-
放牧是草地的主要利用方式之一,主要通过牲畜的采食、践踏、卧息和排泄粪便等方式对地表植被、土壤养分以及土壤微生物产生影响[1]。土壤微生物在有机质的分解、养分循环与转化等方面具有重要作用[2],其数量和群落功能多样性能够在一定程度上指示土壤质量及其可持续利用性[3]。研究放牧与土壤微生物的关系,有助于揭示过度放牧导致草场沙漠化的机制。根际是植物、土壤、微生物之间进行物质和能量交换的关键区域,是植物和土壤相互作用的重要界面,诸多学者对植物根际与非根际土壤的养分、微生物数量及群落组成的差异性开展了大量的研究[4-7]。邱权等[8]综合比较了4种人工灌木丛根际和非根际土壤的特性,发现土壤酶活性和微生物数量呈现出根际高于非根际;对宁夏宁南山区猪毛蒿Artemisia scoparia,百里香Thymus mongolicus等9种典型植物[9]和内蒙古羊草Leymus chinensis,大针茅Stipa grandis和冷蒿Artemisia frigida等典型植物[10]进行研究,表明大多数植物的根际土壤微生物数量、活性及多样性等均高于非根际土壤,是由于植物物种差异所引起。冷蒿是菊科Compositae蒿属Artemisia植物,多年生小半灌木,是退化草场的典型植物,具有强烈的耐牧生存能力,高强度放牧干扰后仍能够生长繁殖并维持一定的生产力,这与其自身的生物学特性[11]及根系代谢产物[12]对土壤微环境的调控密切相关。目前,关于放牧干扰对冷蒿根际土壤微生物群落多样性影响的研究尚未报道。本研究拟采用微生物传统培养法和Biolog-ECO板技术,对不同放牧强度下冷蒿根际土壤化学性质、微生物数量及其群落功能多样性进行研究,分析冷蒿根际土壤微生物数量及代谢功能多样性对放牧干扰的响应,探讨冷蒿耐牧性与土壤微生物群落多样性之间的关系,为揭示冷蒿成为草场退化阻击者提供土壤生态学方面的理论依据。
HTML
-
本研究依托内蒙古锡林浩特市毛登牧场(内蒙古大学草地生态学研究基地)进行,其地理位置为44°10′02.4″ N,116°28′56.8″ E,海拔1 160 m,属半干旱大陆性气候,冬季寒冷干燥,夏季在一定程度上受海洋季风气候影响。全年平均气温为-0.4 ℃,最冷月(1月)平均气温-22.3 ℃,最热月(7月)平均气温18.8 ℃,≥0 ℃年积温为2 410 ℃,≥10 ℃积温为1 597.9 ℃,无霜期91 d,草场植物生长期为150 d左右。全年平均降水量为365.6 mm,集中于6-9月,占年降水量的80%左右,但年度间的变幅较大,多雨和少雨的年份降水量相差1倍以上。该地雨热同期,有利于植物的生长,土壤为栗钙土。本研究区域主要草原植物为羊草,糙隐子草Cleistogenes squarrosa,克氏针茅Stipa krylovii,大针茅,防风Saposhnikovia divaricata,冷蒿,瓣蕊唐松草Thalictrum petaloideum,阿尔泰狗哇花Heteropappus altaicus等。
-
于2012年5月至2014年7月连续2 a对草场进行不同放牧强度处理,每年放牧时间为5-9月。试验按照放牧强度设置不放牧为对照(ck),5月和7月每月21日放牧1 d为轻度放牧(light grazing,LG),5-9月每月21日放牧1 d为重度放牧(heavy grazing,HG),3个处理;受天气因素的影响,每次放牧时间延后。分别设置重复3个·处理-1,面积为33.3 m × 33.3 m·小区-1。试验用羊为当年生乌珠穆沁羊Capra hircus ‘Ujimqin’,各个放牧季节羊放牧率为6只·小区-1。
-
土壤样品采集于2014年7月冷蒿生长高峰期,在各个小区采用5点取样法。随即选取4~5丛冷蒿,以冷蒿株丛为中心,将冷蒿纯植株丛完整挖起(0~10 cm),轻轻抖动根系并去除粘附在根系上的较大颗粒土,作为冷蒿非根际土壤(NRS),采集粘附在根际上根系表面的土壤作为冷蒿根际土壤(ARS)。各个小区采集的土壤混合在一起作为该小区样地的土样,各个小区用5点取样法采样2次以获得同一小区的2份土样。将土壤装入无菌封口塑料袋,带回实验室。土样分成2份,1份于4 ℃保存,用于可培养微生物的分离、计数及Biolog-ECO板测定,另1份风干过2 mm筛,用于测定土壤理化性质。
-
土壤可培养微生物数量(colony forming units,cfu)用稀释平板法分离计数。细菌采用牛肉膏蛋白胨固体培养基;真菌采用马丁培养基;放线菌采用高氏1号培养基,30 ℃恒温培养,细菌培养1 d后计数,真菌、放线菌培养3 d后计数。
-
土壤微生物代谢功能多样性采用Biolog-ECO板方法进行分析。称取新鲜土样1 g于9 mL磷酸缓冲液中,在摇床上震荡30 min,在接种前按10倍稀释法制成10-4土壤稀释液,使用8通道移液器,从V型槽中吸取150 μL稀释液至ECO板的微孔中,接种后的板置于30 ℃恒温培养,每隔24 h在Biolog读板仪上用Biolog Reader 4.2软件(Biolog,Hayward,CA,美国)读取590 nm波长的吸光度D(590),培养时间为168 h。
-
测定参照鲁如坤[13]的土壤农化分析方法进行。有机质(organic matter,OM)采用重铬酸钾容量法;全氮(total nitrogen,TN)采用半微量凯氏定氮法;碱解氮(hydrolysis nitrogen,HN)采用碱解扩散法;全磷(total phosphorus,TP)采用氢氧化钠碱溶-钼锑抗比色法;速效磷(available phosphorus,AP)采用碳酸氢钠浸提钼锑抗比色法;全钾(total potassium,TK)采用氢氧化钠碱溶-火焰光度法;速效钾(available potassium,AK)采用乙酸氨浸提-火焰光度法;pH值采用酸度计法,土壤悬液为水土比为m(水): m(土)=5:1。
-
土壤微生物群落利用碳源的整体能力(即代谢活性)用平均孔颜色变化率(average well color development,AWCD)表示。AWCD=[∑(Ci-R)]/n,其中:Ci为测定的31个碳源孔吸光值,R为对照孔吸光值,n为碳源数目。土壤微生物群落功能多样性采用Shannon指数、Simpson指数、丰富度指数和McIntosh指数进行分析。
所有的数据均为5次重复的平均值±标准误差,利用Origin 8软件(美国Origin Lab公司)对96 h的AWCD值进行统计分析和作图。统计方法采用单因素方差分析(one-way ANOVA)进行检验,并进行Fisher最小显著差数法(LSD)多重比较(P<0.05)。采用双因素方差分析(two-way ANOVA)分析土壤×放牧处理之间相互作用的影响,利用SPSS 16.0进行主成分分析[14]。
1.1. 研究地区概况
1.2. 样地设置及土样采集
1.2.1. 试验设计
1.2.2. 土壤样品的采集与保存
1.2.3. 微生物分离与记数
1.2.4. 微生物代谢功能多样性测定
1.3. 土壤理化性质测定
1.4. 数据处理
-
由表 1可知:放牧对2种土壤中的有机质、全磷、碱解氮、速效钾和pH值具有极显著影响,对全氮和全钾具有显著影响。放牧特别是重度放牧后,NRS土壤中各养分质量分数均显著增加,pH值显著下降;ARS土壤中有机质和其他养分质量分数均显著增加,重度放牧后,有机质、全氮、全磷、碱解氮、速效磷和速效钾与对照相比分别增加20.0%,29.1%,17.7%,13.0%,3.4%和6.7%,但pH值显著降低,重度放牧后呈弱碱性;相同放牧处理下ARS土壤各养分质量分数均显著高于NRS土壤,pH值明显低于NRS土壤。

Table 1. Soil chemical properties under different grazing intensity
-
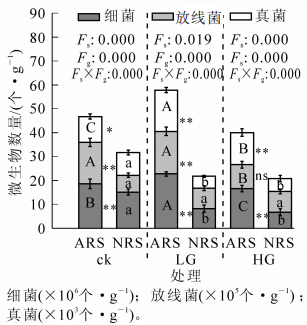
不同放牧强度下2类土壤中微生物的总量、主要类群数量差异显著(图 1),微生物总量以LG-ARS和ck-ARS最高,分别为24.6×106个(cfu)·g-1和20.4×106个(cfu)·g-1,显著高于其他处理组。三大类异养微生物数量在各土壤微生物组成中均以细菌类群占绝对优势,但细菌、真菌、放线菌间的组成比例差异较大,其中细菌占微生物总数88%~95%,放线菌其次,占微生物总数的3%~12%,真菌最少。放牧后NRS土壤中细菌、真菌数量显著下降,而ARS土壤中真菌数量增加显著,且显著高于NRS,细菌数量在轻度放牧后显著增加,重度放牧后下降。
-
图 2为土壤微生物群落代谢活性(AWCD)随培养时间的变化曲线:在培养初始的24 h内土壤微生物活性较低,24 h后AWCD值快速增长,168 h时各处理的AWCD值均达到最大,利用碳源能力的顺序为LG-ARS>ck-ARS>ck-NRS>LG-NRS>HG-ARS>HG-NRS,平均值分别为0.999,0.918,0.861,0.769,0.695,0.310;相同牧压下ARS土壤微生物的AWCD值均显著高于NRS,对照、轻度和重度放牧后AWCD值分别是NRS的1.07倍、1.30倍和2.24倍。
-
放牧强度不同,土壤微生物对不同种类碳源利用强度存在显著差异(表 2);冷蒿根际土壤中,轻度放牧可增加微生物对不同种类碳源的利用能力,碳源代谢的优势群落与对照相同,依次为糖类>氨基酸>羧酸>聚合物>胺类>酚酸代谢群落,土壤微生物群落结构稳定;重度放牧后,微生物对各类碳源的利用率显著下降,优势群落发生改变;冷蒿非根际土壤中,放牧强度增强,土壤微生物对不同种类碳源利用率变化较大,未显示一定的规律性,土壤微生物群落功能多样性变化很大,不稳定。

Table 2. Effect of soil microbial on the ability to utilize six types carbon source under different grazing intensity (96 h)
-
随放牧强度的增加,冷蒿根际和非根际土壤微生物的4种指数均显著降低(表 3)。Shannon指数和碳源利用丰富度指数表明放牧降低微生物群落功能多样性,减少碳源的利用数目,而冷蒿根际土壤微生物种类多且较均匀,利用的碳源数量较多;冷蒿根际土壤的Simpson指数显著高于非冷蒿根际,表明冷蒿能够显著提高优势菌的数量,削弱放牧对常见微生物物种的不良影响;LG-ARS和HG-ARS的McIntosh指数显著高于LG-NRS和HG-NRS,说明LG-ARS和HG-ARS的土壤微生物种类更为丰富,碳源利用程度较高;重度放牧后McIntosh指数最低,表明过度放牧会降低土壤微生物种类丰富度和碳源利用程度。

Table 3. Functional diversity indices for soil microbial community under different grazing intensity (96 h)
-
运用SPSS软件对培养96 h测定的AWCD数据进行主成分分析,得到2个与土壤微生物利用碳源多样性相关的主成分,累计贡献率达到69.3%。其中第1主成分(PC1)的方差贡献率为52.1%,权重最大,第2主成分(PC2)贡献率为17.2%。因其他主成分贡献率较小,因此只用PC1和PC2得分作图来表征微生物群落碳源代谢特征(图 3)。由图 3可知:不同处理在PC轴上出现明显的分布差异,HG-ARS位于PC1负方向,得分系数为-0.308,其他处理均位于PC1正方向,得分系数为0.160~1.030;HG-NRS位于PC2负方向,得分系数为-0.357,其他处理位于PC2正方向,得分系数范围为0.300~0.950。可见,提取的2个主成分基本上能够区分不同放牧强度ARS和NRS土壤类别的微生物群落功能多样性。另外,将主成分PC1和PC2的得分系数与31种单一碳源做相关性分析,其中与PC1相关的碳源有16种,其中11个呈负相关,主要是糖类、羧酸类和聚合物,肝糖与PC1显著负相关;5个呈正相关,主要是氨基酸类和胺类。与PC2相关的碳源有17种,其中15种呈正相关,主要是糖类和羧酸类碳源,L-苯丙氨酸与其相关性显著。可见羧酸类和氨基酸类碳源在主成分分离中具有主要贡献作用。
2.1. 不同放牧强度下土壤部分化学性质的变化
2.2. 不同放牧强度下土壤微生物的组成
2.3. 土壤微生物群落代谢活性的变化
2.4. 土壤微生物对不同类型碳源利用强度分析
2.5. 土壤微生物群落功能多样性指数分析
2.6. 不同放牧强度下土壤微生物碳源利用的主成分分析
-
土壤养分是土壤健康状况的重要指标,放牧对土壤有机质、元素循环产生影响。对中国西藏高原高山草甸[15]、科尔沁沙漠化草地[16]、松嫩平原羊草草甸[17]和玉树隆宝滩地区高寒草甸[18]的研究中均发现土壤有机质质量分数在放牧后显著降低,但是REEDER等[19]和WIENHOLD等[20]的研究结果与之相反,认为放牧能够增加土壤中的有机质等。安慧等[21]认为放牧过程动物通过粪便将消耗的植物养分大部分返还到土壤中,增加土壤碳氮输入,使有机质、氮素和速效钾、速效磷等增加。本研究结果与上述研究结果相似,放牧后土壤中有机质、全氮、全磷、全钾、碱解氮、速效钾和速效磷等增加,土壤pH值下降。同时,本研究还发现冷蒿根际土壤中有机质、全氮、碱解氮、速效磷和速效钾等养分显著高于非根际,而土壤的pH值显著低于非根际(P<0.05),冷蒿的生长能够有效改善土壤健康状况,降低放牧对土壤的扰动,可能是由于冷蒿的生物量、盖度、根茎比与牧压成正比[22],较大的根茎比增加了碳素等向地下的分配量[23],使得土壤中各化学组分的量增加;根系分泌大量的酸性物质[12],降低土壤pH值的同时增加各种元素的可溶性和可被利用性。
-
关于放牧对草原土壤微生物影响的研究很多,但结果不一致。WANG等[24]在美国佛罗里达州一草地的研究结果表明,放牧草地土壤微生物数量显著高于未放牧草地,停止放牧微生物数量也随之下降;BARDGETT等[25]研究表明:放牧能增加高原草地土壤的微生物量。有更多研究认为,适度放牧有助于土壤微生物数量的增加,过度放牧则会导致微生物数量显著降低[26]。与此类研究结果不同,本研究中放牧干扰降低了非根际土壤微生物数量,但冷蒿的生长却使土壤微生物数量在放牧后显著升高,并且相同放牧强度下,冷蒿根际微生物的数量均高于非根际(图 1)。表明冷蒿的生长能够降低放牧对土壤微生物产生的干扰,使土壤微生物能够较正常的生长繁殖,这可能是冷蒿在放牧后较其他草原植物仍能生长良好引起的。冷蒿被动物践踏破坏顶端优势后,其半匍匐型枝条生成不定根进行克隆生长[22, 27],其发达的根系及丰富的根系分泌物能够为土壤微生物提供丰富的生长基质和有利的生存环境,丰富了土壤微生物的能量来源。另外,冷蒿地上茎叶部分常释放出具有抑制动物采食、其他牧草种子萌发、幼苗生长和繁衍能力的挥发性物质,增强冷蒿的生存竞争力[28-29],这些特性为构建稳定土壤生态群落提供了坚实基础。冷蒿的良好生长为土壤微生物提供了良好的生存环境以及丰富的碳源,而对非根际土壤微生物来说土壤微环境破坏严重且可用的碳源较少而不能大量繁殖。本研究表明:土壤微生物量与冷蒿的有无具有密切关系,冷蒿能够为微生物提供相对稳定的微生态环境以及相对丰富的土壤养分,降低放牧对土壤的扰动,使土壤微生物量显著升高。
-
本试验采用Biolog-ECO板技术对内蒙古典型草原不同放牧强度下冷蒿根际和非根际土壤微生物功能多样性进行了研究,结果显示放牧后土壤微生物活性、4种微生物多样性指数及碳源利用能力均显著下降。导致微生物群落功能多样性下降的可能原因是放牧改变了地表植被多样性,使输入地下的植物残体、根系分泌物成分改变,加之牧畜践踏增强对土壤团聚体及地表的破坏,改变了土壤结构,使土壤微生物生境改变,从而影响土壤微生物的活性及群落多样性。当前,诸多研究证明草地利用、管理条件和植被类型的变化能显著改变土壤微生物群落组成、活性及功能多样性[30]。张海芳等[31]对内蒙古贝加尔针茅草原在放牧、刈割和围封等3种不同利用方式下土壤微生物功能多样性进行研究,发现放牧后土壤微生物代谢活性降低,但功能多样性增强;刈割与放牧方式下微生物群落碳源利用情况及代谢功能相似,而围封土壤微生物群落代谢活性最高,碳源利用模式及代谢强度也不同于放牧和刈割。李玉洁等[32]认为随着休牧年限增加,贝加尔针茅草原土壤微生物的代谢功能增强,数量增大;毕江涛等[33]研究荒漠草原5种不同植被类型土壤微生物活性、主要利用碳源类型、群落功能多样性均存在显著差异。可见,植物根际土壤微生物多样性不仅随着利用方式改变,还随着植物类型改变,具有很强的植物种的特异性。
本研究还发现:不同放牧强度处理下冷蒿根际土壤微生物活性、碳源利用能力及功能多样性等都高于非根际土壤。主成分分析表明:对照和轻度放牧后冷蒿根际与非冷蒿根际土壤差异不显著,重度放牧后两者差异显著,羧酸类和氨基酸类碳源在分异中起重要作用,植被类型和多样性的改变影响微生物的碳源利用,尤其体现在对这两类碳源的利用上[34-35]。其中羧酸类和氨基酸类碳源是根系分泌物的主要成分,分别与植物抗胁迫和土壤养分有效性有关[36]。根际土壤与非根际土壤微生物之间的差异与植物凋落物和根系分泌物息息相关,植物凋落物和根系分泌物是土壤微生物生长基质和有利环境的提供者。而不同植物的凋落物和根系分泌物化学组分差异很大[37],是植被类型影响土壤微生物活性及功能类群的主要推进力量[38]。王纳纳等[10]对内蒙古草原典型植物对土壤微生物群落影响的研究发现,植物不同土壤微生物群落组成不同,并且土壤微生物群落结构在根际和非根际间的差异大于不同物种间的差异,说明植物根际和非根际土壤性质和微生物群落功能多样性存在巨大不同。杨阳等[7]对宁夏荒漠草原不同植物根际与非根际微生物量分布特征的研究也发现,长芒草Stipa bungeana,蒙古冰草Agropyron mongolicum,甘草Glycyrrhiza uralensis等6种地带性优势植物根际土壤微生物量显著高于非根际土壤。滕应等[39]发现矿区土壤根际微生物数量、群落功能多样性、碳源利用类型及群落结构因种植牧草种类不同而发生相应变化,且根际土壤微生物代谢活性均显著高于非根际土壤。
3.1. 放牧对冷蒿根际土壤养分的影响
3.2. 放牧对冷蒿根际土壤微生物数量的影响
3.3. 放牧对冷蒿根际土壤微生物群落功能多样性的影响
-
本研究结果表明:放牧处理后,在冷蒿根际和非根际土壤化学性质、微生物量、群落结构和代谢功能上存在不同程度的差异。冷蒿根际土壤微生物量、代谢活性、碳源利用能力和多样性指数均高于非根际。LG-ARS微生物代谢活性最高,ck-ARS微生物多样性指数最高,对31种碳源利用最强,而HG-NRS微生物多样性指数均最低。冷蒿根际土壤微生物优势代谢群落为糖类、氨基酸类和羧酸类,相对稳定,而非根际优势代谢群落变化较大,不稳定。放牧处理后,冷蒿根际土壤pH值均低于pH 8.0,土壤养分均高于非根际。总之,冷蒿的“纯植株丛”生长方式能够改善土壤微环境,增加其根际土壤微生物群落功能多样性,从而增强抵抗放牧胁迫的能力,成为草场的优势群落。












 DownLoad:
DownLoad:

