-
薄壳山核桃Carya illinoensis又称长山核桃、美国山核桃,为世界著名的高档干果、油料树种和材果兼用优良树种,其果实营养丰富,保健价值高[1]。研究发现:长期食用薄壳山核桃有明显的防衰老、健肠胃、防治心血管疾病等作用[2]。然而,薄壳山核桃树体高大,不利于人工采摘果实及日常的养护管理,培育矮化砧木可为薄壳山核桃育种工作及果品的推广提供便利。魏灵珠等[3]提出:赤霉素 (gibberellins,GA3) 是调控植物株高的重要激素,相关株高基因的克隆与分子机制研究对于合理调控植物生长发育和农业生产具有极其重要的利用价值。赤霉素作为一类二萜类植物激素,调节种子萌发[4−5]、茎杆伸长[6]、花粉管生长[7]、花和种子发育[8]等多种生理活动,也能在环境胁迫下调控植物的生长和发育[9]。其中,值得关注的是赤霉素通过促进节间伸长来调控植株的营养生长[10]。目前,已经克隆到赤霉素生物合成大多数步骤催化酶的基因,构建了从前体分子到活性赤霉素的整体合成框架[11]。研究发现:赤霉素后期的代谢酶(GA20ox、GA3ox、GA2ox)对活性赤霉素的精确调控具有关键作用[12]。GA20氧化酶(GA20-oxidase, GA20ox)是赤霉素合成过程中关键的限速酶,在棉花Gossypium spp.[13]、毛白杨Populus tomentosa[14]和山茶Camellia reticulata[15]中过表达会使得植株增高,抑制该基因在烟草Nicotiana tabacum[16]、苹果Malus domestica[17]等植株中表达则会使植株矮化。邓伟等[14]通过农杆菌介导法在毛白杨中过量表达棉花GA20ox使得杨树的茎伸长,但减少了叶片和根的生长。赵恺[17]通过农杆菌介导法将GA20ox的干扰信号转入苹果中,获得了苹果的矮化砧木。GA3氧化酶(GA3-oxidase, GA3ox)则是赤霉素合成过程中的一个关键酶。研究发现:抑制小麦Triticum aestivum[18]和水稻Oryza sativa[19]中的GA3ox基因表达,植株会呈现矮化的性状。刘颖等[18]发现:在矮杆小麦‘宁98-2105’中,GA3ox1和GA3ox2在茎的倒一节结节中的转录水平显著高于对照,推测是赤霉素传导通路受到影响导致植株突变。而过量表达赤霉素合成过程中的另一个关键酶赤霉素氧化酶(GA2-oxidase, GA2ox),也会使麻疯树Jatropha curcas[20]、荔波连蕊茶Camellia lipoensis[21]和矮牵牛Petunia hybrida[22]等植株矮化。HU等[20]运用农杆菌介导法在麻疯树中过表达JcGA2ox发现:植株呈现矮化,更小更深的叶片,花序果实都变小的性状。本研究以薄壳山核桃幼苗为实验材料,于生长期喷施100 mg·L−1的赤霉素,研究其对薄壳山核桃幼苗生长的影响和赤霉素合成代谢关键基因的时空变化,初步了解薄壳山核桃GA20ox、GA3ox和GA2ox基因的时空表达特征及在赤霉素合成中的调控作用,为薄壳山核桃矮化新种质育种奠定基础。
HTML
-
实验薄壳山核桃种子是半同胞家系,2018年10月采摘于浙江农林大学同一株树,挑选大小一致的种子作为实验材料。晾晒后置于塑封袋内,置于4 ℃保存。2019年4月,将薄壳山核桃种子种在基质土[m(泥炭)∶m(珍珠岩)∶m(蛭石)=1∶1∶1]内,在亚热带森林培育国家重点实验室的驯化室[(25±2) ℃,湿度为75%]进行萌发培养。2019年5月,选取生长势、株高、节间长度和主根长度等形态指标基本一致的幼苗54株,叶面喷施100 mg·L−1的赤霉素(CAS: 77-06-5, BC, 上海生工)进行处理(24 h内不浇水),以去离子水为对照,3次重复。
-
处理28 d 后测量植株高度、顶芽至第1叶节间长度和主根长度,均用直尺测量,保留1位小数。采用SPSS 17.0中的单因素方差分析对所有指标进行统计处理,P<0.05为差异显著,P<0.01为差异极显著。
-
薄壳山核桃、山核桃Carya cathayensis和核桃Juglans regia 的GA20ox、GA3ox和GA2ox均由浙江农林大学省部共建亚热带森林培育国家重点实验室张启香课题组克隆。从美国国家生物信息中心(NCBI)(http://www.ncbi.nlm.nih.gov/)上分别搜索已报道的植物 GA20ox、GA3ox、GA2ox 同源基因的氨基酸序列,通过软件DNAMAN进行氨基酸序列比对。利用ITOL (https://itol.embl.de/)和MEGA制作系统进化树。
-
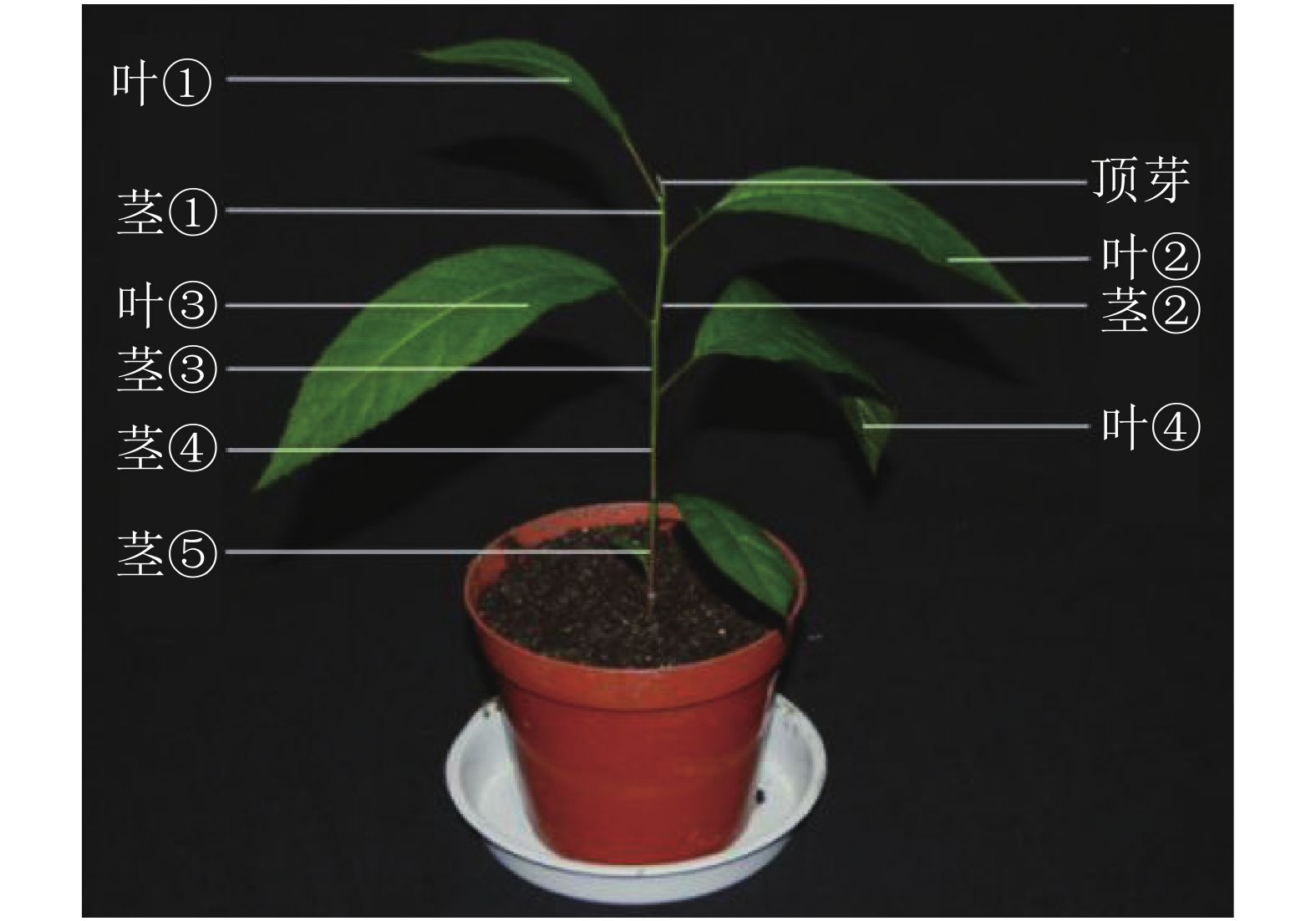
对处理 0、7、14、21和28 d后植株的叶片进行采样,用于基因不同时间表达变化;基因不同空间表达变化采用处理0和28 d后植株的顶芽、叶①、茎①、叶②、茎②、叶③、茎③、叶④、茎⑤和根(图1)。样品采集后立即放入液氮,总RNA的提取方法按照RNAprep pure Plant Kit(北京天根)及其说明书提供的方法进行。用Prime ScriptTM RT Master Mix (TaKaRa公司)反转录合成cDNA。
根据基因序列,利用Primer3Input设计定量引物,以山核桃Actin基因为内参,引物序列见表1。荧光定量PCR反应体系为10.0 μL,包含cDNA 0.4 μL,TBGREEN (TaKaRa公司)5.0 μL,正向引物0.2 μL,反向引物0.2 μL,双蒸水 4.2 μL。反应条件为:95 ℃ 10 min;95 ℃ 10 s,60 ℃ 31 s,40个循环;95 ℃ 15 s,60 ℃ 1 min,95 ℃ 30 s,60 ℃ 15 s。数据分析采用7300system软件和2−∆∆Ct的方法,ΔΔCt = (Ct靶基因−Ct内参)处理组−(Ct靶基因− Ct内参)对照组[23-24]。图表由 Excel 2007 软件制作。
引物名称 上游引物(5′→3′) 下游引物 (5′→3′) RTCiGA20ox GCACACCGACCCACAAATCATT TGAGTTCTGATCAGGTGGGACT RTCiGA3ox CACTCGAACAATTCCGCCAACT TGCCCAAGGAGCCTAGCATTAG RTCiGA2ox CAGGTAGGTGGGCTTCAAGTGT CCCGATGCAAGCAACTTTTGTA CcActin TGCGGGTGCTCGCTTCGGCAGC GGGCAGCCAAGGATGACT Table 1. Primers sequences
1.1. 材料与处理
1.2. 方法
1.2.1. 处理后薄壳山核桃幼苗形态指标测定
1.2.2. 赤霉素代谢关键基因的生物信息学分析
1.2.3. 赤霉素相关基因的时空表达分析
-
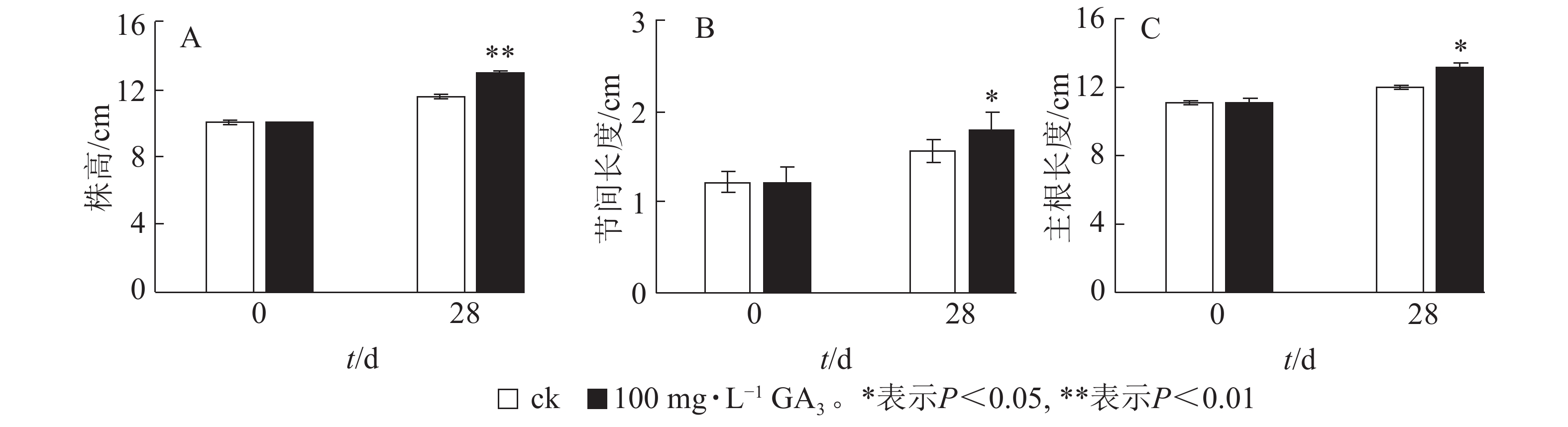
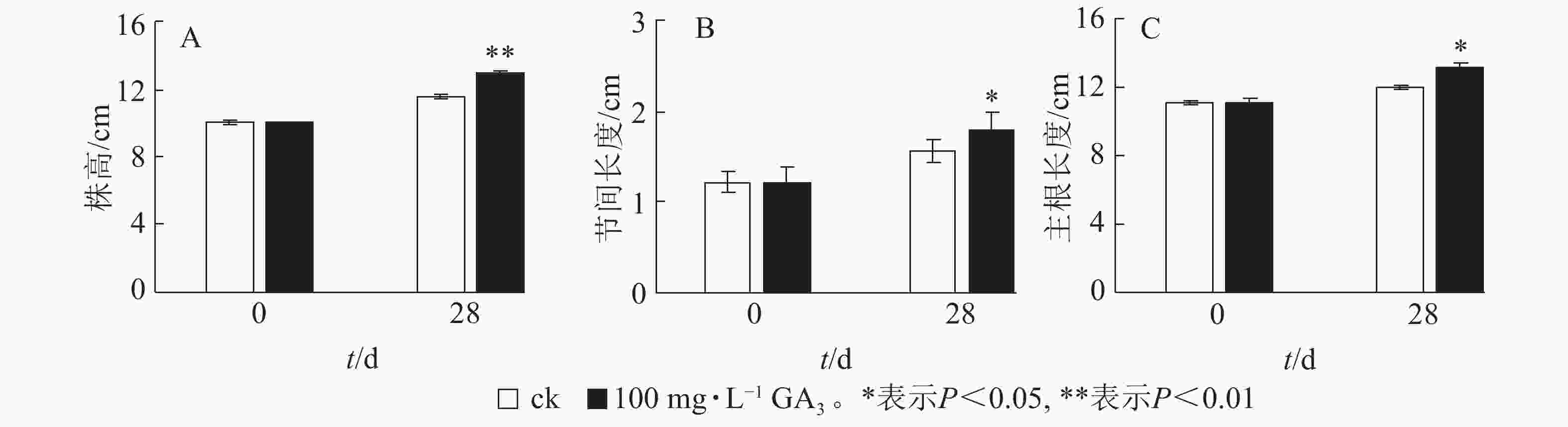
由图2A可知:处理28 d后,薄壳山核桃植株平均生长量达2.9 cm,对照组平均生长量仅为1.5 cm,处理后为对照的1.93倍,差异极显著(P<0.01)。外源赤霉素可以有效刺激薄壳山核桃的营养生长,显著促进了茎的生长。经过赤霉素处理后,薄壳山核桃从顶芽至第1叶茎节间长度伸长量为对照的1.14倍,差异显著 (P<0.05),但节的数量没有发生变化(图2B)。此外,赤霉素对根系生长也有良好的促进作用。赤霉素处理28 d后薄壳山核桃主根长伸长量是对照组的1.09倍,差异显著(P<0.05)(图2C)。
-
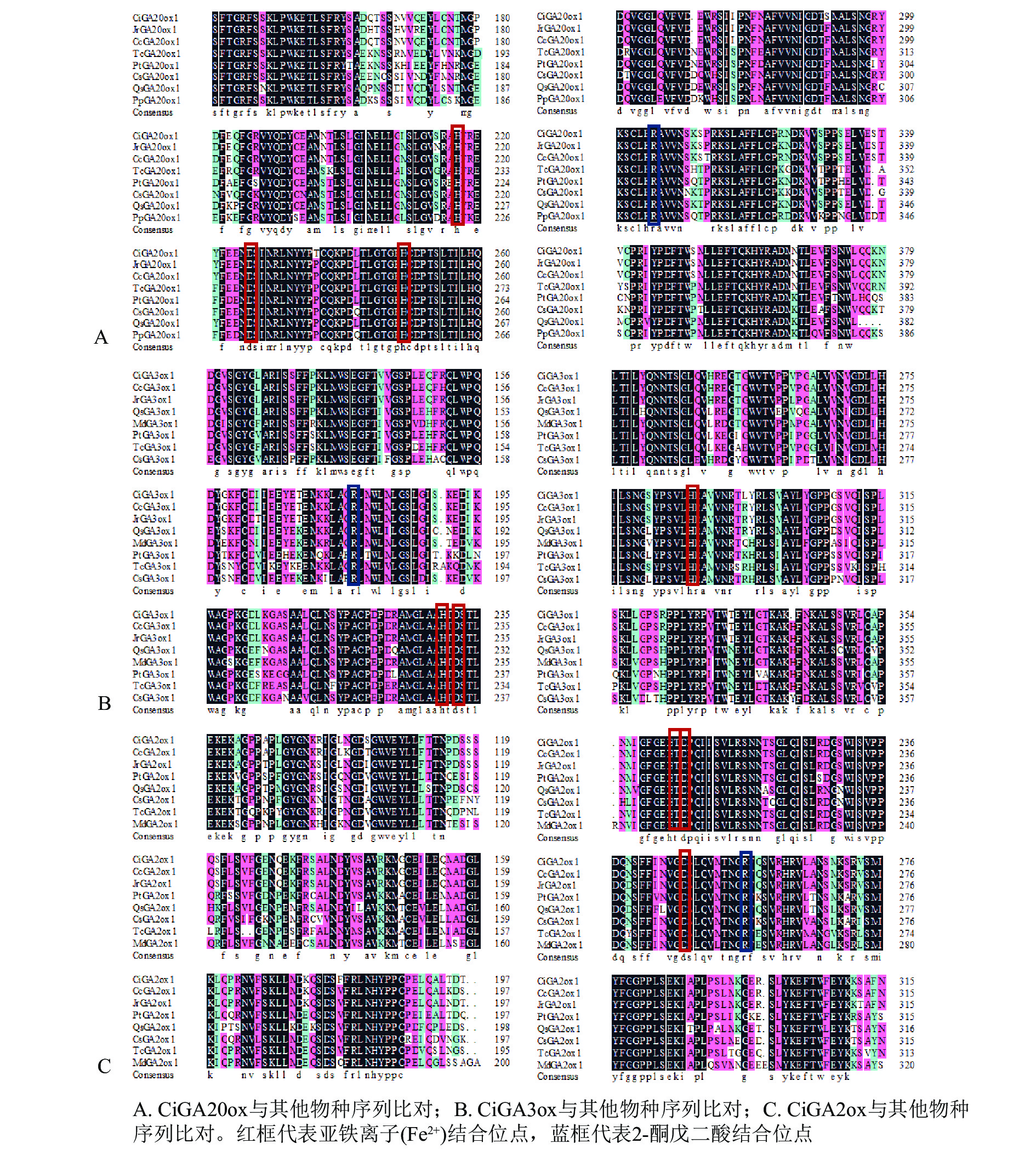
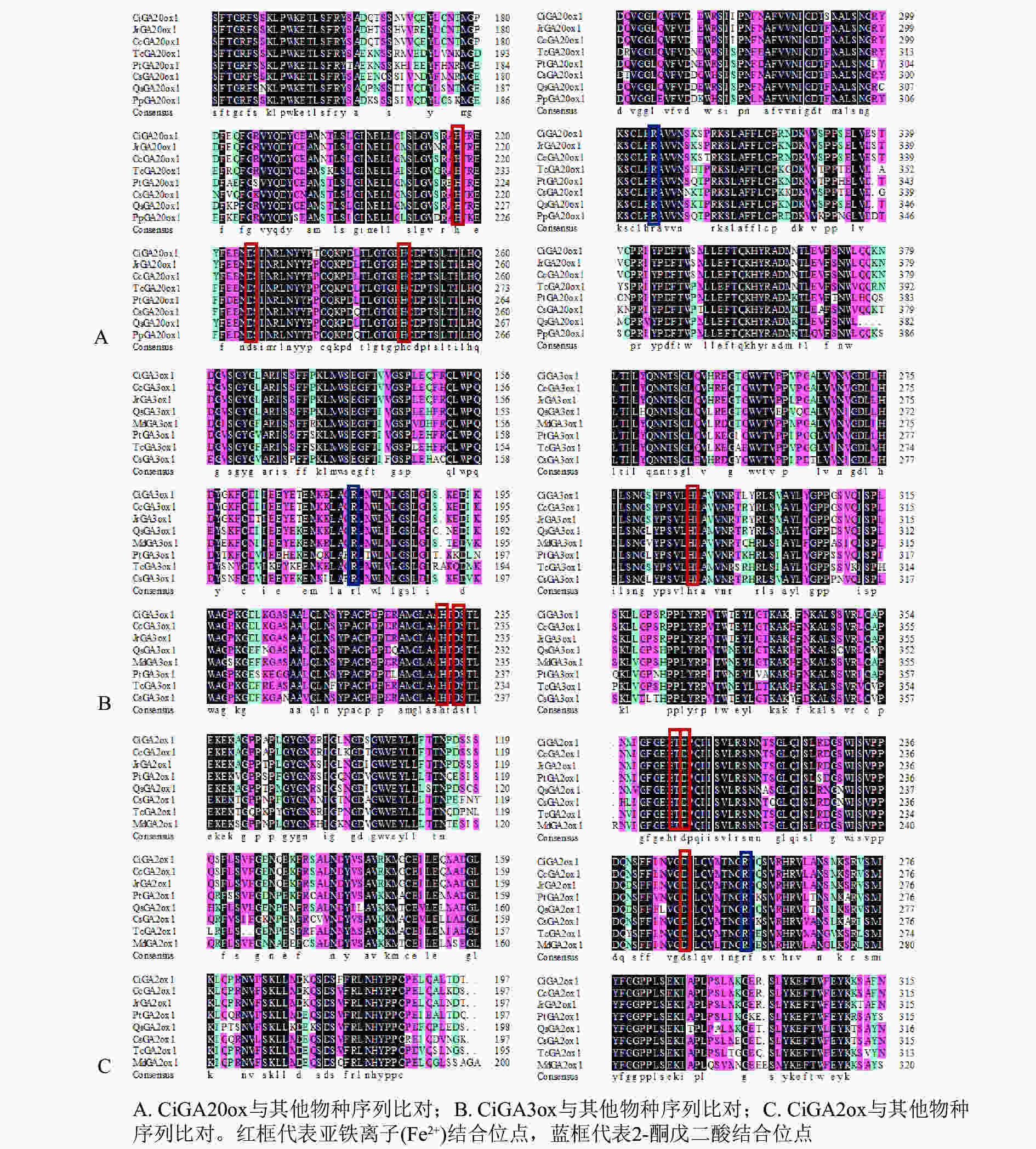
将CiGA20ox、CiGA3ox和CiGA2ox基因编码的氨基酸序列与NCBI数据库的GA20ox、GA3ox和GA2ox蛋白氨基酸序列进行比对分析(图3)。发现CiGA20ox(图3A)、CiGA3ox(图3B)和CiGA2ox(图3C)都有着相应的保守20G-FeⅡ-Oxy蛋白结构域,以及2-酮戊二酸结合位点和亚铁离子(Fe2+)结合位点。说明CiGA20ox、CiGA3ox和CiGA2ox属于相应的赤霉素氧化酶家族。
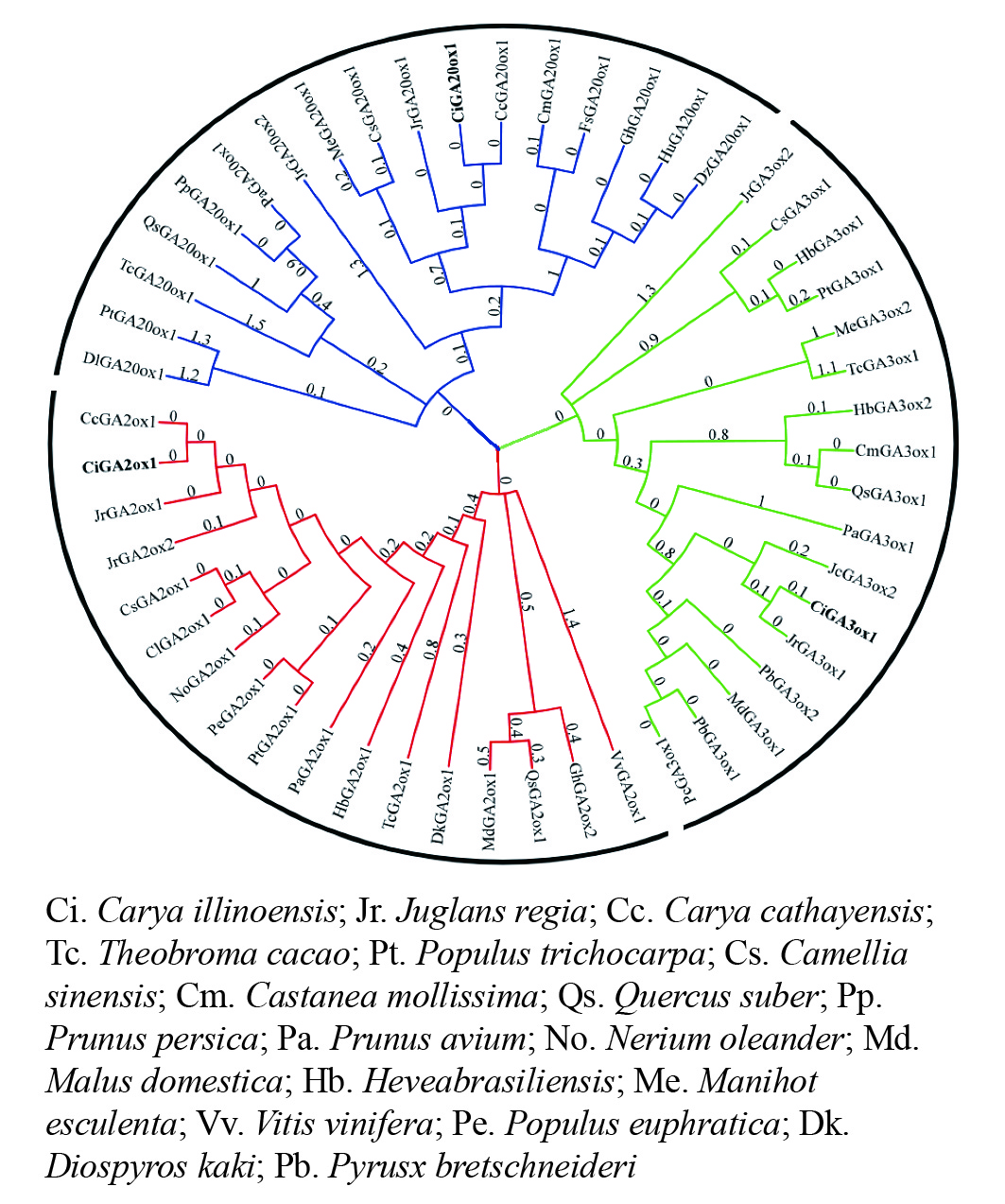
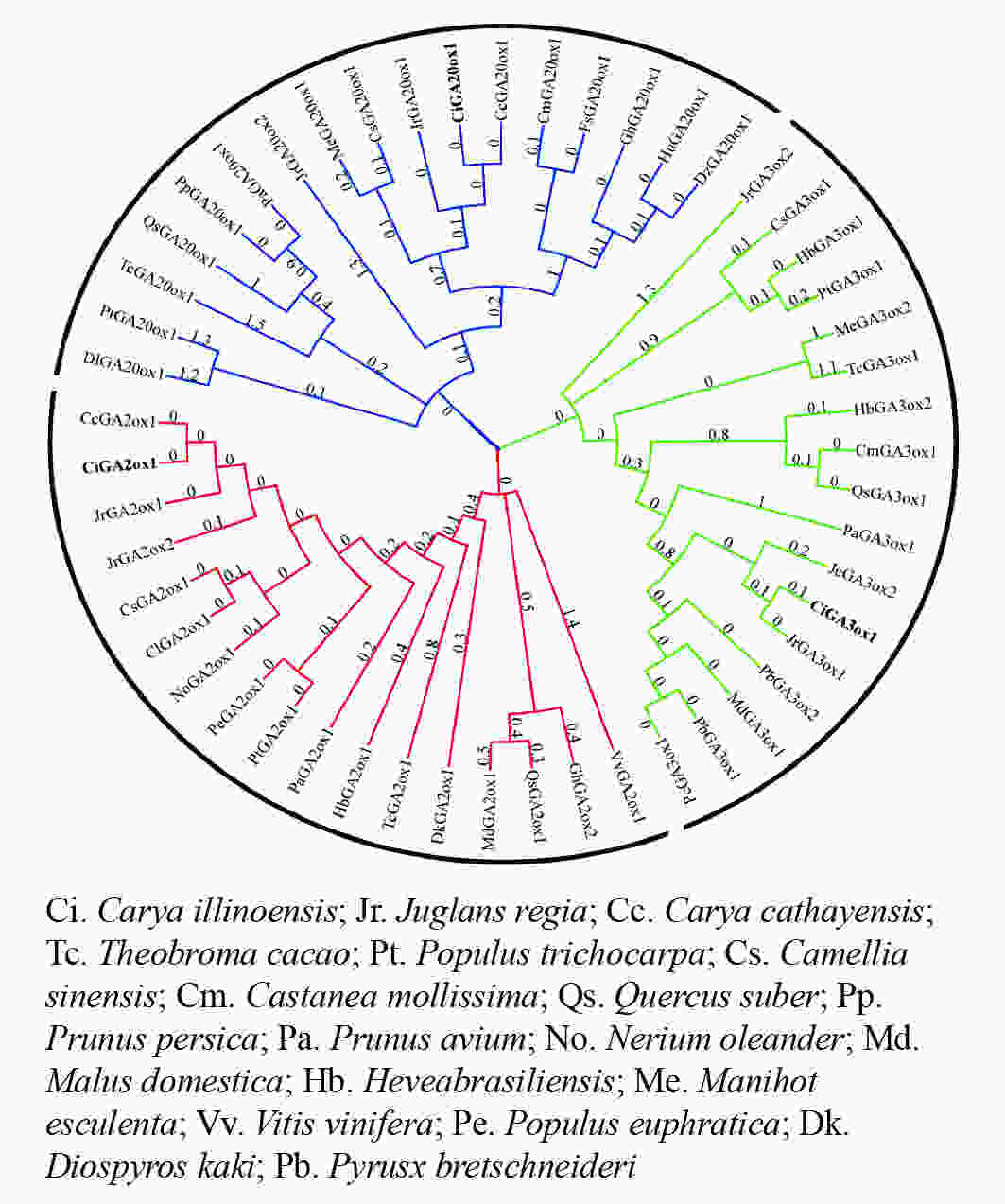
在进化关系图(图4)中,CiGA2ox、CiGA20ox和CiGA3ox与山核桃和核桃的亲缘关系较近,且与山核桃的亲缘关系最近。可见同科植物的GA20ox、GA2ox和GA3ox蛋白同源关系较近,这与该蛋白的序列相似性分析结果相一致,表明薄壳山核桃GA20ox、GA2ox和GA3ox蛋白在进化方面较为保守。
-
对薄壳山核桃幼苗喷施100 mg·L−1赤霉素,通过qRT-PCR检测并分析其在处理后不同时期(0、7、14、21、28 d) CiGA20ox、CiGA3ox和CiGA2ox的相对表达量特征。
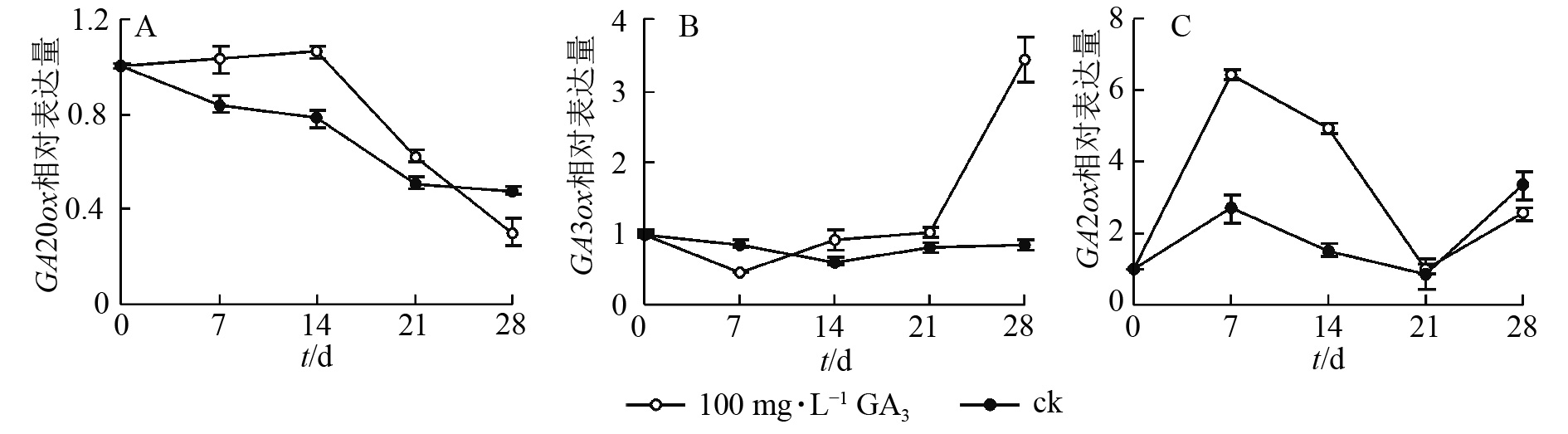
定量结果显示:喷施100 mg·L−1赤霉素可在短期内抑制CiGA20ox的转录。在前期,CiGA20ox表达量相对平稳,在处理14 d后,CiGA20ox的表达量快速下降,处理21 d后,表达量为初始值的59.1%,之后持续缓慢下降,28 d后的表达量仅为初始值的38.6%(图5A)。
在赤霉素处理后CiGA3ox 表达量呈先下降后上升的趋势。处理7 d后表达量到达最低点,为对照的48.6%,之后缓慢升高,于处理21 d后回升至处理前水平。后快速上升,于处理28 d后到达顶点,表达量为初始值的350%。与GA20ox基因表达情况不同的是在赤霉素处理21 d后,GA3ox表达量迅速提高到较高的水平,可能是GA3ox在赤霉素质量浓度下降时起更重要的反调节作用,以保持植物体内的赤霉素水平,具体调控机制尚不明确(图5B)。
赤霉素代谢过程中的另一个关键酶CiGA2ox表达量总体则呈波浪形变化,在7 d后达到顶峰,之后缓慢下降,14 d后快速下降至21 d时恢复到了对照水平,28 d后再次上升为初始值的220%(图5C)。
-
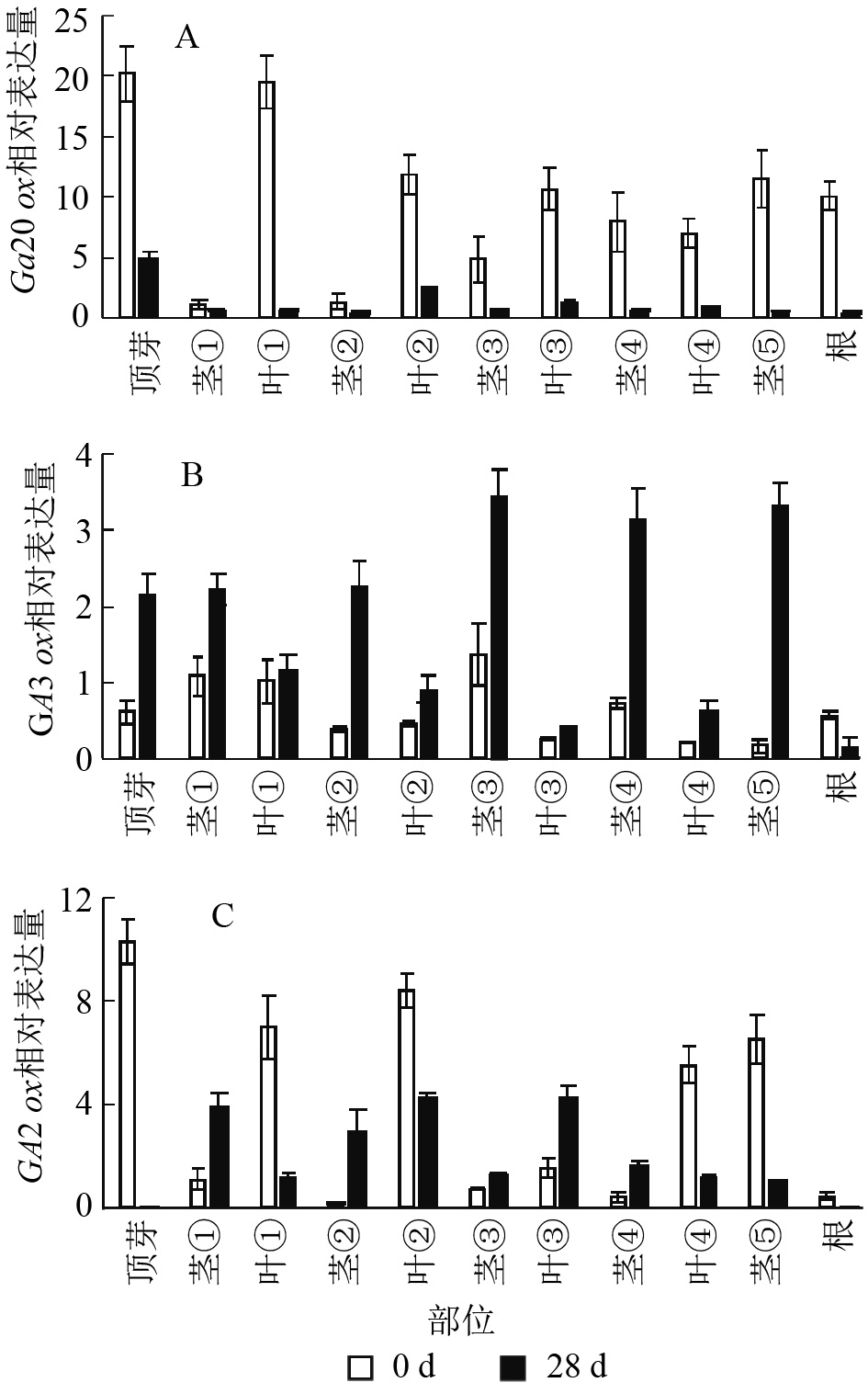
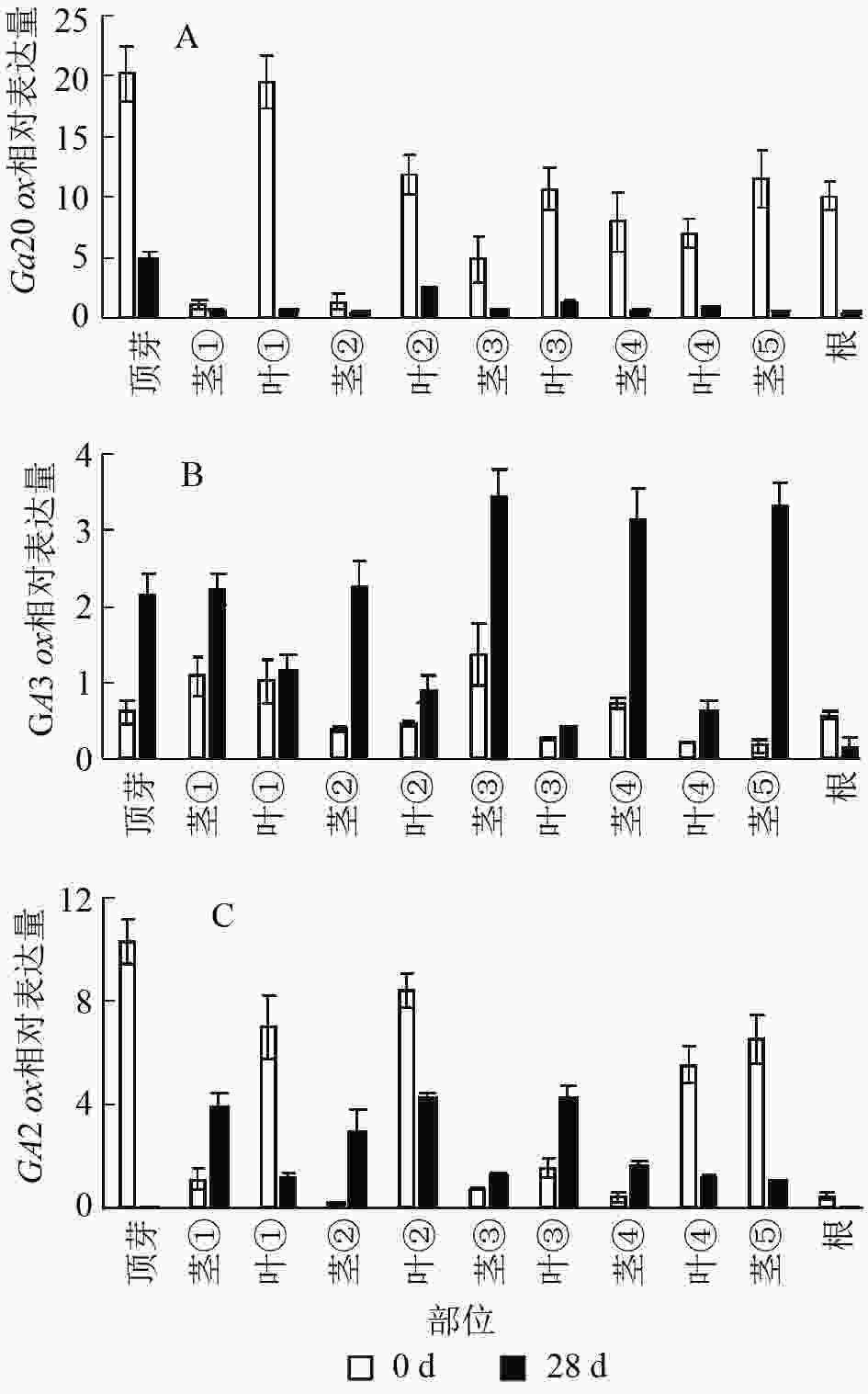
本研究通过qRT-PCR对薄壳山核桃幼苗不同部位赤霉素代谢关键基因GA20ox、GA3ox和GA2ox的相对表达量开展了研究。由植株上端至下端命名不同部位 (图1)。
结果显示:薄壳山核桃GA20ox在不同部位均有表达,但是不同部位分布不均,主要分布在植株的顶芽、叶片和根内,分布规律大致为在叶片中是由顶芽向叶④逐渐递减,在茎中是由茎①至茎⑤逐渐递增(图6A)。赤霉素处理过后,各个部位的GA20ox基因表达量均下降,说明在赤霉素处理28 d后,CiGA20ox的各部位表达均受到了抑制,但是表达模式没有发生变化,顶芽中最多,其次是叶片内表达量大于茎秆中的表达量。
薄壳山核桃GA3ox基因表达量相对较少,存在组织特异性,主要集中在植株顶芽和茎中,在叶片中表达量较少(图6B)。但是在外施赤霉素后可以发现:除了根部,GA3ox在其他部位均有上升,而在茎中的增加量大于在叶片中的增加量。
薄壳山核桃GA2ox在各个部位均有分布,主要分布在薄壳山核桃的顶芽和叶片内,在茎杆部位几乎不表达(图6C)。经过赤霉素处理后,CiGA2ox在靠近植株顶端的茎中,相对含量发生了累积,而叶片中的GA2ox转录受到了抑制。
2.1. 外源赤霉素处理对薄壳山核桃幼苗生长指标的影响
2.2. 薄壳山核桃赤霉素代谢关键基因CiGA20ox、CiGA3ox和CiGA2ox的生物信息学分析
2.3. 薄壳山核桃赤霉素代谢关键基因CiGA20ox、CiGA3ox和CiGA2ox表达的时间变化
2.4. 薄壳山核桃赤霉素代谢关键基因CiGA20ox、CiGA3ox和CiGA2ox的空间变化
-
在本研究中,外施赤霉素使得薄壳山核桃茎杆伸长,植株增高,并且在一定程度上促进了主根系的生长。李俊南等[25]用不同浓度的赤霉素浸泡薄壳山核桃种子后,增加了薄壳山核桃的发芽率,并且对薄壳山核桃幼苗的根茎影响较大。此外,刘芳等[26]对甜樱桃Prunus avium外施赤霉素后,新梢生长量显著高于对照,但是调节新梢长度的影响主要体现在调节其节数,对平均节间长度的作用并不显著。这可能是植株新梢生长程度与植株整体并不一致造成的。
外施赤霉素使得薄壳山核桃幼苗内的赤霉素生物合成基因CiGA20ox和CiGA3ox表达量显著下调,而CiGA3ox的表达量在处理后期出现了升高的现象,可能是因为GA3ox作为赤霉素生物合成的最后一步,在赤霉素质量浓度骤然升高或降低时,GA3ox起着更重要的反调节作用,从而维持植物体内赤霉素的平衡。该结果类似于赤霉素合成过程中的负反馈调节现象。而CiGA2ox则是呈现先上升后下降的波浪形趋势,说明在外源赤霉素的作用下,随着活性赤霉素物质量的增加,GA2ox表达量也在增加。郝鹏博[27]在对桃树Prunus persica外施赤霉素后,桃树的GA20ox、GA3ox呈下降趋势,并于后期增加,GA2ox则是呈先上调后下降的趋势,与本研究基本一致。这些结果均表现出赤霉素合成代谢酶GA20ox和GA3ox参与赤霉素负反馈调节,而GA2ox则是参与了正反馈调节。
本研究选取了薄壳山核桃的不同部位进行qRT-PCR 分析,通过组织特异性分析发现:赤霉素合成相关基因CiGA20ox和CiGA3ox均在顶端大量表达,参与合成活性赤霉素;而调控赤霉素代谢的基因家族CiGA2ox在中部和顶部也高表达,表明薄壳山核桃顶部的生命活动最旺盛。外施赤霉素后,薄壳山核桃顶端维持的赤霉素稳定程度被打破,CiGA20ox被抑制转录,CiGA3ox大量生成调控了植株生长,而CiGA2ox在茎中表达量上升以降解体内过多的赤霉素,以达到稳定的状态保证植株稳定生长[28]。而这些基因相对含量的变化都与植株生长有着一定的联系。
本研究通过对薄壳山核桃喷施100 mg·L−1赤霉素,对薄壳山核桃赤霉素代谢关键基因GA20ox、GA3ox和GA2ox进行了系统深入研究,为薄壳山核桃矮化分子育种提供了理论基础。目前,张佳琦等[29]通过农杆菌介导法构建核桃的GA2ox同源转化体系,已经得到了核桃的矮化再生植株。薄壳山核桃和核桃亲缘关系较近,可以通过转化矮化目的基因到植物体胚内,以期待得到薄壳山核桃良种矮化砧木。










 DownLoad:
DownLoad: