-
光是植物进行光合作用的重要因素之一,对植物的生长发育及形态建成具有重要影响[1]。长期庇荫必然影响阳性植物的生长发育,但适度遮光则有利于阴性植物的更新繁育和进展演替。在不同的光环境下,植物往往会在形态结构、生长发育、光合作用、生理生化和形态解剖等方面发生相应的可塑性改变,以维持其正常的生命活动[2]。叶片可塑性的大小是衡量植物对异质环境适应能力的重要指标,叶片可塑性常与植物对环境具有高的潜在适应能力紧密联系[3]。因此,研究遮光条件下植物生理特点和解剖结构的变化,可以反映某种特定植物对光照强度的反应,进而推断其对庇荫环境的忍耐性,这对于指导农林业生产,提高种苗繁育的成活率和保存率,改进生产管理措施具有重要意义。连香树Cercidiphyllum japonicum是连香树科Cercidiphyllaceae连香树属Cercidiphyllum植物,中国特有第三纪孑遗单科属植物。该种雌雄异株,结实较少,天然更新困难,资源稀少,已濒临灭绝状态,因此被列入国家Ⅱ级重点保护野生植物种[4-5]。在中国,连香树分布于山西西南部、河南、陕西、甘肃、安徽、浙江、江西、湖北及四川等地[6],但多零星分布,成片资源很少。近年来,由于大规模旅游开发和人为干扰的影响,中国连香树资源趋于萎缩,亟待采取人为措施予以保护。目前,连香树自然结实率低,天然更新困难,林下幼树极少,种群的挽救和保护依然存在一定困难。有关连香树的生物学特性已有一定文献报道,但多集中在形态解剖[7]、种群结构[8]、种子萌发[9]及群落遗传[10]等方面。与种群更新和保育紧密相关的需光性及耐荫性研究较少。本研究以2年生实生苗为研究对象,通过人为遮光创造不同梯度的光环境,分析夏季遮光条件下连香树幼苗生理生态参数和叶片超微结构的变化,旨在为连香树种苗培育和野生种群的更新保护提供一定的参考。
HTML
-
试验地位于江苏省林业科学研究院实验林场(南京市江宁区东善桥,31°51′25′′N,118°46′37′′E)。地处北亚热带的边缘,季风气候,年均气温16.1 ℃,极端最高气温43.0 ℃,极端最低气温−14.0 ℃,年相对湿度78%~80%,年降水量1 116.3 mm,年均日照时数1 912.8 h,无霜期224.0 d。试验土壤为山地黄棕壤,pH为6.8。
-
供试材料来自湖北省宜昌市五峰县,为生长健壮、规格一致的2年生实生苗。于2018年3月下旬定植,经过3个月的缓苗生长后,当年6月20日开始用黑色遮阳网进行遮光处理,并一直维持到10月下旬。遮光棚南北设置,棚高2.0 m,两边覆盖苗床并延伸到地面,南北敞开,便于通风透气。试验设4个处理:1层遮光(L1,辐射光强相当于全光照55%)、2层遮光(L2,辐射光强相当于全光照25%)、3层遮光(L3,辐射光强相当于全光照10%),全光照(L0,辐射光强100%)为对照处理。采用单因素随机区组设计,重复3次(即3个区组),不同区组间平行排列,区组内随机设置遮光小区,每小区处理幼苗30株,并挂牌标记。为避免交错影响,不同处理间留有1.5 m的间距。苗木生长期间正常土壤管理,确保苗田无杂草、地面不干旱,不同处理间栽培技术措施一致。
-
8月选取连续晴朗的白天,利用LI-6400型便携式光合作用测定仪进行光合作用日变化测定。不同处理选择发育完好的幼苗各3株,每株选择3片位置相当、发育成熟的功能叶(顶端数第4片,叶柄涂标)测定,连续测定3 d。自7:00−18:00,隔2 h测定1次,每次测定40 ~ 60 s。测定均采用自然光源,标准叶室(2 cm×3 cm)。测定光合指标有:净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)、胞间二氧化碳(CO2)摩尔分数(Ci ), 利用公式Pn / Tr计算叶片的水分利用效率(EWUE)。同时记录不同处理的环境参数:光合有效辐射(PAR)、气温(Ta)、大气相对湿度(HR)、CO2摩尔分数(Ca)等。
-
自7月10日开始,选取标记叶片同等位置的功能叶测定光合色素。隔20 d取样1次,取样持续到9月底。叶绿素采用质量分数为80%丙酮提取,分光光度法,并根据Arnon公式计算[11-12]。基径(D)和苗高(H)采用卷尺和游标卡尺测量,利用生物量模型D2H比较不同处理下幼苗的生物量差异[13]。
-
9月末剪取标记的功能叶测定叶片含水量与叶片形态。叶面积采用AM-300手持式叶面积仪测定;叶片含水量(CLWC)采用烘干法测定,并用以下公式计算:CLWC = (叶鲜质量−叶干质量)/叶干质量×100%;比叶面积(SLAM)和比叶重(mLMA)由以下公式换算:SLAM = (叶面积/叶干质量);mLMA = (叶干质量/叶面积)×100%[14]。
-
10月上旬测定叶片超微结构。不同处理采集涂标的功能叶(顶端数第4片),中部近叶脉处取材,取材大小为1.5 mm×1.5 mm。放入预冷的质量分数为2.5%戊二醛中固定12 h (4 ℃),0.1 mol·L−1磷酸缓冲液(pH 7.2)漂洗3次,每次20 min。然后转移至质量分数1%的锇酸中后固定4 h (4 ℃),丙酮梯度脱水,之后用SPI-812树脂渗透、包埋,Leica UC6超薄切片机切成 60~80 nm超薄切片,醋酸铀-柠檬酸铅双染色,用日立H-7650型透射电镜观察,并用Gatan公司生产的832SC1000W数字成像系统拍照。
-
所有数据采用平均值±标准差的形式表示,应用Excel 2003作图,SPSS 17.0进行方差分析和相关分析,多重比较采用最小显著极差法(LSD法)。
1.1. 试验地自然条件
1.2. 试验设计
1.3. 指标测定与方法
1.3.1. 光合作用参数
1.3.2. 光合色素及生长指标
1.3.3. 叶片含水量与叶片形态
1.3.4. 叶片超微结构
1.4. 数据处理
-
从表1和图1可见:遮光对连香树幼苗的叶片发育有显著影响。遮光与全光照相比,其SLA有显著差异(P<0.05),表现为遮光后个体SLA显著增大,尤以L3处理的SLA最大(34.88 cm2),为全光下SLA的2.024倍,L1和L2处理的SLA与全光下相比也有显著差异(P<0.05)。全光下单叶鲜质量(mSLWf)与L1、L2差异不显著(P>0.05)。单叶干质量(mSLWd)也呈现类似的变化。从叶片含水量(CLWC)的变化来看,随着遮光强度的增强,叶片CLWC显著增加(P<0.05),这可能与遮光后林内气温下降、相对湿度适量增加有关。
处理 单叶面积(SLA)/cm2 单叶鲜质量(mSLWf)/g 单叶干质量(mSLWd)/g 叶片含水量(CLWC)/% 比叶重(mLMA)/(g·dm−2) 比叶面积(SLAM)/(dm2·g−2) L0 17.26±5.31 b 0.36±0.11 a 0.14±0.04 a 159.27±6.46 c 0.81±0.19 a 1.27±0.27 b L1 29.65±6.03 a 0.45±0.15 a 0.16±0.06 a 190.97±18.52 b 0.52±0.09 b 1.96±0.38 a L2 28.36±3.93 a 0.41±0.02 a 0.13±0.01 a 210.45±19.29 a 0.47±0.04 b 2.14±0.17 a L3 34.88±3.11 a 0.45±0.08 a 0.14±0.03 a 221.22±7.38 a 0.40±0.05 b 2.55±0.38 a 说明:数据为平均值±标准差,同列不同字母表示差异显著(P<0.05) Table 1. Changes of leaf water content and morphology indexes of C. japonicum leaves under different shading treatments
-
比叶重(mLMA)为单位面积叶干质量,反映了叶片干物质积累的多少[14]。从表1可见:遮光对mLMA和SLAM的影响较大,表现为随着遮光强度的增强mLMA日趋减小,而SLAM则日渐增大,呈现出显著差异(P<0.05)。遮光引起幼苗SLA和mSLWd的改变,进而导致了mLMA和SLAM的显著变化。
-
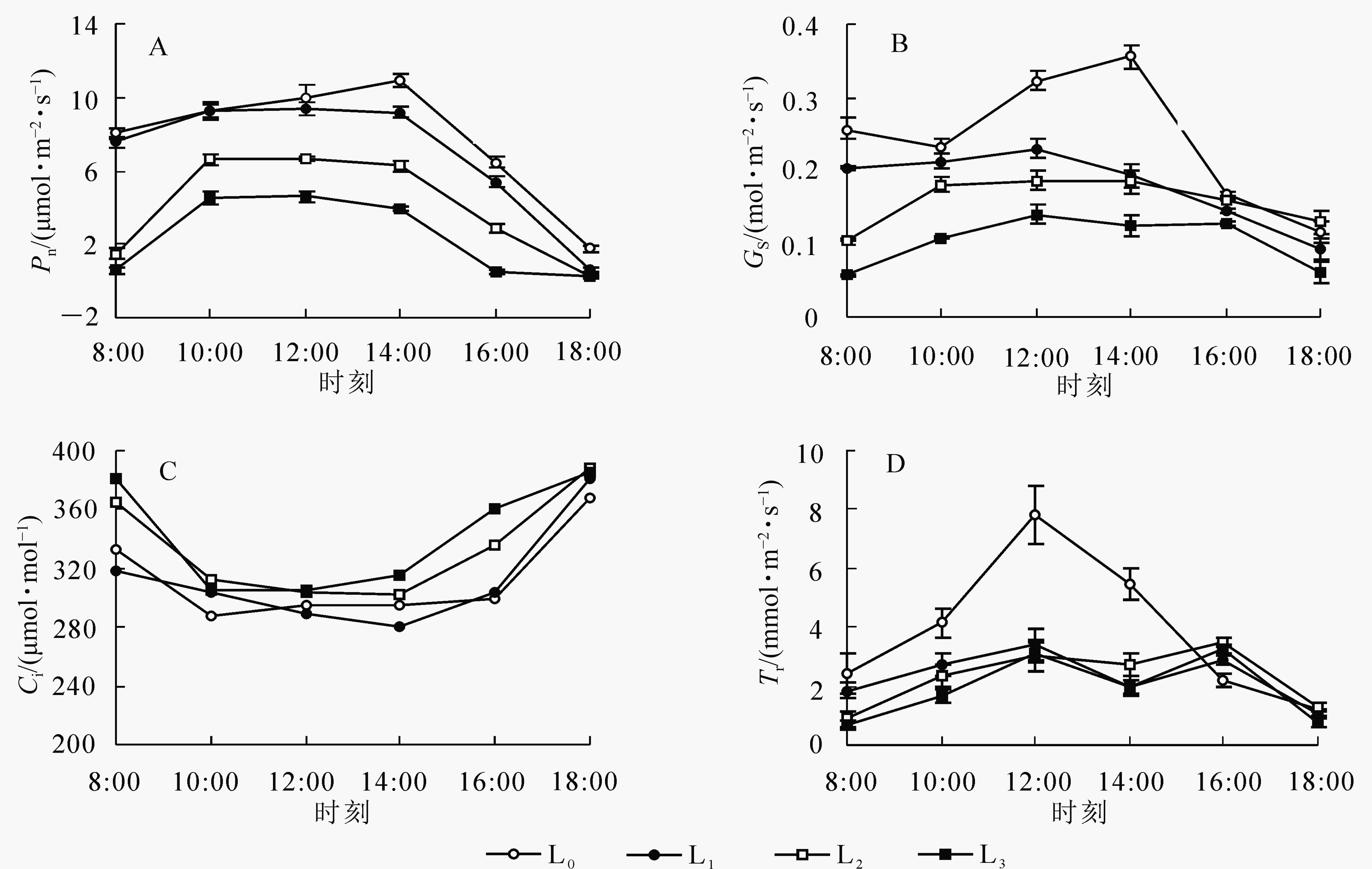
全光和遮光处理下Pn呈现出不同的变化特征(图2A)。比较而言,全光下Pn变化呈现不对称的“几”字形,12:00之前处于高位变化,而且最大值出现在14:00,14:00后急剧下降;L1处理下Pn的变化与L0类似,但其变化较为缓和,峰值出现在12:00;L2和L3处理下Pn则呈现近对称的“几”形变化。由于遮光的影响,林内光合辐射下降,12:00时 Pn最大值仅为全光下0.61和0.42倍。日均Pn从大到小排序为L0、L1、L2、L3。方差分析表明:不同处理下日均Pn有显著差异(P<0.05)。
-
一般而言,气孔导度反映大气二氧化碳和水汽与植物叶片传导、交换能力的高低。由图2B可见:遮光处理下Gs呈现先升高后降低的变化特征,但与对照相比起伏较小。遮光处理下,11:00时之前叶片中Gs基本是缓慢升高的,12:00呈现最大值,午后开始下降,这一点与Pn的变化基本相似,表明其变化与Pn呈一定相关性。从全光下Gs的日变化进程来看,10:00之前略有下降,但10:00之后就迅速升高,14:00达最大值,在所有时间段内Gs一直高于其他遮光处理。日均Gs从大到小排序也为L0、L1、L2、L3。方差分析表明:不同处理间日均Gs有显著差异(P<0.05),但L1与L2间差异不显著。
-
不同遮光下Ci呈基本一致的凹形变化,即中午之前缓慢降低,但午后逐渐抬升(图2C)。不同处理下日均Ci从大到小依次为L3、L2、L1、L0。方差分析表明:不同处理间Ci差异显著(P<0.05)。L3处理强度遮光,14:00以后光照强度已经很低,而且Gs下降,二氧化碳同化率低,致使较多的二氧化碳积聚在细胞间,导致Ci的快速升高。
-
由图2D可见:全光下,Tr的变化幅度较大,尤以12:00的Tr增加最甚,并显著高于遮光处理下的测定值(P<0.05);而遮光处理下Tr的变化较为缓和,并在14:00出现了下降,表明遮光显著抑制了幼苗的蒸腾作用。就日均Tr从大到小依次为L0、L1、L2、L3,分别为遮光处理下的3.48(L1)、2.58(L2)、2.51(L3)倍。方差分析表明:L1与L2间差异不显著,但L0与3种遮光处理间均有显著差异(P<0.05)。
依据Pn和Tr可以估算幼苗的EWUE。由于L1处理具有较高的Pn和相对稳定的Tr,在所有处理中EWUE最大(2.82),显著高于重度遮光(L2、L3)下的测定值(1.73、1.27)(P<0.05)。
-
将不同遮光处理下连香树幼苗净光合速率与光合有效辐射(PAR)、叶片含水量(CLWC)及叶片形态指标作相关分析见表2。结果表明:Pn与PAR、mLMA呈显著正相关(P<0.05),而与CLWC呈极显著负相关(P<0.01),与SLA呈显著负相关(P<0.05);Pn与mSLWf和mSLWd相关性均不显著。可见,遮光条件下,尽管植株SLA增加,但由于mLMA减小,Pn却随之下降,进而影响到幼苗正常的生理生长。
指标 Pn PAR SLA mSLWf mSLWd CLWC mLMA Pn 1 0.889* −0.661* −0.205 0.110 −0.800** 0.674* PAR 1 −0.796** −0.295 0.058 −0.895** 0.862** SLAM 1 0.704* 0.427 0.640* 0.701* mSLWf 1 0.919** 0.184 −0.055 mSLWd 1 −0.208 0.297 CLWC 1 −0.833** mLMA 1 说明:*,**分别表示相关性达0.05和0.01水平 Table 2. Relationship of the photosynthesis parameters with relative water contents and morphology indexes of C. japonicum leaves
-
叶绿素是植物光合作用的最有效的光能吸收色素,而类胡萝卜素也是不可缺少的色素,因此叶绿素和类胡萝卜素质量分数是分析植物光合作用能力的主要参数。反过来,环境中光照条件的变化也影响到叶绿素及类胡萝卜素的合成与分解,通过增加单位叶面积色素来提高其捕光能力[15-16]。由表3可知:遮光对连香树叶片叶绿素及类胡萝卜素质量分数均有显著影响(P<0.05)。遮光初期(20 d)不同处理间连香树叶片内类胡萝卜素已有显著差异(P<0.05),表现出遮光条件下叶绿素和类胡萝卜素显著增加,说明遮光处理促使了叶片内叶绿素的快速积累;随着遮光时间的延续,不同处理间叶片内叶绿素a、叶绿素b、叶绿素a+b、类胡萝卜素差值逐渐变小,但多重比较显示仍有显著差异(P<0.05)。总的趋势来看,遮光条件下叶片内叶绿素a、叶绿素b、叶绿素a+b、类胡萝卜素均有增加,且随着遮光强度的加大其质量分数递增。同时,随着遮光处理的持续叶绿素及类胡萝卜素质量分数递减,说明短时间内遮光对色素质量分数的影响较小,随着遮光时间的延长叶片内光合色素的合成受到抑制,质量分数呈现递减趋势,尤其是叶绿素a、叶绿素b、叶绿素a+b的变化较为明显。
处理 指标 不同遮光时间叶片光合色素质量分数/(mg·g−1) 20 40 60 80 100 d L0 叶绿素a 3.04±0.04 Ba 2.64±0.78 Ba 1.44±0.04 Db 1.76±0.04 Bb 1.73±0.10 Cb 叶绿素b 1.33±0.02 Ba 1.06±0.37 Bb 0.58±0.04 Dc 0.71±0.02 Bc 0.70±0.06 Cc 叶绿素a+b 4.37±0.04 Da 3.69±1.15 Ca 2.02±0.08 Dc 2.47±0.06 Bb 2.43±0.16 Cb 类胡萝卜素 0.67±0.02 Ba 0.61±0.17 Ca 0.30±0.01 Db 0.36±0.02 Bb 0.35±0.02 Bb L1 叶绿素a 3.22±0.25 Ba 3.87±0.11 Aa 2.22±0.24 Cb 1.70±0.03 Bc 2.04±0.04 Bb 叶绿素b 1.48±0.10 Aa 1.48±0.03 Aa 0.90±0.10 Cb 0.70±001 Bc 0.92±0.12 Bb 叶绿素a+b 4.71±0.36 Ca 5.35±0.14 Aa 3.12±0.34 Cb 2.41±0.03 Bc 2.96±0.09 Bb 类胡萝卜素 0.72±0.08 Ba 0.84±0.01 Ba 0.39±0.04 Cb 0.35±0.01 Bb 0.38±0.05 Bb L2 叶绿素a 4.04±0.02 Ba 2.79±0.01 Bb 2.79±0.03 Bb 1.95±0.06 ABd 2.47±0.04 Ac 叶绿素b 2.04±0.01 Aa 1.04±0.02 Bc 1.18±0.02 Bb 0.83±0.02 ABd 0.96±0.00 Ac 叶绿素a+b 6.08±0.02 Ba 3.83±0.02 Bb 3.97±0.05 Bb 2.78±0.08 ABd 3.43±0.04 Ac 类胡萝卜素 1.46±0.01 Aa 1.14±0.02 Aa 0.47±0.01 Bb 0.39±0.01 Bc 0.47±0.01 Ab L3 叶绿素a 5.96±1.56 Aa 2.86±0.04 Bb 3.30±0.10 Aa 2.27±0.52 Ac 2.29±0.17 Ac 叶绿素b 1.43±0.67 Aa 1.01±0.02 Bb 1.46±0.05 Aa 1.00±0.26 Ab 1.11±0.08 Ab 叶绿素a+b 7.40±0.93 Aa 3.86±0.04 Bc 4.76±0.15 Ab 3.27±0.78 Ac 3.40±0.14 Ac 类胡萝卜素 1.73±0.30 Aa 1.13±0.01 Ab 0.53±0.02 Ac 0.45±0.05 Ac 0.48±0.05 Ac 说明:数据为3次测定平均值±标准差;同行不同小写字母表示同一处理不同处理时间差异显著(P<0.05);同列不同大写字母表示同 一时间内不同处理间有显著差异(P<0.05) Table 3. Changes of chlorophyll and carotenoid content of C. japonicum leaves under different shading treatments
-
遮光降低了林内光照辐射强度,影响到连香树幼苗的生长形态。遮光处理后,连香树幼苗的分枝数目变化不大,但苗高(H)和基径(D)依次递减,最终影响到幼苗的生物量(表4)。多重比较表明:L1与对照相比,H、D、H/D、D2H和分枝数目均没有显著差异,说明轻度遮光(L1)没有对幼苗的生长带来明显的生长抑制。但是,重度遮光(L2和L3)下,H、D和D2H明显小于对照(L0)(P<0.05),说明遮光对幼苗的生长产生显著的抑制作用,对幼苗的成长不利。
处理 分枝数目/条 H/cm D/mm H/D D2H/cm3 L0 19.71±4.08 a 140.20±16.01 a 14.37±1.96 a 99.13±18.17 a 295.68±97.64 a L1 21.29±3.99 a 146.41±19.67 a 14.07±1.71 a 105.65±19.61 a 293.38±79.22 a L2 23.03±5.07 a 138.97±14.49 b 13.11±1.98 b 107.77±16.64 a 246.80±94.58 b L3 22.02±4.77 a 121.08±12.88 c 12.08±2.40 c 103.82±21.93 a 183.06±75.64 c 说明:同列不同字母表示同一指标不同处理间差异显著(P<0.05) Table 4. Effects of shading on growth indexes of C. japonicum seedlings
-
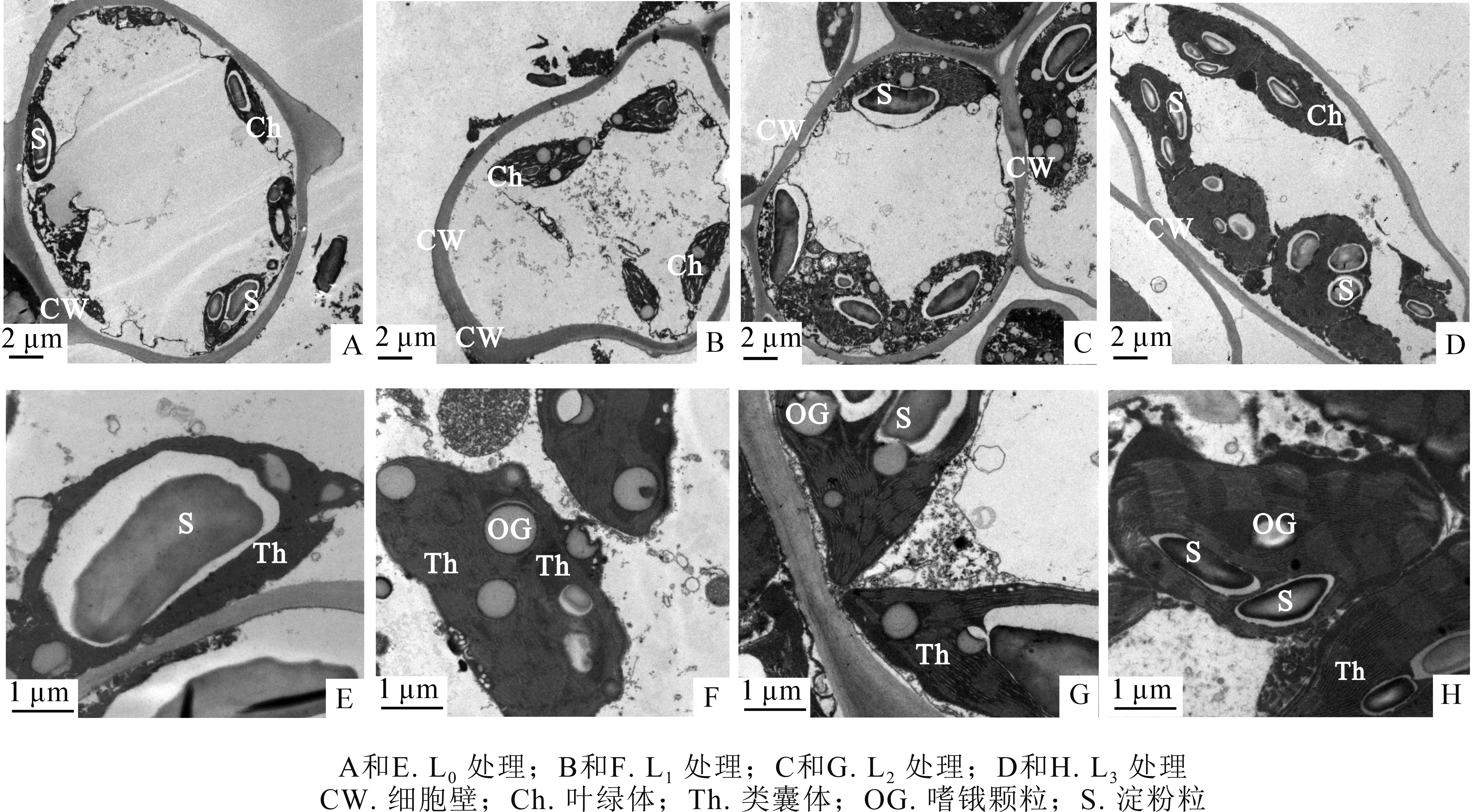
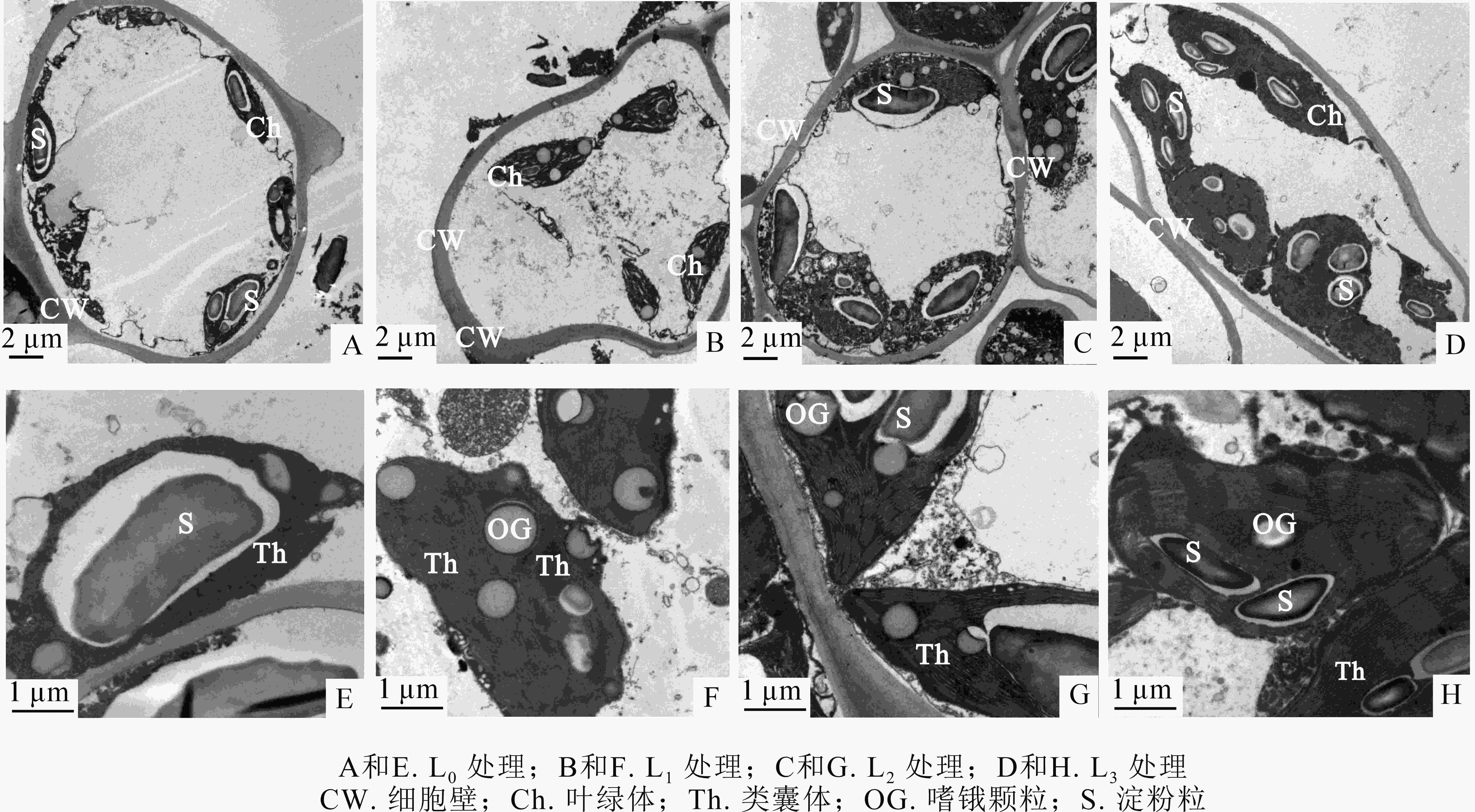
叶绿体是植物进行光合作用的场所,也是细胞中对逆境最敏感的细胞器[17]。因此,分析叶片中叶绿体的结构变化可以为植物遭受不良生境下的生理反应作出解释。从叶片超微结构(图3)可以看出:全光(L0)条件下,高强度光辐射叶肉组织中细胞轮廓可鉴,叶绿体(Ch)数量少,紧靠细胞壁(CW)边缘成平行分布,形状如梭形,中央淀粉粒(S)明显。由于叶绿体边缘分布,细胞中央形成有大的空腔,类囊体片层与叶绿体长轴近似平行排列,基粒类囊体(Th)略有膨胀,基质片层有模糊,未见有嗜锇颗粒(OG)分布(图3A、E);L1处理下细胞内轮廓清晰,Ch数量有所增加,在细胞中占有面积增加,但与CW之间的间隙加大,OG相应数量明显增加,S数量减少,叶绿体中Th排列紧密,之间通过基粒片层相连(图3B、F);L2、L3处理细胞结构与叶绿体变化不大,但叶绿体数量显著增多,细胞内空腔面积减小,叶绿体外形逐渐变为圆球形或椭圆形,S数量较多,在细胞中占有比例较大,零散分布有OG,Th结构尚清晰,片层厚度加大,但排列位置不规则,出现了部分黑色斑块(图3C、G、D、H),这可能与过度遮光的阴湿环境有关。
2.1. 遮光对连香树单叶面积(SLA)、单叶干质量(mSLWd)、单叶鲜质量(mSLWf)及叶片含水量(CLWC)的影响
2.2. 遮光对连香树叶片比叶面积(SLAM)和比叶重(mLMA)的影响
2.3. 遮光对连香树叶片光合作用日变化的影响
2.3.1. 净光合速率(Pn)
2.3.2. 气孔导度(Gs)
2.3.3. 胞间二氧化碳摩尔分数(Ci)
2.3.4. 蒸腾速率(Tr)和水分利用效率(EWUE)
2.4. 净光合速率与叶片含水量及叶片形态指标的相关关系
2.5. 遮光对连香树光合色素及幼苗生长的影响
2.5.1. 不同遮光条件下连香树光合色素的变化
2.5.2. 遮光对连香树幼苗生长指标的影响
2.6. 遮光对连香树叶片叶肉细胞超微结构的影响
-
光是影响植物生长发育最主要、最直接的外界因素之一[17-18]。因此,光照条件的改变直接影响植物个体的外部形态及生长发育。不同强度的遮光处理对连香树幼苗的形态产生了显著影响,表现为随着遮光强度的增大,CLWC、SLA显著增加,mLMA明显减小,但SLAM增大,这与文献[19]的研究结果基本类似。全光下,幼苗适应高强度的光辐射,叶片具有较高的mLMA,较低的SLAM,单叶面积较小,说明叶片充分发育,叶片较厚,从而减缓了光在叶片内部的传导。这样可以避免强光对叶片内部机构造成破坏,是一种生理适应;而弱光下叶片蒸腾较弱,CLWC较高,具有较低的mLMA和较高的SLAM,单叶变得薄而具有较大的SLA,说明幼苗通过扩大叶面积而获取更多的光强,以弥补庇光环境下的光照不足。
遮光对连香树幼苗的光合作用产生了显著影响。全光和L1处理下Pn的变化曲线相似,在1 d中呈现不对称的“单峰”,L2和L3处理下Pn则呈现近“几”形变化,Tr、Gs也出现与Pn类似的特征。据研究,植物光合作用日变化有单峰[20]、双峰、三峰[21]等类型,这与不同植物自身生物学特性和试验测定时幼苗所处的环境条件有关[22]。全光和L1处理下,早晨太阳有效辐射升高很快,造成幼苗Pn快速上升,这是Pn呈现不对称“单峰”变化的重要原因。全光下,白天外界有效辐射长时间在2 000 μmol·m−2·s−1以上,但过剩的强光并未有对连香树幼苗的组织器官造成明显灼伤,未出现明显的“午休”和光抑制,而L1下,幼苗生长生理的小环境得到一定改善,Tr变弱,尽管光照辐射强度减弱,幼苗仍能够维持较高的Pn 和相对较高的EWUE。从这一点来看,连香树对外界强光具有一定的忍耐性,轻度遮光也未对幼苗的生长带来明显的抑制性影响。
叶绿素质量分数及相对比例同光合作用的关系十分密切,在一定程度上可以反映植物同化物质的能力[23]。本研究表明:遮光条件下连香树叶片叶绿素a、叶绿素b、叶绿素a+b、类胡萝卜素均有一定程度增加,而且随着遮光强度的加大其质量分数递增。李霞等[23]认为:耐荫性植物品种遮光后植株叶片叶绿素质量分数增加,而不耐荫的植物品种叶绿素质量分数降低。从这一理论可以推断,连香树又表现出一定的耐荫性[24]。同时,遮光时间的长短也影响叶片内光合色素的变化,短期内连香树叶片内光合色素已存在显著增加,说明连香树对短期庇荫具有敏感性。长期庇荫的光环境将引起连香树叶片光合色素的适应性增加,增强了叶片的捕光性,这对于幼苗的营养积累和健壮生长是有利的,说明连香树对外界庇荫的环境具有一定的适应能力。
大量研究表明:遮光不仅会造成植物光合生产能力的降低,而且会对光合器官的微观结构产生影响[25]。长期生活在弱光条件下,植物叶绿体根据光线的强弱将做出形态上的调整,甚至发生结构的变化,以增强对弱光的吸收利用能力[26−27]。因此,分析叶肉细胞超微结构可以为遮光处理对幼苗的生理功能的影响机制找到解释。本研究表明:遮光对连香树叶肉细胞超微结构带来一定影响,进而影响到幼苗光合产物的积累。全光下连香树叶绿体紧靠细胞壁平行分布,形状如梭形,细胞中央形成有大的空腔,但叶绿体数量偏少。而遮光条件下,特别是强度遮光,细胞内空腔面积减小,叶绿体数量明显增加,叶绿体外形逐渐变为圆球形或椭圆形,淀粉粒数量增多,这将有利于叶绿素片层附着更多的补光色素与光合酶,有利于植物吸收较多的光能以弥补弱光的不足,提高其光合作用效率,这与HIKOSAKA等[28]和LIN等[29]的研究结论基本一致。遮光条件下,叶绿体中淀粉粒数量增多,这可能是由于光合产物合成后迅速转化为淀粉暂时储存于叶绿体中,而弱光下同化产物的运输速度相应降低,使得淀粉积累在叶绿体内形成较大的颗粒。同时,遮光条件下叶绿体类囊体叶绿体膜发育不完全,嗜锇颗粒增多,类囊体结构尚清晰,但排列位置不规则,出现了部分黑色斑块,这可能与过度庇荫的阴湿环境有关。
由于遮光降低了林内光合辐射强度,影响了幼苗的光合作用效率,改善了林内小环境,最终会影响连香树幼苗的生长形态。本研究表明:强度遮光条件下,连香树H和D递减,生物量模型D2H明显下降,说明强度遮光会影响幼苗正常的生长发育,不利于幼苗的健康。同时,轻度遮光与对照相比,H、D、H/D和D2H均未出现显著差异。可见,连香树对光照条件的改变具有一定的自我调节能力。轻度遮光下,幼苗仍能维持较高的Pn,以确保植株生长良好;而中度和重度遮光则会严重降低连香树的Pn,并导致植株营养缺乏,生长受抑,影响植物个体的质量。因此,在育苗实践中,夏季可以对其进行适度的遮光处理,以降低高温和日灼危害,并可以减少土壤水分蒸发,缓和高温干旱对幼苗生长的制约,但遮光强度不能过大,有效辐射光强以不低于自然光强的55%为宜,以满足幼苗正常生长发育所需要的环境条件。










 DownLoad:
DownLoad: