-
喜树Camptotheca acuminata是中国特有树种,属于蓝果树科Nyssaceae喜树属Camptotheca植物,因叶片含有抗癌物质喜树碱(camptothecin),被认为是重要药用植物。施肥是高效培育喜树、增加叶片产量的重要措施之一[1]。氮素是植物生长和产量形成的首要因素,植物吸收氮素的形式主要有铵态氮(NH4+-N)和硝态氮(NO3--N)。研究表明:合理增施氮肥可以促进植物生长,提升植物品质[2];除了影响光合作用,氮素还影响植物中抗氧化酶和膜脂过氧化物的生理代谢。过量氮肥会打破植物内活性氧代谢的平衡,破坏膜系统结构[3],导致植物生理系统的紊乱,影响植物类囊体数量和类囊体蛋白形成。psbA,psbB和psbC是光系统Ⅱ核心复合体(PSⅡ)中重要的蛋白编码基因。卡尔文循环中,核酮糖-1, 5-二磷酸羧化酶(rubisco)是光合特性中碳同化的关键酶,主要由大、小2个亚基组成,在植物碳同化过程中具有重要作用,影响植物的光合特性[4]。不同植物在不同环境和生育期表现出对不同氮素形态吸收的显著性差异[5-6]。三叶青Tetrastigma hemsleyanum[7]和铁核桃Juglans sigillata[8]都表现出对NO3--N吸收的偏向性,而水稻Oryza sativa的叶绿素荧光动力学参数在2种氮素营养下没有差异[9];出现差异的原因可能与研究对象和条件都存在一定的相关性[10],但氮素形态对植物叶绿素荧光动力学过程及其参数的影响机制,目前尚无明确定论。总的来说,不同植物对氮素形态吸收具有偏向性,而后者直接影响了植物生长和叶绿素荧光特性,从而影响植物生长的产量和品质,因此合适的氮肥水平及形态对植物生长具有重要意义。近年来,关于氮肥对喜树生长的研究,主要集中在氮肥处理的不同水平[11],而尚未采用不同氮素形态的不同水平对喜树进行处理研究。因此,本研究以2年生喜树实生苗为实验材料,采用不同水平的铵态氮和硝态氮施肥处理,通过研究叶片生长、叶绿素荧光特性和叶绿体相关基因的表达,来探究适于喜树生长的最佳氮素水平和氮素形态,为喜树施肥栽培提供一定的理论依据。
HTML
-
于2017年4月5日选取无病虫害、生长健壮、规格基本一致的优质2年生喜树实生苗270株(江西九江森淼绿化苗木公司),栽植在直径30.0 cm,高40.0 cm的花盆中。栽植基质为V(泥炭):V(珍珠岩):V(园土)=1:1:3,填土(10.0±0.2)kg·盆-1,土壤pH值为6.8,土壤电导率(EC)为0.305 mS·cm-1,水解氮为86.6 mg·kg-1,速效磷为3.2 mg·kg-1,速效钾为94.6 mg·kg-1。苗木于浙江农林大学平山试验基地(30°15′50.09″N,119°42′54.67″E)缓苗2月,缓苗期间正常浇水,待全部成活后,于6月18日搬于浙江农林大学温室内(30°15′30.39″N,119°43′26.92″E)进行施肥处理。
试验以清水组为对照(ck),铵态氮处理组使用硫酸铵[(NH4)2SO4]施肥,施氮量分别为2.5(T1),5.0(T2),7.5(T3)和10.0(T4)g·株-1,硝态氮处理组使用硝酸钾(KNO3)施肥,施氮量分别为2.5(W1),5.0(W2),7.5(W3)和10.0(W4)g·株-1。所有盆栽苗均随机摆放,叶片之间无重叠,各盆配有直径40.0 cm的托盘,防止养分流失,每处理设置10株,重复3次。试验从6月18日至10月4日,隔15 d施肥1次。供试氮肥均为分析纯(AR99%),购自国药(硫酸铵CAS#: 7783-20-2,硝酸钾CAS: 7757-7)。施铵态肥时,肥料中加入7.0 μmol·L-1二氰二氨(C2H4N4, DCD)以抑制硝化反应,于0,30,60,90,105 d测定相关数据。试验期间进行正常的管理。
-
叶长:分别于处理前后(0,105 d)选第2轮叶的倒数第3,4,5片叶子,用直尺测量苗木的叶长(测量3株,取平均值),从叶片与叶柄连接基部到叶片尖端,精确到0.1 cm。叶面积:于处理前后(0,105 d)选第2轮叶的倒数第3,4,5片叶子,用方格纸测定苗木的叶面积(测量3株,取平均值),从叶片与叶柄连接基部到叶片尖端的整个范围,精确到0.01 cm2。叶绿素a和叶绿素b:采集喜树顶端第5,6片叶,参照李合生[12]乙醇浸提法测定叶绿素质量分数。叶绿素荧光参数:采用Li-6400便携式光合仪(LI-COR,Lincoln,美国),于晴天上午9:00-11:00测定植株上位叶(第2轮叶倒数3,4片)叶绿素荧光参数。叶片暗适应30 min后测定初始荧光产量(Fo),随后施加1次强闪光(6 000 μmol·m-2·s-1,脉冲时间0.7 s)测最大荧光产量(Fm),然后在自然光下适应20 min,待荧光基本稳定再测得稳态荧光产量(Fs),最后给予1次强闪光,获得光适应下的最大荧光产量(Fm′)和最小荧光(Fo′)。各处理均3次重复。暗反应下PSⅡ最大光化学效率Fv/Fm=(Fm-Fo)/Fm,光系统下PSⅡ最大捕获效率Fv′/Fm′=(Fm′-Fo′)/Fm′,光化学猝灭系数qP=(Fm′-Fs)/(Fm′-Fo′),非光化学猝灭系数qNP=1-Fv′/Fm′[13]。测定叶绿素荧光参数时,同时任选3株,从各株顶端6~9片叶中任意采集3片,放入液氮罐中带回实验室置于-80 ℃冰箱中保存,用于测定光合酶基因的表达。叶绿体基因的表达:采用RNAperp Pure Plant Kit(TIANGEN,北京)试剂提取喜树RNA;参照Reverse Transcriptase M-MLV(Takara,大连)试剂说明书合成cDNA,于-20 ℃储存备用。从美国国立生物技术信息中心(NCBI)上获得喜树叶绿体基因序列,以Actin为内参基因,用Primer Express software(Applied Bio systems)设计引物(表 1)。反转录产物cDNA稀释10倍后,用SYBR®Primix Ex TaqTM试剂盒(Takara,大连)进行实时荧光定量聚合酶链式反应(qRT-PCR),利用Light Cycler 480 Ⅱ(Roche)荧光定量仪器进行目的基因qRT-PCR表达分析,反应体系为20.0 μL,其中SYBR®Primix Ex TaqTM荧光染料10.0 μL,cDNA模板2.0 μL,上下游引物(10 μmol·L-1)各0.8 μL,双蒸水6.4 μL。两步法PCR扩增标准程序:预变性95 ℃ 30 s;聚合酶链式反应95 ℃ 5 s,61 ℃ 30 s,40个循环,每个样品重复3次,采用2-ΔΔCt算法分析结果。
基因 正向引物(5′→3′) 反向引物(5′→3′) psbA ACAGATTCGGTCAAGAGGAAGA CAGTGAACCAGATACCTACTACAG psbB GGAGGAATCGCTTCTCATCATAT CGGACGCTAAGATGGAATAGAC psbC GTCAATTATGTCTCGCCTAGAAGT GACCTACGAAGAAGAAGAATCCTAA RbcL CTTGGCAGCATTCCGAGTAAC GTTGTCCATGTACCAGTAGAAGATT Actin GGTACTCGTTCACAACAACTGCTG CTGTCCATCGGGCAACTCATAG Table 1. Primers sequence of target genes
-
采用Excel 2007和SPSS 20.0软件对数据进行统计分析。采用单因素(one-way ANOVA)和Duncan法进行方差分析和多重比较(α=0.05)。利用Origin 9.0软件作图。图表中数据为平均值±标准差。
1.1. 供试材料与试验方法
1.2. 测定项目和方法
1.3. 数据统计与分析
-
表 2显示:喜树生长至105 d,叶长和叶面积均增加,处理组叶长和叶面积均显著高于ck(P<0.05)。随着氮素处理水平的提高,叶长和叶面积均呈先上升后下降趋势。与其他处理相比,T3和W3叶长和叶面积最大,叶长较ck(8.10±0.02)cm分别增加了32.3%和78.5%,叶面积较ck(25.43±0.15)cm2依次增加了130.0%和242.0%;且硝态氮处理水平下的叶长和叶面积均高于铵态氮,说明硝态氮更有利于植物生长。
处理 叶长/cm 叶面积/cm2 ck 13.00 ± 0.12 Ad 58.83 ± 0.14 Ae T1 15.80 ± 0.12 Ac 79.07 ± 0.08 Bd T2 16.70 ± 0.03 Bb 98.93 ± 0.08 Bc T3 16.70 ± 0.03 Bb 124.03 ± 0.12 Ba T4 16.40 ± 0.05 Bb 116.37 ± 0.31 Bb ck 13.00 ± 0.12 Ad 53.83 ± 0.14 Ae W1 19.20 ± 0.06 Ac 139.73 ± 0.24 Ad W2 20.80 ± 0.08 Ab 152.07 ± 0.35 Ab W3 23.20 ± 0.21 Aa 184.30 ± 0.05 Aa W4 20.50 ± 0.03 Ab 150.97 ± 0.12 Ac 说明:不同小写字母表示同种氮素不同水平间差异显著(P < 0.05),不同大写字母表示同种氮素相同水平下与ck差异显著(P < 0.05) Table 2. Effects of different concentrations of ammonium nitrogen and nitrate on leaf lengh of Camptotheca acuminata
-
由表 3可知:随生长时长,植株叶片叶绿素质量分数总体升高,除T4外,其他处理叶绿素质量分数60 d较30 d时有所下降,90 d时又有所上升,至105 d时达到最大值。相同生长时长下,不同处理水平间差异较小。生长至105 d时,T3叶绿素质量分数显著高于其余处理(P<0.05),较ck,T1,T2和T4依次上升了35.2%,17.2%,23.8%和15.2%。
处理 w叶绿素/(mg·g-1) 0 d 30 d 60 d 90 d 105 d ck 1.61 ± 0.26 Da 1.90 ± 0.26 Db 2.80 ± 0.10 Cb 3.96 ± 0.22 Ba 4.38 ± 0.16 Ac T1 1.63 ± 0.19 Da 4.54 ± 0.83 Aa 4.30 ± 0.08 Ca 3.72 ± 0.85 Ca 5.05 ± 0.12 ABb T2 1.61 ± 0.09 Da 4.78 ± 0.49 Aa 4.36 ± 0.19 Ca 4.72 ± 0.39 Ca 5.14 ± 0.36 ABb T3 1.67 ± 0.14 Da 5.25 ± 0.32 Ba 4.53 ± 0.17 Ca 4.80 ± 0.32 Ca 5.92 ± 0.11 Aa T4 1.61 ± 0.11 Da 4.88 ± 0.11 Ba 4.39 ± 0.15 ABa 4.26 ± 0.23 ABa 4.78 ± 0.22 Ab 说明:不同小写字母表示同种氮素不同水平间差异显著(P < 0.05),不同大写字母表示同种氮素相同水平下与ck差异显著(P < 0.05) Table 3. Effects of different concentrations of ammonium nitrogen and nitrate on chlorophyll content in C. acuminata
-
表 4表明:随处理时间的延长,硝态氮处理下喜树叶片叶绿素质量分数变化显著;但不同处理水平相比,质量分数差异较小。处理30~60 d,所有铵态氮处理下叶绿素质量分数均显著高于ck,各处理水平相比,W3处理下叶绿素质量分数最高,较ck和其他处理依次高出73.9%,44.5%,10.2%和31.3%。处理90~105 d,ck和W1的叶绿素质量分数继续上升,W2,W3和W4先上升后下降,105 d时,W1叶绿素质量分数高于其他处理,W4最低。
处理 w叶绿素/(mg·g-1) 0 d 30 d 60 d 90 d 105 d ck 1.61 ± 0.17 Ca 1.90 ± 0.26 Ba 2.80 ± 0.10 ABc 4.38 ± 0.16 Ba 3.69 ± 0.22 Aab W1 1.65 ± 0.24 Ca 2.97 ± 0.84 Ba 3.37 ± 0.16 Ab 4.39 ± 0.10 Aa 4.41 ± 0.13 ABa W2 1.63 ± 0.12 Ba 3.84 ± 0.09 Aa 4.42 ± 0.38 Aab 3.68 ± 0.11 Abc 3.95 ± 0.11 Aa W3 1.67 ± 0.19 Ca 3.89 ± 0.14 Aa 4.87 ± 0.23 Aa 3.15 ± 0.49 Ac 3.83 ± 0.29 Ba W4 1.61 ± 0.11 Ba 3.45 ± 0.10 Aa 3.71 ± 0.28 Ab 3.22 ± 0.13 Ac 3.80 ± 0.35 Aa 说明:不同小写字母表示同种氮素不同水平间差异显著(P < 0.05),不同大写字母表示同种氮素相同水平下与ck差异显著(P < 0.05) Table 4. Effects of different concentrations of ammonium nitrogen and nitrate on the chlorophyll content in C. acuminata
-
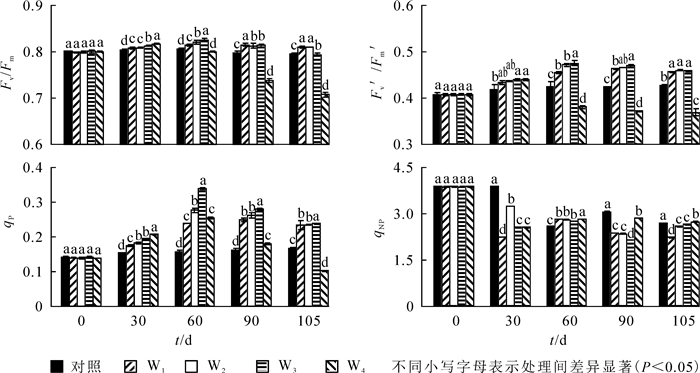
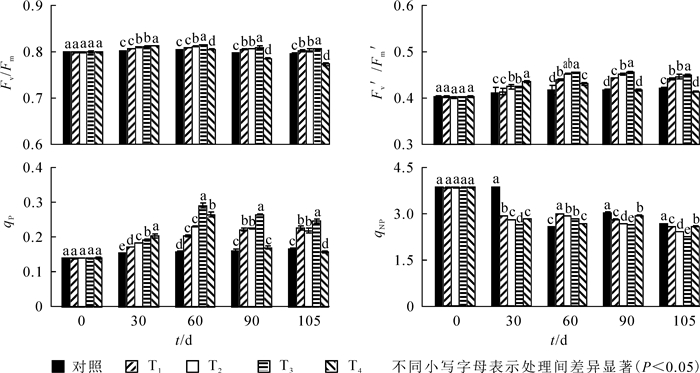
由图 1可知:至30 d,Fv/Fm,Fv′/Fm′和qP呈显著上升趋势(P<0.05),qNP呈下降趋势,T4处理下Fv/Fm,Fv′/Fm′和qP达到最大。到60 d时,T2和T3处理下Fv/Fm,Fv′/Fm′和qP达到最大值,与ck相比,依次提高了1.4%,9.2%和84.8%。60 d时T3处理组各测试参数显著高于其他处理(P<0.05),T4显著低于其他处理。处理90~105 d,T2,T3和T4处理下Fv/Fm,Fv′/Fm′和qP值呈下降趋势,T1处理下Fv/Fm下降,Fv′/Fm′和qP值呈上升趋势,T3依然高于其余处理。90 d时,T3处理下qNP显著低于其他同类处理,与ck相比下降了12.9%;105 d时,T4处理下Fv/Fm,Fv′/Fm′和qP降到最小,显著低于其他处理;与ck相比,依次下降了11.1%,3.3%和5.6%。
-
图 2显示:30 d时,不同硝态氮处理下Fv/Fm,Fv′/Fm′和qP显著上升,qNP显著下降,其中W4处理Fv/Fm和Fv′/Fm′达到最大值,此后显著下降(P<0.05)。60 d时,W2和W3处理下Fv/Fm,Fv′/Fm′和qP达到最大值,与ck相比,依次上升了2.6%,12.3%和115.8%;此时W3处理下这3个参数值显著高于其他处理。处理90~105 d,W2,W3和W4处理Fv/Fm,Fv′/Fm′和qP值呈下降趋势;W1处理Fv/Fm下降,Fv′/Fm′和qP值上升;W3处理Fv′/Fm′和qP值依然高于其他处理,qNP显著低于其他处理,与ck相比下降了29.9%。至105 d时,W4处理Fv/Fm,Fv′/Fm′和qP显著低于其他处理,与ck相比,依次下降了11.1%,3.3%和5.6%。
-
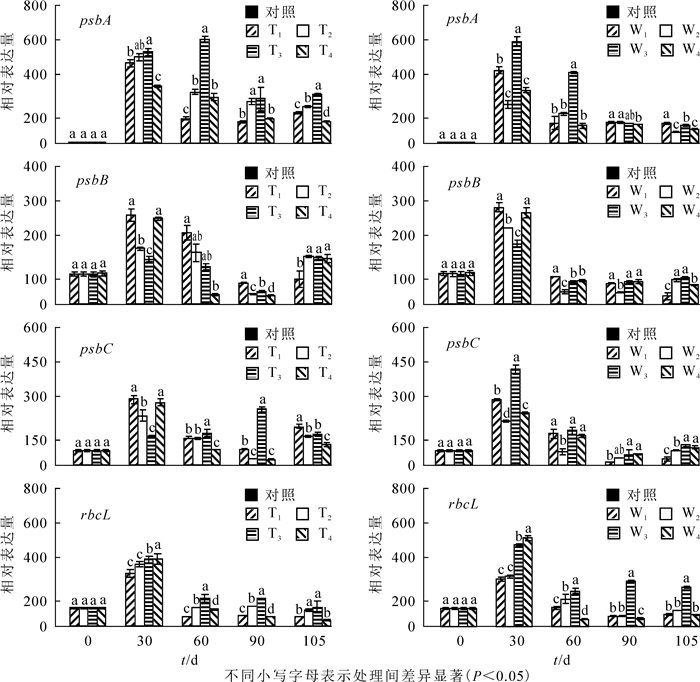
以不同处理在0 d时的表达量为ck,增施氮肥对PSⅡ蛋白编码基因psbA(图 3A和图 3B),psbB(图 3C和图 3D),psbC(图 3E和图 3F)和光合酶基因RbcL(图 3G和图 3H)表达的影响显著(P<0.05)。由图 3可知:生长30 d,所有基因表达量显著提高。处理60 d时,T3处理下psbA表达上调,其余处理下所有基因表达显著下调(P<0.05),T3和W3处理下psbA,psbC和RbcL表达量显著高于其他同类处理(P<0.05),所有铵态氮处理psbB随处理水平的升高而升高,T2处理psbB表达量显著低于其他同类处理(P<0.05);与T1,T2和T4相比,T3处理psbA表达量上升了76.3%,50.8%和55.6%,psbC表达量上升了15.8%,16.5%和52.5%,RbcL表达量上升了66.8%,34.4%和39.9%。与W1,W2和W4相比,W3处理psbA表达量上升了71.9%,57.9%和75.6%;psbC表达量上升了8.7%,61.7%和15.4%;RbcL表达量上升了47.1%,22.3%和79.1%,处理90~105 d,T3和W3也保持显著优势,90 d时,T3较T1,T2和T4依次增加了88.9%,28.7%,379.1%,W3较W1,W2,W4依次增加了201.8%,52.3%和45.5%。105 d时,T4处理psbA和RbcL表达量显著低于其他同类水平处理,达到最小值。
2.1. 氮素处理水平对喜树叶片生长的影响
2.2. 氮素处理水平对喜树叶片叶绿素质量分数的影响
2.2.1. 铵态氮不同处理水平对喜树叶片叶绿素质量分数的影响
2.2.2. 硝态氮不同处理水平对喜树叶片叶绿素质量分数的影响
2.3. 氮素处理水平对喜树叶片叶绿素荧光参数的影响
2.3.1. 铵态氮不同处理水平对喜树叶片叶绿素荧光参数的影响
2.3.2. 硝态氮不同处理水平对喜树叶片叶绿素荧光参数的影响
2.4. 氮素形态和处理水平对喜树叶片核心复合体叶绿体基因和光合酶基因表达的影响
-
植物不同生长过程对氮肥需求不同,在合理范围内增施氮肥,可以显著促进植物生长,提高植物的产量和品质[14]。研究认为:与铵态氮相比,硝态氮具有更好的亲和力、氮转运和吸收效果[15]。本研究发现:铵态氮和硝态氮均提高了喜树的叶面积和叶长生长量,以T3和W3优势最为明显;相比而言,硝态氮处理下喜树生长更优;随着氮素水平的继续提高,喜树叶长和叶面积显著下降,说明植物吸收利用氮素存在一定限度,过量添加不利于生物量的积累[16]。
叶绿素参与光能的吸收传递及分配,是重要的光合色素;不同氮素形态对植物叶绿素合成及对光能吸收、传递、捕获和转换具有重要影响[17]。研究表明:适量增加氮素可促进米老排Mytilaria laosensis[18]和菘蓝Isatis indigotica[19]叶绿素合成,提高光合效率。本研究发现:合理范围内(T1~T3,W1~W3)增施氮肥,喜树叶片叶绿素合成和光合效率显著提高,以T3和W3效果最佳。对油麦菜Lactuca sativa var. longifoliaf[20]和黄瓜Cucumis sativus[21]而言,硝态氮处理下叶绿素相对含量和叶绿素荧光参数高于铵态氮处理;本研究同样发现,处理初期,相同氮素水平下,硝态氮处理组叶绿素质量分数和叶绿素荧光参数显著高于铵态氮,这可能与植物对氮素形态的吸收代谢有关。李晓静等[22]发现铵态氮以被动吸收为主,扩散进入生物膜并破坏生物膜结构,阻碍质子动力势的建立和碳同化物的形成;而硝态氮以主动吸收为主,进入生物膜并储存于液泡中,对植物体内离子平衡、碳同化物的形成有利。RAAB等[23]研究发现:高氮会造成叶绿素酶失活,叶绿素分解,光系统Ⅱ遭到破环,光子传递受阻,使植物发生光抑制现象;如小麦Triticum aestivum在高氮处理下,植物光合结构和功能受到伤害,光合特性降低[24]。本研究发现,处理中后期(60~105 d)高氮水平下(T4和W4),喜树的叶绿素质量分数、叶绿素荧光特性显著下降。原因可能是高铵态氮造成铵离子在体内积累,类囊体结构被破坏,质子形成受阻,形成氧化磷酸化,破坏碳水化合物的形成,导致叶绿素合成受阻和植物光合特性降低[25];高硝态氮处理破坏土壤理化性质,造成喜树缺素症,表现出对铁、镁等元素吸收的拮抗作用,从而影响植物光合特性[26]。不同氮素形态或水平对qNP影响无显著规律,其原因还需要进一步研究。
光系统Ⅱ(PSⅡ)由多亚基复合组成,吸收外界光能并将激发能转移到反应中心;psbA,psbB和psbC是编码PSⅡ反应中心蛋白质基因,RbcL基因编码核酮糖二磷酸羧化酶的大亚基。研究发现:增施氮肥可以提高植物碳同化能力,促进RbcL表达[27]。以甜菜Beta vulgaris[28]为例,铵态氮处理,其叶片中Rubisco酶基因表达高于硝态氮处理。本研究中,处理初期(30 d)不同氮素形态处理均促进了叶绿体基因的表达,相同水平下硝态氮处理RbcL的表达高于铵态氮;至中后期,T1和W1处理下RbcL的表达显著高于其他同类处理,说明适宜的铵态氮和硝态氮处理提高了植物的碳同化能力;相比之下,硝态氮较铵态氮更有利于提高喜树的碳同化能力,进一步说明了喜树是喜硝植物[29]。叶绿素荧光过程极其复杂,受各种刺激调控,植物碳同化和氮同化可能存在相应的竞争关系。本试验60 d时,叶绿体基因表达下调,可能原因是随着植物对长期环境变化的响应和适应,相关叶绿体基因表达受到影响[30-31]。环境胁迫造成PSⅡ中的蛋白质破坏,受蛋白编码基因刺激表达的影响,受损的蛋白质不断被新合成的蛋白质替代。SHAO等[32]发现:2.0 mg·L-1氮处理下,连苯三酚引起的铜绿假单胞菌Pseudomonas aeruginosa损害可通过psbA的刺激表达弥补。本实验中psbA,psbB和psbC在不同氮素水平处理下表达差异不显著,可能是氮素刺激了PSⅡ的蛋白质合成,形成自我保护机制[33]。高氮处理下,植物光电子传递受阻、热耗散能力显著下降、碳同化能力下降,发生了明显的光抑制现象[34],也会造成叶绿体基因的表达显著下调。高铵态氮下铵离子的积累破坏了类囊体蛋白,降低了Rubisco酶活力,而高硝态氮下,植物对三价铁离子、二价镁离子的吸收下降,Rubisco酶活力受损,导致叶绿体基质中类囊体光驱电子的转移受阻碍,从而影响质子中铁硫-氧化蛋白的还原[35]。












 DownLoad:
DownLoad: