-
紫斑牡丹Paeonia rockii为芍药科Paeoniaceae芍药属Paeonia牡丹组Sect.Moutan小灌木[1], 是野生牡丹受威胁程度最高的种类之一, 被列为国家三级保护植物[2]。其种子需沙藏催根, 生根后种子的上胚轴仍处于休眠, 如休眠不解除, 则种子成苗率极低, 这是导致野生紫斑牡丹濒危的重要原因[3]。KENTZER[4]和AMEN[5]认为, 抑制物质的存在是植物种子休眠的主要原因之一。周理平等[6]发现, 紫斑牡丹的种皮、胚乳中含有抑制油菜Brassica campestris种子萌发及幼苗生长的物质, 认为种子内的抑制物质是紫斑牡丹种子休眠的重要原因。周仁超等[7]通过紫斑牡丹种皮和胚乳水提液对白菜Brassica pekinensis种子生长影响的研究, 认为其内的抑制物质不是种子萌发的决定因素。上述研究仅测定未生根种子中的抑制物质, 并未动态观察生根及发芽的种子, 因此, 单纯靠生根前种子各部位浸提液得出种子中抑制物质是否为制约紫斑牡丹种子萌发的结论值得商榷。本研究选用与紫斑牡丹种子结构类似, 具明显上下胚轴、同时又可迅速生根、发芽的豌豆Pisum sativum种子为材料,观察紫斑牡丹种子生根及发芽前后的种皮、胚、胚乳浸提液对豌豆种子萌发的影响,测试紫斑牡丹各提取物的抑制强度,并结合种皮透性及种子离体培养探究紫斑牡丹种子休眠原因,为紫斑牡丹的繁育和保护提供理论支持。
HTML
-
紫斑牡丹种子于2015年9月采集于甘肃省临洮县新添镇上街村, 湿沙中变温层积(20 ℃暖温层积90 d生根, 4 ℃低温层积40 d催芽, 20 ℃暖温层积60 d发芽)备用。豌豆种子为市售。
-
随机取饱满无损伤种子60粒, 分完整种子组和去皮种子组, 30粒·组-1。称量后分别放入20 ℃的恒温水中, 使种子吸水膨胀。隔6 h称量1次, 直至种子恒量。分别计算每组种子不同阶段的吸水率。3次重复, 取平均值。每次各组称量后均用小篮子法[8]测定种子呼吸速率, 分别计算完整种子与去皮种子的呼吸速率。3次重复, 取平均值。
-
取生根前、生根后及发芽后3类种子, 分离其种皮、胚乳、胚。80 ℃下烘干至恒量后粉碎。称取粉碎品10 g, 分别加入蒸馏水定容至100 mL(新鲜种子胚较小, 取5 g, 定容至50 mL), 保鲜膜封口后于20 ℃浸提24 h, 5 000 r·min-1离心10 min, 取上清液即得比例为100%的浸提液, 4 ℃保存。将均匀、饱满的豌豆种子置于蒸馏水及上述浸提液中, 于25 ℃下培养, 保持蒸馏水及浸提液浸润种子的2/3。72 h后统计豌豆生根率和根长, 144 h后统计豌豆上胚轴萌发率和上胚轴长。豌豆种子为30粒·处理-1, 3次重复, 取平均值。
-
将流水冲洗后的紫斑牡丹种子用体积分数为75%的乙醇消毒30 s, 蒸馏水冲洗1次, 再用体积分数为0.4%的次氯酸钠消毒5 min, 无菌水冲洗5次。将完整种子(处理1), 种皮置于一旁的去皮种子(处理2), 去皮种子(处理3), 完整裸胚(处理4), 去子叶裸胚(处理5)等5个处理接种到MS培养基上, (20±1) ℃下培养, 定期观察生根率及生长情况。30粒·处理-1, 3次重复, 取平均值。取处理4和处理5中根长大于3 cm的胚, 分2组:一组置于(4±1) ℃中, 30 d后放回(20±1) ℃下继续培养; 另一组始终放置在(20±1) ℃下, 30 d后观察2组发芽情况。30粒·处理-1, 3次重复, 取平均值。
-
用Excel和SPSS 10.0进行统计分析, 采用单因素方差分析和最小显著差异(LSD)法比较差异分析, 显著水平P<0.05。
1.1. 材料
1.2. 方法
1.2.1. 种皮透水性和透气性测定
1.2.2. 种子萌发抑制物质活性测定
1.2.3. 种胚离体培养
1.2.4. 数据处理
-
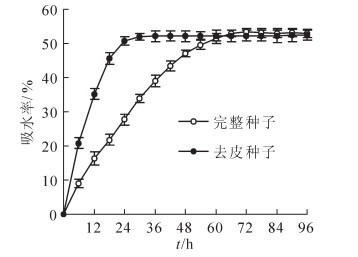
由图 1可见:去皮种子吸水率在前18 h内增长非常快, 至24 h时, 吸水率已接近饱和, 此后一直维持在54%左右; 完整种子吸水率增长较慢, 浸种24 h时仅约28%, 72 h后才近饱和。可见, 种皮在浸种前72 h内对种子吸水率的影响较大。
-
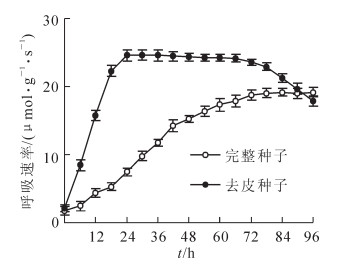
从图 2可以看出:浸水前完整种子与去皮种子的呼吸速率均很低, 去皮种子的呼吸速率在浸种24 h内迅速增大, 到24 h时达最大值, 约24 μmol·g-1·s-1, 72 h后, 其呼吸速率又迅速下降, 解剖发现其种胚变黄软化; 完整种子的呼吸速率增长速度慢, 浸种72 h后才达最大值, 约19 μmol·g-1·s-1, 此后保持不变。完整种子所能达到的最大呼吸速率值明显低于去皮种子。可见, 种皮阻碍了种子的呼吸。
-
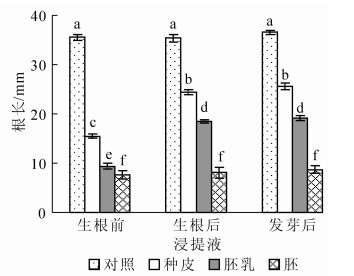
从图 3和图 4可见:在生根前紫斑牡丹种子胚乳、胚、种皮浸提液中豌豆的生根率、根长依次升高, 但都小于对照, 说明紫斑牡丹种子胚乳、胚、种皮都会抑制豌豆生根及根生长, 但抑制物质强度依次降低。在生根后紫斑牡丹种子的胚乳、种皮浸提液中豌豆的生根率及根长均显著大于在生根前紫斑牡丹种子浸提液中的, 而在发芽后的紫斑牡丹种子的胚乳、种皮浸提液中豌豆的生根率、根长虽有所增加, 但与生根后紫斑牡丹种子浸提液中的并无显著差异。说明紫斑牡丹种子胚乳和种皮中抑制物质对豌豆生根抑制强度随生根进程而降低, 但生根后到发芽过程中对豌豆生根的抑制强度并无显著变化。与胚乳、种皮不同, 在生根前、生根后及发芽后紫斑牡丹种子胚浸提液中豌豆的生根率依次降低, 根长无明显变化。
-
由图 5和图 6可知:在生根前紫斑牡丹种子种皮、胚、胚乳浸提液中豌豆的上胚轴萌发率均为0, 而对照可达87.78%, 可见生根前紫斑牡丹种子种皮、胚、胚乳完全抑制了豌豆上胚轴的生长; 在生根后和发芽后的的紫斑牡丹种子种皮及胚乳浸提液中的豌豆均有部分上胚轴萌发, 且两者无显著差异, 说明生根后的紫斑牡丹种子种皮及胚乳浸提液的抑制物质已经降低到可以使豌豆上胚轴萌发的强度, 且生根后到发芽过程中紫斑牡丹种子种皮及胚乳浸提液对豌豆上胚轴生长具有抑制作用的物质不再发生显著变化。在生根前、生根后及发芽后的紫斑牡丹种子胚浸提液中豌豆的发芽率均为0。
-
从图 7和图 8可见:处理1在60 d内紫斑牡丹种子无生根, 首粒生根时间明显晚于其他处理; 处理2可以消除种皮的机械障碍及透水、透气性影响, 保留种皮中可以影响胚生长的物质, 与处理1相比, 紫斑牡丹种子生根快, 但60 d内仅6%的种子生根; 而处理3比处理2早生根约14 d, 60 d时生根率约38%, 说明去除种皮有利于紫斑牡丹种子生根而种皮中确实有抑制下胚轴萌发的物质; 与处理3相比, 处理4和处理5提早约26 d生根, 两者接种12 d后, 下胚轴即开始伸长、生根, 60 d时生根率约90%, 在5个处理中生根最快, 生根率最高, 且两者无显著差异, 可见子叶对胚的生根并无影响。胚乳也会影响胚根生长, 裸胚(处理4和处理5)较有胚乳的胚(处理3)生根更快更好, 去除种皮及胚乳可以促进下胚轴快速伸长, 使其迅速生根。
从表 1可见:未经4 ℃低温处理的生根胚及生根去子叶胚的上胚轴萌发率均为0, 而低温处理后两者的上胚轴萌发率均达73%以上, 上胚轴长达2.7 cm, 且两者无显著差异。可见仅去除子叶而不经过低温不能打破紫斑牡丹种子上胚轴休眠。这与JING等[9]的研究结果不同, 他认为子叶是控制牡丹Paeonia种子上胚轴休眠的关键部位, 而周仁超等[7]研究表明, 子叶对上胚轴休眠无影响, 这与本试验结论类似。
处理组 上胚轴萌发率/% 上胚轴长/cm 4 ℃低温处理的生根胚 74.44 ± 4.16 a 2.71 ± 0.44 a 未经低温处理的生根胚 0.00 ± 0.00 b 0.00 ± 0.00 b 4 ℃低温处理的生根去子叶胚 73.33 ± 4.71 a 2.81 ± 0.47 a 未经低温处理的生根去子叶胚 0.00 ± 0.00 b 0.00 ± 0.00 b 说明:同列小写字母不同表示差异显著(P < 0.05) Table 1. Epicotyl germination rate and length of Paeonia rockii embryo in different treatments
2.1. 种皮对种子吸水率和呼吸速率的影响
2.1.1. 种皮对种子吸水率的影响
2.1.2. 种皮对种子呼吸速率的影响
2.2. 不同浸提液对豌豆种子萌发的影响
2.2.1. 不同浸提液中豌豆种子生根率及根长
2.2.2. 不同浸提液中豌豆种子上胚轴萌发率及上胚轴长
2.3. 种胚离体培养结果
-
种皮透性研究表明:种皮对紫斑牡丹种子的吸水率及呼吸速率有一定影响, 去除种皮能提高其吸水速率和呼吸速率, 加快吸水进程。
豌豆种子萌发研究表明:紫斑牡丹种子的种皮、胚、胚乳均含有抑制物质, 其中, 种皮的抑制物质强度最弱, 胚乳的抑制物质强度最强, 这与凤丹牡丹Paeonia ostii[10], 四川牡丹P.decomposita[11]类似, 而与大花黄牡丹P.ludlowii不同, 其种皮的抑制物质最强, 而胚乳的抑制物质较弱[12]; 种皮及胚乳对豌豆生根及上胚轴生长的抑制强度在生根过程中均明显下降, 生根后到发芽过程中并无明显变化, 且在生根种子种皮及胚乳浸提液中的豌豆上胚轴均有大量萌发率。因此认为, 紫斑牡丹种子生根与种皮及胚乳中抑制物质强度下降有关, 而其上胚轴的萌发与生根后到发芽过程中种皮及胚乳中抑制物质强度的变化无明显关系。
本研究进行了不同处理的胚培养试验, 结合豌豆萌发试验的生物测定方法初步揭示了紫斑牡丹种子休眠的原因。结论如下:种子下胚轴休眠的原因包括种皮的机械障碍和透性, 种皮和胚乳的抑制物质, 去除种皮及胚乳可以解除下胚轴休眠, 促进生根; 种子上胚轴休眠的原因与生根种子种皮及胚乳的抑制物质无关, 去除种皮、胚乳及子叶无法解除其休眠, 必须经过低温处理方可解除上胚轴休眠。
-
种子萌发抑制物质的测定方法有很多, 常见的方法如高效液相色谱法(HPLC), 气相色谱法(GS)和酶联免疫吸附检测法(EIISA)等[13]。这些方法可以将抑制物质具体分类到不同激素种类进行测定, 但操作较为复杂, 且需要一定的试剂、仪器等支持。而传统的生物测定法虽不能将抑制物质具体分类到特定激素进行测定, 却可以简单易行地测定出抑制物质的强弱。在以往的生物测定试验中, 人们多数采用白菜Brassica pekinensis籽作为萌发试验材料, 如白花延龄草Trillium kamtschaticum种子的甲醇和水浸提液对白菜籽幼根的生长均有明显的抑制作用, 而乙酸乙酯浸提液对白菜籽生长无显著影响, 说明其种子中的抑制物质是极性化合物[14]; 乌桕Sapium sebiferum种子种皮或胚乳浸提液明显抑制白菜籽的生长, 且胚乳浸提液的抑制性强于种皮的[15]。其他, 如麻栎Quercus acutissima[16], 红豆杉Taxus chinensis[17], 茖葱Allium victorialis[18], 青钱柳Cyclocarya paliurus[19], 紫椴Tilia amurensis[20]等种子休眠的研究中均有人采用白菜籽作为萌发试验材料来探究其抑制物质的强弱。也有部分人采用油菜Brassica campestris籽作为萌发材料, 如田胜尼等[21]利用油菜种子萌发法测定了南方红豆杉Taxus mairei种皮及胚乳中的抑制物质强弱, 而杨勇等[22]、周理平等[6]用油菜种子萌发法分别测定了四川牡丹、紫斑牡丹种子中的抑制物质。另有少数人采用小麦Triticum aestivum作为萌发材料进行抑制物质的生物测定。如SONG等[23]对四川牡丹的研究表明, 其种皮及胚乳中均含有强烈抑制小麦种子生长的物质, 且胚乳具有更强的抑制性。上述研究中的白菜、油菜、小麦种子的胚无明显上胚轴分化, 测定抑制物质时仅可以观察其生根情况而无法观察其上胚轴的生长。牡丹种子有明显的上下胚轴之分,具有双胚轴休眠特性,且以上胚轴休眠更为突出[24]。因此, 本试验采用与牡丹种子胚结构相似、具有明显上、下胚轴之分的豌豆种子代替传统的萌发试验材料, 可以更清楚分析浸提液对豌豆种子上、下胚轴生长的影响。
种子休眠与萌发是一个十分复杂的过程。大量研究表明:在休眠种子中通常存在发芽抑制物质, 且该类物质可以通过水或有机溶剂萃取出来, 主要包括激素、萜类、香豆素类化合物和脂肪酸等[25-29]。激素在种子的休眠与萌发中具有十分重要的作用。赤霉素(GA)和脱落酸(ABA)是植物种子萌发调控中的2类重要植物激素, 两者具有拮抗作用, 相互协同调节种子的休眠与萌发[30-31]。GA可以增强内源水解酶活性, 削弱种皮及胚乳等的机械障碍, 并能够促进储藏物质的分解, 促进胚胎的生长, 与种子休眠的解除有关[32]。ABA促进并维持休眠, 抑制种子的萌发, ABA生物合成酶相关基因的表达可以导致休眠加重[33-35]。芍药Paeonia lactiflora胚培养的研究中发现, 在琼脂糖凝胶中加入GA可以打破胚的上胚轴休眠, 若再往培养基中加入100 mol·L-1的ABA, 则GA促进上胚轴休眠的作用就会完全被抑制[36], 有研究表明, ABA可以抑制GA诱α淀粉酶活性[37], 同时能抑制β-1, 3-葡聚糖酶的表达, 影响胚乳中营养物质的代谢, 延缓种子的萌发[38]。此外, 细胞分裂素(CTK), 乙烯(ETH), 油菜素内酯(BR)等也是常见的与种子休眠萌发相关的激素, 如ETH对拟南芥Arabidopsis thaliana, 豌豆, 烟草Nicotiana tabacum等许多植物种子的萌发十分重要[39-40]。除常见的各类激素外, 还存在一些其他对植物种子休眠萌发有影响的化合物。如种子中的多酚、单宁酸、酚类物质可以通过抑制部分代谢途径而影响栎类种子的萌发[41-42]。
本研究发现:豌豆在生根后及发芽后的紫斑牡丹种胚浸提液中的生根率、发芽率及根长、芽长均小于在未生根紫斑牡丹种胚浸提液中的。这可能是生根及发芽的紫斑牡丹种子的根及茎叶释放出了新的化感物质, 从而对豌豆产生了异株克生的作用[43]。有研究表明:牡丹具有自毒效应[44-45], 且牡丹根和叶的浸提物对多种植物的生长具有抑制作用[46-47]。本研究未能准确地测定种子萌发抑制物质种类, 不能明确紫斑牡丹种子上胚轴休眠是胚内部抑制物质还是胚内其他原因。建议在以后的研究中分析种子各部分, 尤其是胚内抑制物质的具体成分, 并尝试分离化感类抑制物质。

















 DownLoad:
DownLoad: