-
桢楠Phoebe zhennan是樟科Lauraceae楠属Phoebe的常绿乔木,材质优良,树姿优美,属上等的材用和庭院绿化树种,为中国著名“金丝楠木”的重要原植物,产于四川、贵州西北部及湖北西部[1]。桢楠对低温敏感,严重制约其栽培地域。为此,研究桢楠的耐寒特性,掌握它在低温胁迫下的生理生化指标变化,为选育抗寒品种提供科学基础,对扩大桢楠的栽培区域具有重要意义。低温作为重要的非生物胁迫因子,对植物生长发育具有重要影响,可以造成植物矿质营养吸收、光合作用、呼吸作用和新陈代谢等生理过程的紊乱,对质膜和细胞结构造成损害[2-4]。林木抗寒性生理方面的研究已有诸多报道,在杨树Populus,葡萄Vitis vinifera,梨Pyrus,核桃Juglans regia等植物[5-8]的抗寒性研究发现,植物的抗寒性与相对电导率、相对含水率、水分饱和缺、超氧化物歧化酶(SOD)和过氧化物酶(POD)活性、丙二醛(MDA)和可溶性糖等具有相关性。目前,有关楠属植物抗寒性方面的研究还较少,滕开琼[9]研究发现超氧自由基产生速率,MDA,SOD和过氧化氢酶(CAT)活性能较好地反映桢楠幼苗的耐低温能力,可作为鉴定其抗寒性的生理指标。宗卫[10]研究发现,自然低温下氯化钙和水杨酸处理可以降低闽楠Phoebe bournei和桢楠幼苗的相对电导率和丙二醛含量,提高POD活性及可溶性糖、可溶性蛋白与叶绿素质量分数,提高植株的耐寒性。自然桢楠群落中存在大叶与小叶2种叶型,大叶型桢楠其正常叶较大,先端渐尖;小叶型桢楠的叶片较小,尾尖,观测发现两者的耐寒性不同。桢楠不同叶型对低温的生理响应比较尚未见报道。本试验选取了2种叶型的1年生桢楠,研究低温胁迫对其叶片膜系统、抗氧化酶活性及渗透物质含量的影响,并运用主成分分析法和隶属函数法对其耐寒性进行综合评价,为桢楠的抗寒品种评价、选育与引种栽培提供科学依据。
HTML
-
以大叶和小叶2种叶型桢楠的1年生实生苗为供试材料,选择生长健壮、长势相近的容器苗各12株。大叶桢楠采种于四川省雅安市(29°46′08.1″N,102°52′13.4″E,海拔为860 m);小叶桢楠采种于贵州省黔南布依族苗族自治州瓮安县(27°06′57.7″N,107°40′32.7″E,海拔为813 m)。
-
2015年12月10日将试验材料移入Snijders MC1000HE-EV智能低温人工气候箱(荷兰Snijders公司)中进行低温胁迫处理。将2个叶型桢楠容器苗分成3组,即设3次重复,4株·处理-1。温度梯度设置为8.0 ℃(对照),4.0 ℃,0.1 ℃(该仪器不可设置0 ℃),-4.0 ℃,-8.0 ℃,-12.0 ℃,各个温度梯度处理24 h。
-
在各温度梯度处理结束后采集叶片,用蒸馏水擦净表面污物,将叶片剪碎、混匀后,进行生理指标的测定,重复3次。
-
质膜透性的测定:参照蔡庆生[11]的方法,采用电导仪法测定相对电导率。相对电导率(%)=(E1-E0)/(E2-E0)×100%,其中:E0为对照电导率值,E1为处理电导率值,E2为处理煮沸后电导率值。丙二醛(MDA)测定采用硫代巴比妥酸法。
抗氧化酶活性测定:过氧化物酶(POD)活性采用愈创木酚法测定[12],超氧化物歧化酶(SOD)活性采用总超氧化物歧化酶(T-SOD)测试盒测定(南京建成生物研究所)。
有机溶质测定:参照张治安等[12]的方法,可溶性糖采用蒽酮法测定,可溶性蛋白采用考马斯亮兰染色法测定,脯氨酸采用脯氨酸(Pro)测定试剂盒测定(南京建成生物研究所)。
-
参照刘杜玲等[13-15]的方法,运用主成分分析和隶数函数法对桢楠的抗寒性进行综合分析。抗冻系数=处理测定值/对照测定值×100%。隶属函数值U(Xj)=(Xj-Xmax)/(Xmax-Xmin),j=1, 2, …, n。其中:Xj表示第j个综合指标;Xmin表示第j个综合指标的最小值;Xmax表示第j个综合指标的最大值。权重${W_j} = {P_j}/\sum\limits_{j = 1}^n {{P_j}} $,j=1, 2, …, n。其中:权重Wj表示第j个综合指标在所有综合指标中的重要程度;Pj为各类型桢楠第j个综合指标的贡献率。综合评定值${D_i} = \sum\limits_{j = 1}^n {\left[{U\left( {{X_j}} \right) \times {W_j}} \right]} $,i=1, 2, …, k; j=1, 2, …, n。其中:Di值为各类型桢楠在低温条件下用综合指标评价所得的耐寒性综合评价值,k为桢楠类型数。
-
运用Excel和DPS软件进行数据处理和统计分析,用Origin软件作图。
1.1. 试验材料
1.2. 试验方法
1.3. 样品采集
1.4. 测定方法
1.5. 桢楠耐寒性综合评价方法
1.6. 数据处理
-
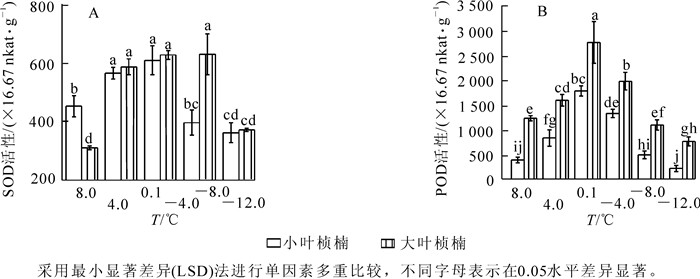
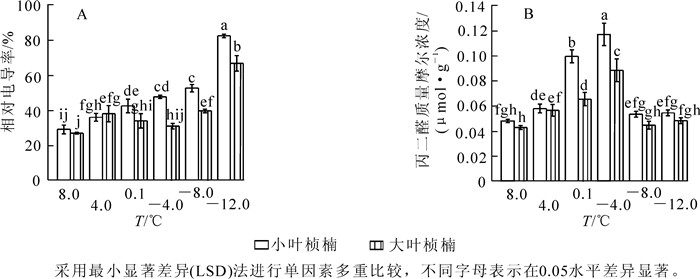
2种叶型桢楠在8.0 ℃时相对电导率差异不显著,随温度降低小叶桢楠相对电导率呈持续上升规律,4.0 ℃时已达显著水平,-8.0 ℃后急剧上升,-12.0 ℃时升高到峰值82.0%,为对照的284.7%;大叶桢楠相对电导率至4.0 ℃时显著升高,之后略有下降,-4.0 ℃后缓慢升高,-8.0 ℃时急剧升高,-12.0 ℃时升高到峰值66.4%,相当于对照的249.7%(图 1A)。
随处理温度降低,2种叶型桢楠丙二醛质量摩尔浓度变化均呈先上升后下降趋势(图 1B)。8.0 ℃和4.0 ℃时2种叶型桢楠丙二醛质量摩尔浓度差异不显著,4.0 ℃后小叶桢楠的丙二醛质量摩尔浓度急剧增加,到0.1 ℃时丙二醛质量摩尔浓度的增幅达72.4%,是大叶桢楠增幅的4.6倍。-4.0 ℃时2种叶型桢楠丙二醛质量摩尔浓度都到达峰值,分别为0.116 5和0.088 1 μmol·g-1,为对照的2.5倍和2.1倍。之后随温度降低,丙二醛质量摩尔浓度迅速降低,-8.0 ℃和-12.0 ℃时降至较低水平,两者之间差异不显著,各自与对照差异也不显著。由图 1B可以看出:0.1 ℃和-4.0 ℃时,小叶桢楠丙二醛质量摩尔浓度和总体变化幅度均显著高于大叶桢楠,可见低温胁迫对小叶桢楠的破坏更严重。
-
图 2A可以看出:低温处理前期桢楠的SOD活性升高。8.0 ℃时小叶桢楠SOD活性显著高于大叶桢楠,至4.0 ℃时2种叶型桢楠的SOD活性均显著增加到较高水平。其中大叶桢楠SOD活性上升速度更快,之后保持在较高水平,4.0 ℃和0.1 ℃时,两者差异不显著,-4.0 ℃时大叶桢楠SOD仍保持较高活性,而此时小叶桢楠SOD活性已显著降低,两者差异显著。POD活性变化趋势2种叶型桢楠相似,呈先上升后下降(图 2B),从8.0 ℃至4.0 ℃间,小叶桢楠POD活性增加了116.3%,达到显著水平,大叶桢楠仅增加了29.3%,未达到显著水平。可见小叶桢楠比大叶桢楠对低温更敏感。之后,两者POD活性都急剧上升,至0.1 ℃时大叶桢楠和小叶桢楠的POD活性都达到峰值,分别为2 753.3×16.67 nkat·g-1,1 776.7 ×16.67 nkat·g-1,为对照的224.5%和464.8%,且在各温度下大叶桢楠的POD活性均显著高于小叶桢楠。从图 2可见:低温胁迫下大叶桢楠的SOD和POD活性均明显高于小叶桢楠,-4.0 ℃时活性分别是小叶桢楠的1.6倍,1.5倍。
-
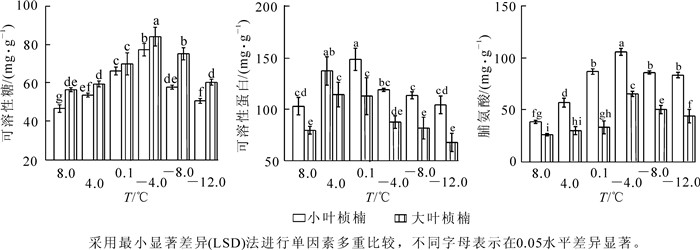
不同低温胁迫下2种叶型桢楠的可溶性糖质量分数的测定结果见图 3A。处理温度在不低于-4.0 ℃时,2种叶型桢楠的可溶性糖质量分数随温度降低均连续上升,-4.0 ℃时2种叶型桢楠可溶性糖质量分数都升高到峰值,分别为77.52,84.27 mg·g-1,是对照的1.7倍和1.5倍。小叶桢楠可溶性糖质量分数在4.0 ℃,0.1 ℃,-4.0 ℃时增幅分别为14.9%,23.4%,16.7%,变化均达到显著水平;大叶桢楠可溶性糖质量分数4.0 ℃时变化未达到显著水平,之后增幅较大,0.1 ℃和-4.0 ℃时分别为17.6%和20.1%,差异显著。各处理温度下,大叶桢楠可溶性糖质量分数始终高于小叶桢楠,除0.1 ℃外,差异都到达显著水平。

Figure 3. Effect of low temperature stress on the content of soluble sugar%A), soluble protein (B) and proline (C)
2种叶型桢楠不同处理温度下的可溶性蛋白质量分数如图 3B所示,8.0 ℃至4.0 ℃间均显著升高,4.0 ℃时均达到了显著差异水平。随着处理温度的进一步降低,小叶桢楠进一步升高,0.1 ℃时达到峰值;而大叶桢楠4.0 ℃后已呈下降趋势。0.1 ℃后2种桢楠的可溶性蛋白质量分数均下降,-4.0 ℃,-8.0 ℃,-12.0 ℃时与对照差异都不显著。低温胁迫下小叶桢楠可溶性蛋白质量分数和变化幅度都大于大叶桢楠。
不同处理温度下2种叶型桢楠的脯氨酸质量分数变化如图 3C所示,均呈先升高后降低的变化趋势。其中小叶桢楠增长幅度显著大于大叶桢楠,4.0 ℃,0.1 ℃和-4.0 ℃时脯氨酸质量分数增长都达到显著水平,-4.0 ℃时质量分数最大为116.54 μg·g-1,较对照增长174.3%。大叶桢楠在0.1 ℃之前脯氨酸质量分数变化呈缓慢增加,0.1 ℃至-4.0 ℃间急剧上升,达到峰值58.65 μg·g-1,较对照增长149.2%。小叶桢楠对低温响应较早,且相对增幅显著高于大叶桢楠。
-
植物在逆境条件下产生复杂的生理变化过程,单一生理生化指标难以科学评定植物对逆境的适应能力[5, 13]。对各指标进行相关性分析,得出相关系数矩阵(表 1)。由相关系数矩阵可以看出,各项生理指标之间存在相关性,使得它们提供的信息发生重叠,各单项生理指标在桢楠耐寒中发挥的作用也不尽相同,为此采用主成分分析法进行综合性评价。综合考虑各种温度处理下各生理生化指标变化趋势,选取-4.0 ℃低温处理下的各项指标进行2种叶型桢楠耐寒性的综合评价。根据各项指标值,计算各项指标的耐寒系数(表 2)。
指标 相对电导率 丙二醛 超氧化物歧化酶 过氧化物酶 可溶性糖 可溶性蛋白 丙二醛 0.047 超氧化物歧化酶 -0.041 0.046 过氧化物酶 -0.415* 0.381* -0.277 可溶性糖 -0.013 0.602** -0.177 0.636** 可溶性蛋白 -0.126 0.321 0.161 0.094 -0.119 脯氨酸 0.492** 0.586** 0.186 -0.195 0.275 0.425** 说明:**表示0.01水平上显著相关 (p < 0.01); *表示0.05水平上显著相关 (p < 0.05)。 Table 1. Correlation matrix of each single index
类型 各指标的耐寒系数/% 相对电导率 丙二醛 超氧化物歧化酶 过氧化物酶 可溶性糖 可溶性蛋白 脯氨酸 小叶桢楠 167.85 244.60 87.71 354.74 115.89 274.38 165.55 大叶桢楠 111.44 200.86 202.52 160.84 109.87 249.21 148.84 Table 2. Freezing resistant coefficient of each single index
对各单项指标进行主成分分析,提取前2个主成分作为综合指标,它们的贡献率分别为:73.17%和19.73%,累积贡献率达到92.90%(表 3)。根据隶属函数计算公式计算出2种叶型桢楠综合指标的隶属函数值,再取各综合指标的贡献率,用权重计算公式计算各综合指标的权重,得出2个综合指标的权重为0.787 6和0.212 4(表 4)。
主成分 相对电导率 丙二醛 超氧化物歧化酶 过氧化物酶 可溶性糖 可溶性蛋白 脯氨酸 贡献率/% 1 0.175 -0.015 -0.184 0.310 0.337 -0.144 0.126 73.17 2 0.124 0.334 -0.129 -0.094 -0.173 0.433 0.202 19.73 Table 3. Coefficient of comprehensive index and their contribution rate
类型 综合指标值CI1 综合指标值CI2 隶属函数值U(X1) 隶属函数值U(X2) 综合评定值D 小叶桢楠 0.848 1 0.600 4 0.358 7 0.619 5 0.414 1 大叶桢楠 -0.848 1 -0.600 4 0.516 8 0.504 8 0.514 3 权重 0.787 6 0.212 4 Table 4. The value of comprehensive index, index weight, U(Xi) and Di
用综合评定值公式计算各类型桢楠低温胁迫下综合评定值Di。根据D值(表 4),对不同类型桢楠的耐寒性进行比较,D值越大,耐寒性越强。结果显示,小叶桢楠和大叶桢楠的综合评定值分别为0.414 1和0.514 3,表明大叶桢楠的耐寒性强于小叶桢楠。
2.1. 低温胁迫对2种叶型桢楠相对电导率和丙二醛质量摩尔浓度的影响
2.2. 低温胁迫对桢楠抗氧化酶活性的影响
2.3. 低温胁迫对有机溶质质量分数的影响
2.4. 2种叶型桢楠耐寒性的综合评价
-
相对电导率和丙二醛在低温胁迫下变化的研究已有诸多报道,植物受到低温逆境胁迫后,其膜脂首先发生相变,由液晶相转变成凝胶相,使膜透性发生改变,胞内离子外渗[16],引起相对电导率升高。丙二醛是膜脂过氧化作用的产物[17],为此,相对电导率和丙二醛质量摩尔浓度常作为反映生物膜受伤害程度的指标[18-20],且与植物的抗寒能力呈负相关。本研究表明,2种叶型桢楠经低温处理后,叶片相对电导率升高,与葡萄,杏Armeniaca vulgaris,藜麦Chenopodium quinoa等植物[6, 21-22]低温胁迫下的测定结果一致,说明在一定低温下,桢楠叶片的细胞膜透性被损坏。2种叶型桢楠丙二醛质量摩尔浓度均先上升后下降,这与黑壳楠Lindera megaphylla,墨西哥玉米Zea mexicana等植物[23-24]低温胁迫下的测定结果一致,前期上升说明低温引起桢楠叶片细胞膜质过氧化,且随着温度降低,过氧化程度不断加剧。2种叶型桢楠相对电导率和丙二醛质量摩尔浓度变化幅度明显不同,表明2种叶型桢楠的耐寒性存在差异,在0.1 ℃时,小叶桢楠虽然叶片、枝干上尚未出现外观变化,但已出现明显的细胞膜伤害,而大叶桢楠则在-4.0 ℃时出现这种生理变化,可以认为大叶桢楠耐寒性显著高于小叶桢楠。
植物体内存在抗氧化酶保护系统,正常条件下活性氧的产生和清除处于动态平衡状态[25]。低温胁迫下,植物会产生大量的活性氧自由基,为抵御活性氧对细胞的伤害,植物抗氧化酶活性增强,以清除活性氧。但这种清除作用存在限制,随着自由基的大量产生,当清除速度小于生成速度时,则动态平衡被打破,对植物细胞造成伤害[26]。本试验发现,SOD和POD活性均呈现先升后降的变化趋势,说明桢楠在胁迫前期,产生了显明的应激反应,通过提高SOD和POD活性来消除体内产生的自由基,抵御低温带来的伤害,与前人的研究结果一致[6, 27-28]。大叶桢楠SOD上升幅度显著高于小叶桢楠,该结果印证了细胞膜透性的变化趋势,证实在0.1 ℃胁迫下,小叶桢楠的的活性氧自由基已超出其自身的清除能力,产生了冷冻伤害,而大叶桢楠此时还处于上升阶段,但到-4.0 ℃后呈下降趋势。
低温胁迫下植物通过积累可溶性糖、可溶性蛋白、脯氨酸等物质降低细胞渗透势,保护生物膜,维持细胞结构提高植物自身对低温的适应性[29-33]。多数研究认为低温胁迫下,可溶性糖、可溶性蛋白、脯氨酸等物质的质量分数与植物抗寒性正相关。但在黑麦草Lolium perenne[34]和结缕草Zoysia japonica[35]等的研究中发现可溶性糖质量分数与植物抗寒性无相关关系;武兰芳[36]在研究低温下玉米Zea mays可溶性蛋白质量分数变化时发现,随着低温处理时间的延长可溶性蛋白质量分数逐渐降低;JACKSON[37],何开跃等[38]认为:脯氨酸的积累可能是植物对低温的一种适应或者细胞受损的一种表现,与植物的耐寒能力无明确相关关系。本研究表明:低温胁迫下,桢楠的可溶性糖、可溶性蛋白、脯氨酸质量分数均逐渐升高,在达到一定的临界低温后,呈现下降的趋势,这与杨玉珍等[39]的研究一致。可以认为:低温胁迫下桢楠可通过生成有机溶质来抵御低温伤害,但3类物质出现峰值的低温不同,且2种叶型桢楠间也不相同。其中大叶桢楠的可溶性糖质量分数显著高于小叶桢楠,而可溶性蛋白和脯氨酸质量分数则显著低于小叶桢楠。由此可见:植物在低温胁迫下可诱导有机溶质的产生,并随细胞功能的损害而下降,但其质量分数直接用于衡量其耐寒能力具有一定的局限性。
植物的耐寒性是受多种因素影响的数量性状[13],在低温胁迫下涉及多种生理生化指标的变化,难以采用单一指标对耐寒性做出准确评价[40]。主成分分析法作为综合评价植物抗性的方法,目前已在茶树Camellia sinesis,葡萄等[41-42]多种植物的耐寒性评价上被采用。本试验采用7个单项指标并根据其贡献率的大小组合成2个综合指标评价桢楠的耐寒性强弱,再结合隶属函数法,得到2种叶型桢楠的综合评定值,对小叶桢楠和大叶桢楠的耐寒性做出综合性评价,证实了大叶桢楠耐寒性优于小叶桢楠。











 DownLoad:
DownLoad: