-
碳水化合物是光合作用的最终产物,不仅为植物体的生长发育和生理代谢提供能量,同时也作为信号分子在物质运输、渗透调节、抵抗胁迫、基因表达等方面起重要作用[1]。樱桃番茄Lycopersicum esculentum var. cerasiforme果实在发育成熟过程中,蔗糖被不断分解,而葡萄糖和果糖逐渐积累[2]。对温州蜜柑Citrus unshiu研究发现[3],蜜柑果实成熟过程中可食组织中糖不断增加,而果糖激酶活性不断下降。拟南芥Arabidopsis thaliana中编码淀粉降解相关基因的表达受光照时间和生物钟调控,持续黑暗条件下,碳水化合物水平、转录水平和蛋白质水平相互协调,糖促进拟南芥下胚轴伸长[4-5]。海藻糖可以诱导光合器官中调控碳水化合物合成酶的激活[6],高浓度海藻糖抑制拟南芥根中碳水化合物分配,抑制根的伸长[7];逆境条件下植物体内海藻糖含量增加以抵抗胁迫[8]。WINGLER等[9]发现海藻糖通过调节糖调控基因的表达影响拟南芥幼苗的生长代谢。海藻糖磷酸合成酶(trehalose-6-phosphate synthase, TPS1)作为一种信号来调控海藻糖-6-磷酸(T6P)的合成,T6P通过磷酸海藻糖磷酸酶(TPP)转化为海藻糖,从而促进植物的生长发育[10]。对水稻Oryza sativa[11],烟草Nicotiana tabacum[12]和小麦Triticum aestivum[13]等植物幼苗研究发现:植物体通过调控TPS1基因的表达来增加体内海藻糖质量分数,从而提高其抗逆性。植物蔗糖非发酵-1-型相关蛋白激酶1(sucrose non-fermenting-1(SNF1)-related kinase 1, SnRK1)是植物中糖信号调控的一种关键激酶,广泛参与植物的细胞周期调控、生长发育、病虫害防御、激素信号传导和非生物胁迫等各种信号的应答反应[14]。能量匮乏条件下,拟南芥SNF1相关蛋白激酶催化亚基KIN10和KIN11促进合成代谢基因的表达,抑制分解代谢基因的表达以响应胁迫[15]。反义表达SnRK1马铃薯Solanum tuberosum叶片丧失蔗糖合成酶的转录功能[16];对水稻和拟南芥研究发现:SnRK1活性的高低严重影响着胁迫诱导型基因的表达,从而增强植物抗逆性[17]。植物中可溶性糖和SnRK1一同作为生物发育的糖信号调控物质,与糖信号途径相互作用。能量缺乏时,SnRK1抑制植物体生长,T6P通过抑制SnRK1的表达来调控基因的表达,保证植物在营养缺乏逆境条件下能够存活生长[18]。ZHANG等[19]研究发现,微摩尔浓度的T6P就可以抑制拟南芥幼苗及其他植物幼嫩组织SnRK1活性。葡萄糖和蔗糖可以缓解高浓度海藻糖对植物生长的抑制作用,蔗糖和海藻糖在糖感受调控基因的表达中有相似的作用,海藻糖能代替蔗糖,调控碳水化合物的代谢[20]。蔗糖调控基因SnRK1与海藻糖相互作用,调控植物碳代谢、基因表达,为植物的生长发育及其代谢提供保证[21]。毛竹Phyllostachys edulis是重要的经济资源、造园材料,关于毛竹的研究目前主要集中在茎秆解剖结构[22]、不同生长期茎秆色素含量[23]、快速生长期水势变化[24]、基因组学及快速生长期节间伸长相关蛋白的表达[25-26]等方面,尚未有关于毛竹快速高生长过程中糖代谢的研究。本研究以快速生长期毛竹笋竹为实验材料,分析毛竹中可溶性糖质量分数及PeSnRK1,PeTPS1基因的表达情况,研究其表达差异性,以期探索毛竹生长过程中糖调控基因如何调控碳水化合物来指导幼笋的快速生长,为毛竹及其他树种的快速生长机制的阐述提供新的理论依据。
HTML
-
供试材料毛竹采自浙江农林大学毛竹示范基地,地理位置为29°56′~30°23′ N,118°51′~119°52′ E,属中亚热带季风气候区,四季分明,温暖湿润,年平均气温为16.4 ℃,气候特点为春多雨,夏湿热,秋气爽,冬干冷,年平均降水量为1 628.6 mm,年平均日照时数1 847.3 h。森林覆盖率为76.5%。
-
2015年4月,在浙江农林大学毛竹试验基地挑选生长健壮的2 m高毛竹笋竹,以18:00为黄昏时,分别于黄昏后0,4,8 h取材。选取3株生长健壮的笋竹3株·次-1,从基部将其伐倒,挖出竹蔸,将地上部分平均分成3段,各段取中间1节记为上部、中部、下部,并对竹蔸中间位置进行取样,将样品迅速放进液氮中冷冻,存于-80 ℃备用。
-
葡萄糖、果糖和蔗糖质量分数测定:称取0.5 g笋竹于研钵中研磨,加蒸馏水8.0 mL,80 ℃恒温水浴30 min,冷却,定容10.0 mL。离心(3 000 g,5 min),上清液为可溶性糖提取液。葡萄糖、果糖和蔗糖质量分数采用可溶性糖试剂盒(南京建成科技有限公司)测定。重复5次·样品-1。
海藻糖质量分数测定:参考苏州科铭生物技术有限公司海藻糖试剂盒,加入5.0 mL提取液,称取0.5 g笋竹于研钵中冰浴研磨,室温静置45 min,常温下8 000 g离心10 min,取上清。取60 μL样本和240.0 μL工作液至EP管中,95 ℃水浴10 min,自然冷却至室温后,取200.0 μL至96孔板中,620 nm波长下测定吸光度D(λ)值。重复5次·样品-1。
-
称取0.5 g笋竹,使用改进Trizol法进行总核糖核酸(RNA)提取。使用分光光度计检测样品RNA的纯度,并用琼脂糖电泳检测RNA的完整性。以上述RNA为模板,冰上配置10.0 μL实时荧光定量聚合酶链式反应(qRT-PCR)体系:包括5×Prime Script RT Master Mix 2.0 μL,总RNA 500 ng,加去RNA酶的水(RNase Free H2O)至总体积10.0 μL。置于PCR仪中,37 ℃孵育15 min,85 ℃加热5 s。反转录得到的cDNA存于-20 ℃用于实时荧光定量分析。
-
根据毛竹基因组数据库中PeTPS1和PeSnRK1基因序列设计基因全长及定量引物,选择毛竹PeNTB基因作为荧光定量的内参基因,引物设计使用Primer 5.0软件,并由上海生工合成以此设计特异性引物(表 1),扩增这2个基因的系列片段。扩增程序为:94 ℃ 5 min;94 ℃ 30 s,60 ℃ 30 s,72 ℃ 30 s,共35个循环;72 ℃ 10 min。质量分数为1.0%的琼脂糖凝胶电泳分离PCR扩增产物。回收纯化目的条带,连接到pMD19-T载体上并转化至大肠埃希菌Escherichia coli(DH5α),菌落PCR鉴定阳性克隆后提取质粒,送往上海生工生物工程服务有限公司测序。
基因名称 序列 用途 PeSnRK1-F ATGGAGGGAGCCGGCAGAGATGCGA 基因开放阅读框(ORF)扩增 PeSnRK1-R TCAAAGGACTCTCAGCTGAGTTAGA PeSnRK1-F AGCTCGACGATGAAACCCTT 荧光定量PCR PeSnRK1-R TTCCATAGAACCGTACTGCCTA PeTPS1-F ATGGACACCTACGCCGCGGAGCCCGCCTC 基因开放阅读框(ORF)扩增 PeTPS1-R TTAATCAGCAGTGCTAGACTGGAAGCCAGT PeTPS1-F ACTCCCTAGTCGGACGGCAA 荧光定量PCR PeTPS1-R CATGCTCTGCCGCCAACCAC PeNTB-F TCTTGTTTGACACCGAAGAGGAG 荧光定量PCR PeNTB-R AATAGCTGTCCCTGGAGGAGTTT Table 1. PCR primers used in this study
-
采用TaKaRa公司SYBR Premix Ex TaqTM(perfect real time)试剂盒,20.0 μL反应体系中包含10.0 μL SYBR Premix Ex TaqTM,0.8 μL正向引物,0.8 μL反向引物,2.0 μL反转录cDNA模版,6.4 μL灭菌蒸馏水。反应于Bio-Rad CFX manager 3.1 PCR仪上进行,以内参为对照,平行反应做5次·样品-1。采用两步法扩增标准程序:95 ℃预变性3 min,95 ℃ 10 s,60 ℃ 30 s,39次循环。反应完成后,得到含所有样品的记录点曲线,得出循环阈值(Ct值)。
-
所得初始数据均经软件进行整理,荧光定量数据按照公式计算:相对表达量=2ΔΔCt[27],使用内参基因校正拷贝数,利用Origin 9.0软件进行统计分析和作图。
1.1. 试验地概况
1.2. 材料
1.3. 可溶性糖测定
1.4. 毛竹笋竹总RNA的分离纯化以及cDNA合成
1.5. 笋竹PeTPS1及PeSnRK1基因全长cDNA克隆
1.6. PeTPS1及PeSnRK1反转录产物的基因表达分析
1.7. 数据处理
-
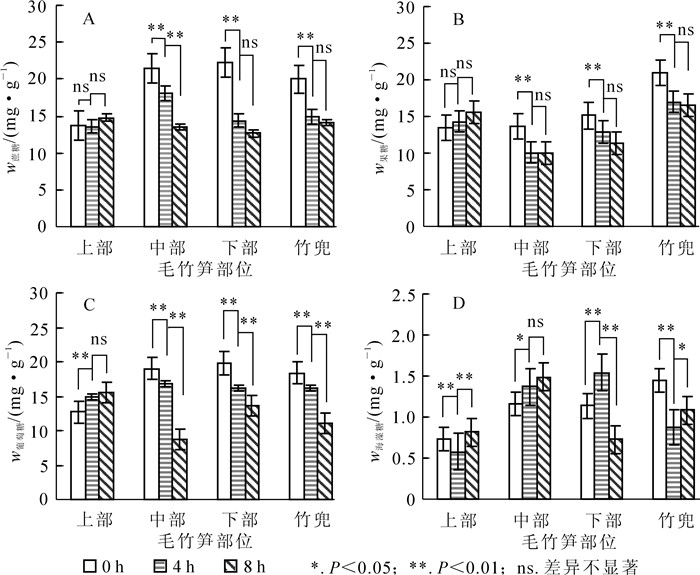
笋竹可溶性糖质量分数变化分析表明,黄昏后0 h到8 h,笋竹中部、下部和竹蔸蔗糖质量分数随黄昏时间的增加均呈现逐渐下降的趋势,其中黄昏后4 h,中部、下部和竹蔸蔗糖质量分数极显著下降(P<0.01);黄昏后8 h蔗糖消耗量极显著高于黄昏时(P<0.01);上部各时间蔗糖质量分数变化无显著差异(图 1A)。黄昏后笋竹果糖与蔗糖质量分数变化相似,黄昏时,笋竹中部、下部和竹蔸果糖质量分数最高,夜间整体呈现逐渐下降的趋势;黄昏时竹蔸中果糖质量分数最高,为上部的1.6倍,上部随黄昏时间增加果糖质量分数变化不显著(图 1B)。葡萄糖质量分数变化如图 1C所示,笋竹上部葡萄糖质量分数呈逐渐上升的趋势,黄昏后8 h达到最大值,此时葡萄糖质量分数为黄昏时1.2倍,中部、下部、竹蔸葡萄糖质量分数随时间变化均呈现极显著下降的趋势(P<0.01),黄昏时葡萄糖质量分数分别为黄昏后8 h的2.2倍、1.4倍和1.6倍。毛竹笋竹快速生长期海藻糖质量分数变化见图 1D。黄昏时,竹蔸海藻糖质量分数为1.45 mg·g-1,分别为下部、中部、上部的1.3倍、1.2倍和2.0倍;黄昏后4 h,笋竹下部、中部海藻糖质量分数极显著升高(P<0.01),均高于上部;笋竹下部海藻糖质量分数呈现先上升再下降的的趋势,在黄昏后4 h达到最大值。

Figure 1. Changes of bamboo shoots soluble sugar mass fraction in different parts of Phyllostachys edulis
上述结果表明:毛竹笋竹各部位中蔗糖、果糖、葡萄糖、海藻糖质量分数变化在不同时间内具有相似的表达模式。夜间笋竹中部和下部处于快速生长期,需要消耗大量碳水化合物,上部基本不生长,可溶性糖质量分数变化不显著;竹蔸是笋竹快速生长期能量的暂存和周转的重要部位,可溶性糖质量分数一直较高。
-
以确定纯度和完整度较好的毛竹总RNA的反转录产物cDNA为模板,用普通PCR扩增笋竹PeTPS1及PeSnRK1基因全长,对扩增产物进行质量分数为1%琼脂糖凝胶电泳检测(图 2),条带清晰且单一,获得与预期片段大小一致、长度分别为1 440 bp和1 476 bp扩增产物。
-
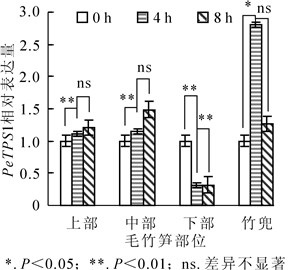
黄昏后笋竹PeTPS1表达量变化如图 3所示。笋竹竹蔸与其他部位相比PeTPS1表达丰度较高,中部和上部PeTPS1表达丰度次之,下部表达量最低。黄昏后4 h笋竹竹蔸PeTPS1表达量达到极显著水平(P<0.05),分别是下部、中部和上部的9.0倍、2.4倍和2.5倍。下部PeTPS1表达量在整个夜间随时间变化被不同程度下调,黄昏时PeTPS1表达量较高,分别为4 h和8 h的3.2倍;中部和上部PeTPS1基因具有相同的表达模式,黄昏时表达丰度较低,黄昏后8 h转录水平达到高峰。上述结果表明:竹蔸在毛竹快速生长阶段为其他部位提供碳源,笋竹下部已完成初生生长,海藻糖需求量小,PeTPS1基因表达量低。
-
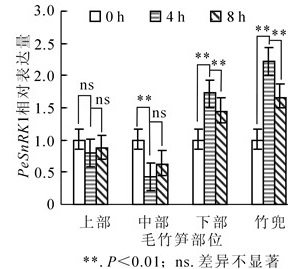
快速生长的毛竹笋竹PeSnRK1基因表达情况如图 4所示。从基部到顶部不同节间PeSnRK1表达量呈先下降再上升的趋势,竹蔸表达丰度最高。上部和中部具有相同的表达模式,黄昏后4 h PeSnRK1表达量下降,黄昏后8 h又呈现上升的趋势。下部和竹蔸PeSnRK1基因转录水平变化相同,黄昏后4 h PeSnRK1基因表达量显著上升,转录水平达到高峰;黄昏后8 h PeSnRK1基因表达量又迅速下降,黄昏后4 h竹蔸种PeSnRK1基因表达量分别是下部、中部和上部的1.3倍、5.2倍和2.8倍。上述结果表明:黑暗条件下,快速生长期的毛竹笋竹中竹蔸和下部已停止生长或生长缓慢,PeSnRK1表达量较高,中部处于快速生长期,PeSnRK1表达丰度相对较低,上部生长速度不明显。
2.1. 毛竹笋竹快速生长过程中可溶性糖质量分数变化
2.2. 笋竹PeTPS1及PeSnRK1基因cDNA全长克隆及验证
2.3. 毛竹笋竹快速生长过程中PeTPS1基因表达模式
2.4. 毛竹笋竹快速生长过程中PeSnRK1基因表达模式
-
糖是植物光合作用的产物,呼吸作用的底物,糖类的合成与分解影响植物体内糖分的运输,作为一种信号分子调节植物的生长发育过程[28]。例如,葡萄糖和蔗糖可以缓解高浓度海藻糖对植物生长的抑制作用,蔗糖和海藻糖在糖感受调控基因的表达中有相似的作用,海藻糖能代替蔗糖,调控碳水化合物的代谢[29]。笋竹茎秆处于快速生长阶段,本研究中,笋竹上部生长发育、老化较晚,主要进行初生生长,耗能较低,可溶性糖质量分数几乎没有变化;中部和下部作为同化产物的利用组织代谢活力大幅度上调,随时间的变化蔗糖、果糖、葡萄糖质量分数均显著下降,而海藻糖质量分数处于上升的趋势,可能是因为中部处于显著伸长期,需要消耗更多的碳水化合物,合成海藻糖以补充能量;黄昏后8 h与4 h相比,可溶性糖及海藻糖质量分数下降均不显著,其原因可能是下部生长逐渐缓慢,所需能量减少;竹蔸作为输出同化产物的“源”输出被上调,黄昏后4 h可溶性糖质量分数显著下降,合成大量T6P以维持竹蔸的生命活动,供应地上部分的快速生长。这与董丽娜[22]结论相似,笋竹各节的伸长活动自下而上,按慢—快—慢的规律,以节间为生长单位进行生长。
-
能量缺乏时,T6P通过抑制SnRK1的表达来调控基因的表达,保证植物在逆境条件下能够存活生长[18]。丁菲等[30]对茶树不同器官中TPS表达量的研究发现不同组织中TPS表达量不同,而这种差异可能是由于组织和器官的生存环境、空间位置、发育状况及代谢活动的不同造成的。在我们的研究中,笋竹各部位PeTPS1基因的表达随时间的变化呈现升高—降低—再升高的趋势。T6P作为一种信号整合由SnRK1调控的碳代谢、酶反应以及相关基因表达重排,从而调控植物的生长发育[31]。例如,拟南芥中Akinl0过量表达会诱导TPS1基因表达[32],通常蔗糖通过促进T6P的积累来抑制SnRK1活性,植物生长发育过程中细胞内蔗糖不断被水解为果糖和葡萄糖,而SnRK1被高水平蔗糖或低水平葡萄糖激活[14]。本研究中,笋竹上部几乎不生长,随时间变化PeTPS1及PeSnRK1基因表达量几乎没有变化。正常条件下植物体内SnRK1水平较低,高水平SnRK1抑制植物生长[33],ZHANG等[19]研究发现,微摩尔浓度的T6P就可以抑制拟南芥幼苗及其他植物幼嫩组织提取物中SnRK1活性。我们的结果显示,笋竹中部随时间的变化PeTPS1表达量呈逐渐上升的趋势,此时PeSnRK1表达量最低;下部已基本完成节间生长,PeSnRK1表达量与上部、中部相比较高,而PeTPS1相对表达量较低,可能是因为下部已完成基本生长,T6P失去对PeSnRK1活性的抑制作用[19];竹蔸是毛竹供应维持生长的碳水化合物的核心部位,需要分解白天储存的淀粉以供应其夜间生长,因此,黄昏后竹蔸中PeSnRK1表达量处于最高。PeTPS1基因表达量呈现出与海藻糖变化相同的趋势,说明毛竹快速生长过程中PeTPS1调控海藻糖的合成。黄昏后4 h竹蔸中PeSnRK1基因被激活,表达量达到峰值,可能是因为夜间毛竹不能进行光合作用,而PeSnRK1的表达则通过T6P含量进行调控。PeTPS1/PeSnRK1基因均存在组织特异性表达,基因的调控表达受不同发育时期的影响。
TPS1不仅参与植物体海藻糖的合成,而且调控糖酵解途径。蔗糖调控基因SnRK1与海藻糖相互作用,调控植物碳代谢及相关基因表达,为植物的生长发育及其代谢提供保证[21]。高蔗糖/低葡萄糖情况下SnRK1被激活,诱导分解代谢基因的表达,激活淀粉合成途径关键酶[34]。本研究中,SnRK1的标记基因TPS1被SnRK1抑制,通过海藻糖上调,这与TSAI等[35]研究结果相似,T6P/SnRK1信号同时参与植物的代谢调控及基因表达调控。笋竹上部伸长生长较慢,对可溶性糖需求量小,糖质量分数及其调控基因变化不显著,PeSnRK1表达量水平较高;黄昏后笋竹中部葡萄糖、蔗糖和海藻糖质量分数较多,PeTPS1表达水平较高,T6P的浓度可能会比较高,进而抑制PeSnRK1的表达量及活性。植物通过调节T6P水平来调控体内蔗糖质量分数,从而促进幼苗在低蔗糖水平下的生长发育[11],中部伸长生长快,可溶性糖消耗较多,PeSnRK1表达量水平较低;竹蔸作为储藏分配碳水化合物的重要部位,黄昏后4 h可溶性糖质量分数显著下降,PeTPS1基因表达量被上调,合成T6P以维持竹蔸的生命活动,供应地上部分的快速生长。综上所述,毛竹快速生长过程中糖调控相关基因之间交错调控共同控制糖分的分配与运输,以保证笋竹的快速生长,T6P/ SnRK1信号通路作为必要的生长机制调,在毛竹笋竹快速生长发育过程的起到重要的调控作用。
通过对快速生长期毛竹笋竹夜间各部分可溶性糖质量分数及PeTPS1和PeSnRK1基因在多个部位中表达的分析可知,笋竹养料储存部位竹蔸可溶性糖质量分数及相关基因表达量始终处于较高的水平;笋竹中部和下部处于快速生长期,可溶性糖质量分数随时间变化不断下降,快速伸长期部位中PeSnRK1基因的表达呈现与PeTPS1相反的模式;上部生长发育缓慢,可溶性糖质量分数及PeTPS1和PeSnRK1基因表达无显著变化。结合上述研究,本实验认为T6P/ SnRK1信号通路通过调节毛竹各部位糖质量分数的分配进而调控笋竹的快速生长。T6P/ SnRK1信号通路在植物生长发育中的生物学功能还需要进一步研究。












 DownLoad:
DownLoad: