生型的27.0倍,四分体到有丝分裂Ⅰ时期表达量是野生型的600.0倍。本研究中‘无籽’瓯柑在小孢子母细胞时期(第I时期)的表达异常升高,在花蕾中是瓯柑的140.0倍,花药中更是高达300.0倍,反而在四分体时期(第Ⅱ时期)显著降低,仅为瓯柑的1/2。
YANG等[21]利用花椰菜Brassica oleracea var. botrytis花叶病毒35S启动子(CaMV35S)使MS1基因过量表达,得到一些花器官及花粉变异的拟南芥类型,并对ms1变异花芽进行基因芯片分析,其中3个有关油脂合成和花粉壁发育的基因(At5g07550,At5g07410和At5g07560)经RT-PCR验证,发现在MS1过表达的拟南芥类型中均表现为下调表达,推测ms1变异株中这些油脂蛋白的缺失影响了TAGs从内质网上的释放,导致了油质的积累和不正常分泌。张敏等[22]通过qRT-PCR发现‘无籽’瓯柑中脂转移酶基因(CsLTP)和脂氧合酶基因(CsLOX)的相对表达量在花粉粒形成期显著下降,LTP是MYB99的下游基因,而MYB99是受MS1调控的转录因子[7, 21],‘无籽’瓯柑成熟花粉粒时期花药的绒毡层附近有大量的脂类物质残存[3],因此,‘无籽’瓯柑败育花粉的形成与MS1基因过表达引起油脂分泌、转运异常密切相关。
-
本研究利用花蕾与花药2种组织材料对RAD51和MS1基因进行表达量比较,发现同一基因在同一时期的花蕾和花药中相对表达量差异极显著(P<0.01)(图 6)。任磊等[23]采用相对荧光定量的方法对 7 个花器官发育相关基因在牡丹Paeonia suffruticosa根、茎、叶、花瓣、萼片、雄蕊和心皮中的表达差异进行分析,其中PsPI和 PsMADS1主要在花瓣和雄蕊中表达,其他器官几乎没有表达,表明不同的基因在不同的器官中有不同的表达模式,具有一定的组织特异性。本研究的重点是‘无籽’瓯柑减数分裂异常的分子机制,因此,采用花药作为研究材料可以更准确地反映减数分裂特性基因的表达水平。
3.1. RAD51的基因功能和表达分析
3.2. MS1的基因功能和表达分析
3.3. 不同组织材料的比较分析
-
在花粉母细胞形成期(Ⅰ),四分体时期(Ⅱ),单核花粉粒时期(Ⅲ),双核花粉粒时期(Ⅳ),花粉粒成熟期(Ⅴ)等5个时期的花蕾和花药中,同种(品种)中各时期RAD51和MS1的表达量均是花药极显著(P<0.01)高于花蕾。第Ⅰ时期花药中,‘无籽’瓯柑RAD51的相对表达量为瓯柑的3.7倍,说明‘无籽’瓯柑的小孢子母细胞时期和四分体时期可能存在某种机制引发的DNA受损现象,从而引起RAD51的异常高量表达。第Ⅰ时期花药中MS1的表达量为瓯柑的300.0倍,但第Ⅱ时期(四分体时期)锐减为瓯柑的1/2;MS1基因的提早过量表达可能引起油脂分泌、转运的异常,与‘无籽’瓯柑败育花粉的形成密切相关。
CHEN等[24]收集拟南芥性母细胞进行RNA-Seq测序,结果显示:减数分裂特性基因MND1,AtSPO11-2,AtSRP2,MSS等的表达量在性细胞中是花药中的2.0倍,AtSPO11-1,AtDMC1,AtRAD51C,AtXRCC3等在性细胞和花药中表达量又比种子高,说明基因表达量与组织特性密切相关。在后续的减数分裂分子机制研究中,可尝试利用激光显微切割技术获取花药中的性母细胞进行转录组测序,以获得更精确的基因信息,推进‘无籽’瓯柑减数分裂异常的研究进程。















 DownLoad:
DownLoad: