-
碳水化合物是自然界分布最广的物质之一,是植物生长发育过程中构成碳骨架,提供能量的主要物质,同时起到渗透调节和信号传导的作用[1]。STITT等[2]研究发现:拟南芥Arabidopsis thaliana白天同化的碳以淀粉形式积累,夜间淀粉转化成可溶性糖为生长提供能量,黎明前几乎完全利用。PANTIN等[3]研究拟南芥幼嫩叶片生长发现:夜间生长由于受到碳的限制,生长速度慢于白天。GRAF等[4]研究拟南芥叶片淀粉代谢发现:夜间淀粉降解模式受生物钟调控。拟南芥植株淀粉代谢研究中显示,糖代谢和淀粉降解可受光周期长度的影响[5-8]。RIOU-KHAMLICHI等[9]研究拟南芥幼苗生长发现:糖作为一种信号物质诱导细胞周期蛋白CYCD2和CYCD3的表达。糖代谢和淀粉降解是由一系列酶介导的。STREB等[10]研究拟南芥瞬时淀粉分解发现:α-淀粉酶[α-Amylase (AMY)]、极限糊精酶[limit dextrinase(LDA)]及异淀粉酶3[isoamylase 3(ISA3)]相互独立地直接作用于淀粉颗粒。植物叶片淀粉分解过程α-淀粉酶作用不明显,而β-淀粉酶[β-Amylase(BAM)]起关键作用[11-14]。小麦Triticum aestivum和烟草Nicotiana tabacum种子萌发过程中淀粉酶活性和AMY基因表达量逐渐上升,导致淀粉降解加速,可溶性总糖含量逐渐增加[15-16]。MARUYAMA等[17]研究拟南芥中mRNA水平,发现脱水和冷胁迫分别导致bam 1和bam 3表达增加。ISA3和LDA、AMY3和BAM等共同协调糖和淀粉的合成与分解[2]。近年来,毛竹Phyllostachys edulis的生理生态研究取得了许多进展,主要涉及光合作用、组织构造、糖的时空变化、不同生长期茎秆色素含量等方面[18-21],而对毛竹快速生长期分子水平的调节机制研究较少。因此,本研究以毛竹笋竹快速生长期的茎秆为材料,分析了非结构性碳水化合物(nonstructural carbohydrate, NSC)质量分数的变化,AMY和BAM的活性以及PeAMY和PeBAM基因表达模式,为进一步揭示毛竹快速生长期的调节机制提供理论依据。
HTML
-
在2019年4月下旬毛竹快速生长阶段,选取生境条件一致、生长状况良好和高度在(3.0±0.2) m的毛竹笋竹30株,从10:00开始每隔4 h随机取5株,每株作为1个独立实验,共5次重复。将茎秆从基部往上每间隔2节依次编号为 1、4、7、10、13、16、19、22。采样后在液氮中冷冻,之后放入−80 ℃超低温冰箱内备用。
-
称取毛竹茎秆0.5 g,研磨至匀浆,加蒸馏水9 mL,沸水浴提取10 min,不断搅拌。冷却,3 000 r·min−1离心5 min,取上清液。葡萄糖、果糖和蔗糖分别采用葡萄糖试剂盒(上海荣盛生物药业有限公司生产)、果糖和蔗糖试剂盒(南京建成科技有限公司生产)测定。具体方法参照说明书。
-
将上述可溶性糖提取后的沉淀用体积分数为80%乙醇冲洗1遍,加蒸馏水3 mL,搅拌均匀,沸水15 min。冷却后,加入9.2 mmol·L−1冷高氯酸4 mL,搅拌提取20 min,蒸馏水定容25 mL,混匀,3 000 r·min−1离心10 min。取上清液,测定方法采用蒽酮硫酸法。
-
参照BRADFORD[22]方法测定蛋白质质量分数。
-
称取毛竹茎秆0.5 g,加10 mL柠檬酸缓冲液(0.1 mol·L−1, pH 7.0),冰浴研磨,放在常温下充分提取15~20 min,10 000 r·min−1离心10 min(4 ℃),上清液为酶提取液。采用3,5-二硝基水杨酸法测定酶活力。40 ℃恒温水浴保温15 min,测定总淀粉酶活性,70 ℃加热15 min,钝化β-淀粉酶,测定α-淀粉酶活性。酶活力以1 min分解淀粉形成1 mol麦芽糖所需酶量为1个活性单位,以U·g−1·min−1(1 U=16.67 nkat)表示。
-
称取毛竹茎秆0.5 g,用 MES 缓冲液(20 mol·L−1, pH 6.2)进行提取,冰浴研磨,提取定容至5 mL,10 000 r·min−1离心10 min(4 ℃),上清液为酶提取液。酶活性测定采用麦芽糖酶试剂盒(南京建成科技有限公司生产)。具体方法参照说明书。
-
①RNA 提取。称取样品0.5 g,使用宝生物工程(大连)有限公司RNAiso Plus试剂盒提取RNA。微量分光光度计检测样品RNA的浓度和纯度,1.2%琼脂糖电泳检测RNA的质量。②反转录及cDNA质量检测。采用宝生物工程(大连)有限公司PrimeScriptTM RT reagent Kitwith gDNA Eraser(perfect real time)试剂盒。具体方法参照说明书。③cDNA全长及启动子克隆。参照毛竹基因组数据库中PeAMY和PeBAM基因序列设计基因全长和定量引物(表1),选择毛竹NTB基因作为内参基因。具体方法参照说明书。④基因表达检测(qRT-PCR)。采用宝生物工程(大连)有限公司SYBR Premix Ex TaqTM(perfect real time)试剂盒。具体方法参照说明书。
基因名称 序列 用途 PeAMY-F CATTTCTTCGACTGGGGCCT 基因全长扩增 PeAMY-R GATCACCTTGCCGTCGATCT PeAMY-F TTTTTGCGGTGGGCGAATAC 荧光定量PCR PeAMY-R AAATGCGGCACACAACACTC PeBAM-F CGGCAGGATTCTACAACCCT 基因全长扩增 PeBAM-R CCTTCAATGTTCTGGGTAGCC PeBAM-F CAGCGAAGCCGAGGAATGAT 荧光定量PCR PeBAM-R AATGGGGGTAGCTGACGGTA PeNTB-F TCTTGTTTGACACCGAAGAGGAG 内参基因 PeNTB-R AATAGCTGTCCCTGGAGGAGTT Table 1. Primer information
-
所有数据均为5次重复的平均值±标准误差。荧光定量数据按照下列公式计算:相对表达量=2−ΔΔCt[23],使用内参基因校正拷贝数,利用Origin 9.0软件(Origin Lab公司, 美国)进行统计分析和作图。
1.1. 材料
1.2. 方法
1.2.1. 可溶性糖质量分数测定
1.2.2. 淀粉质量分数测定
1.2.3. 蛋白质质量分数测定
1.2.4. α,β-淀粉酶活性测定
1.2.5. 麦芽糖酶活性测定
1.2.6. 基因表达检测
1.3. 数据处理
-
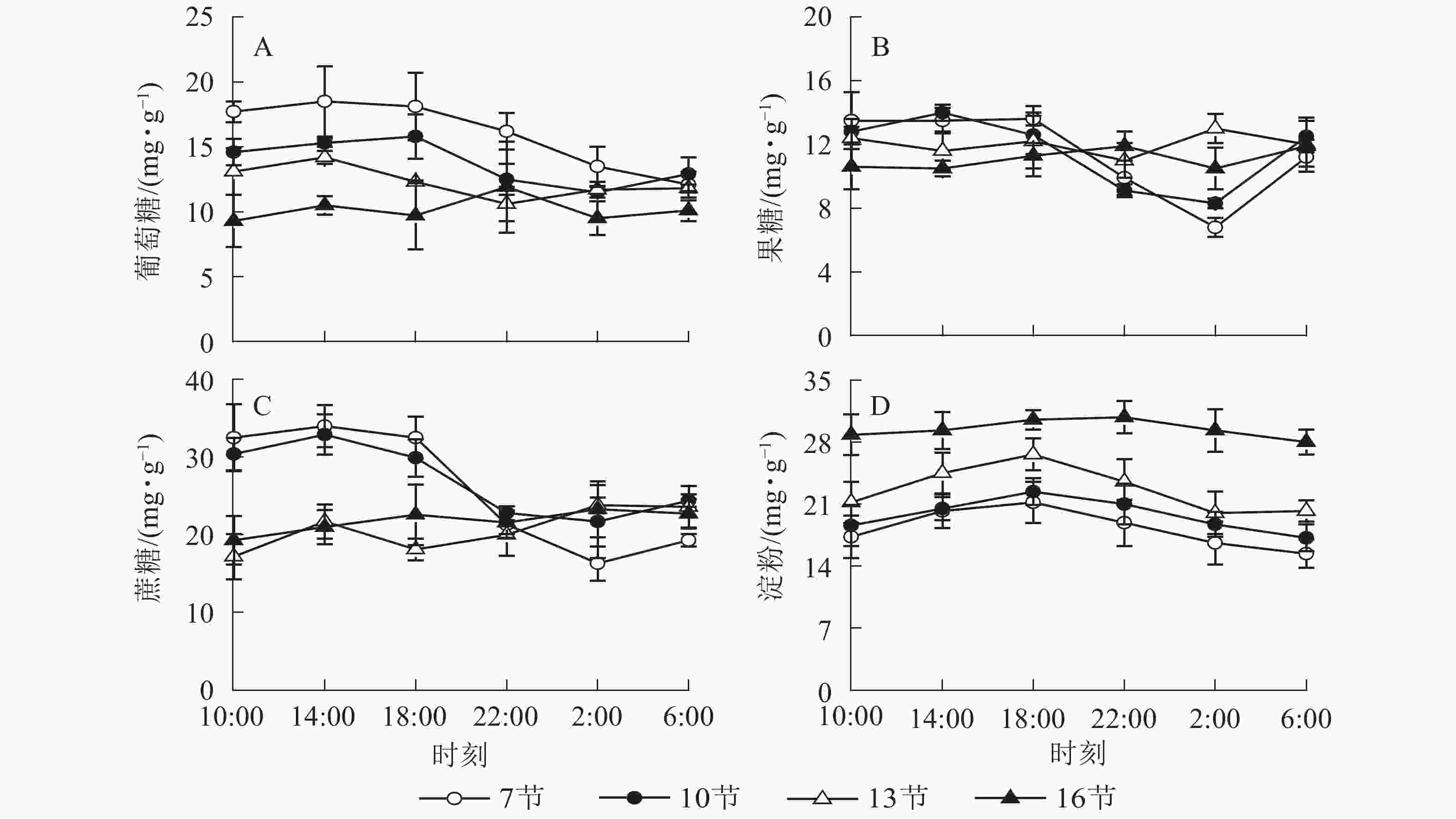
由图1A可以看出:随时间变化,葡萄糖质量分数白天较高,18:00之后逐渐降低,第7节和第10节2:00分别比18:00降低了25.4%和27.2%(P<0.01)。第13节和第16节变化不显著。由图1B可以看出:随时间推移,第7节和第10节果糖质量分数白天较高,18:00后极显著下降,2:00最低,与18:00相比分别降低了50.0%和34.1%(P<0.01),之后极显著上升,6:00比2:00分别高出64.7%和50.6%(P<0.01)。第13节和第16节变化不显著。由图1C可以看出:蔗糖质量分数在第7节和第10节较高,第13节和第16节较低且变化不显著。第7节和第10节白天蔗糖质量分数较高,18:00后极显著下降,2:00最低,分别比18:00低了49.8%和27.4%(P<0.01)。如图1D所示:淀粉质量分数在第7节和第10节较低,第13节较高,第16节最高且变化不明显。第7、10和13节白天淀粉质量分数高,18:00达最高,之后逐渐下降,6:00分别比18:00降低了27.3%、23.2%和24.0%(P<0.01)。
-
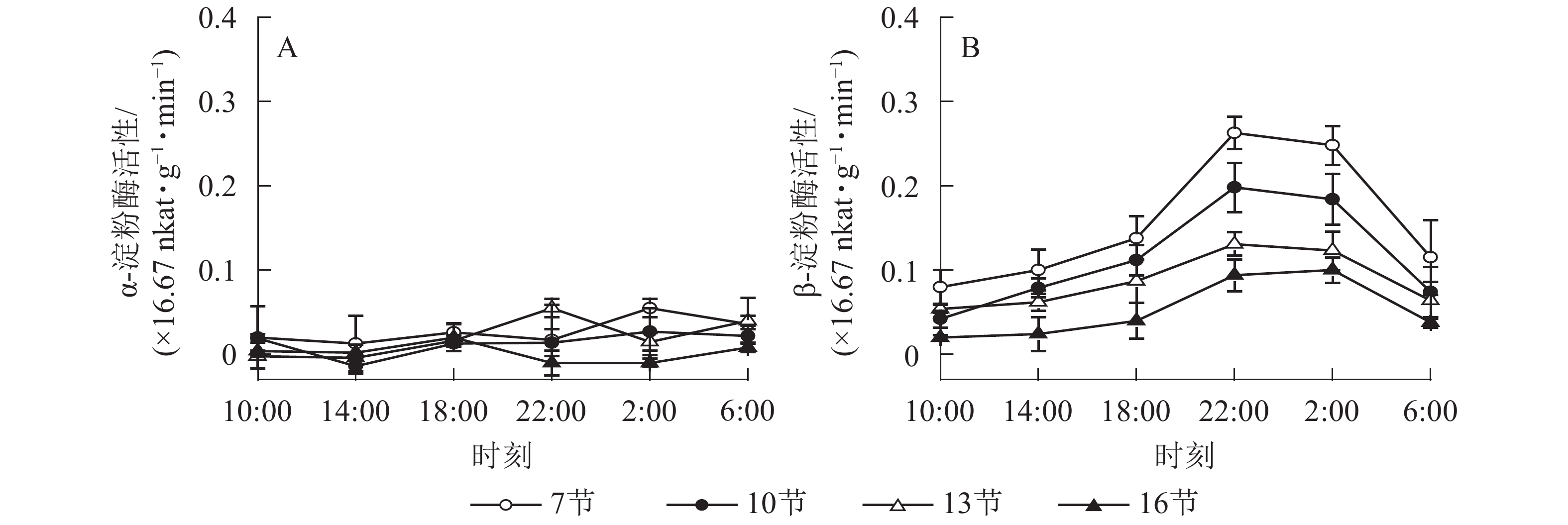
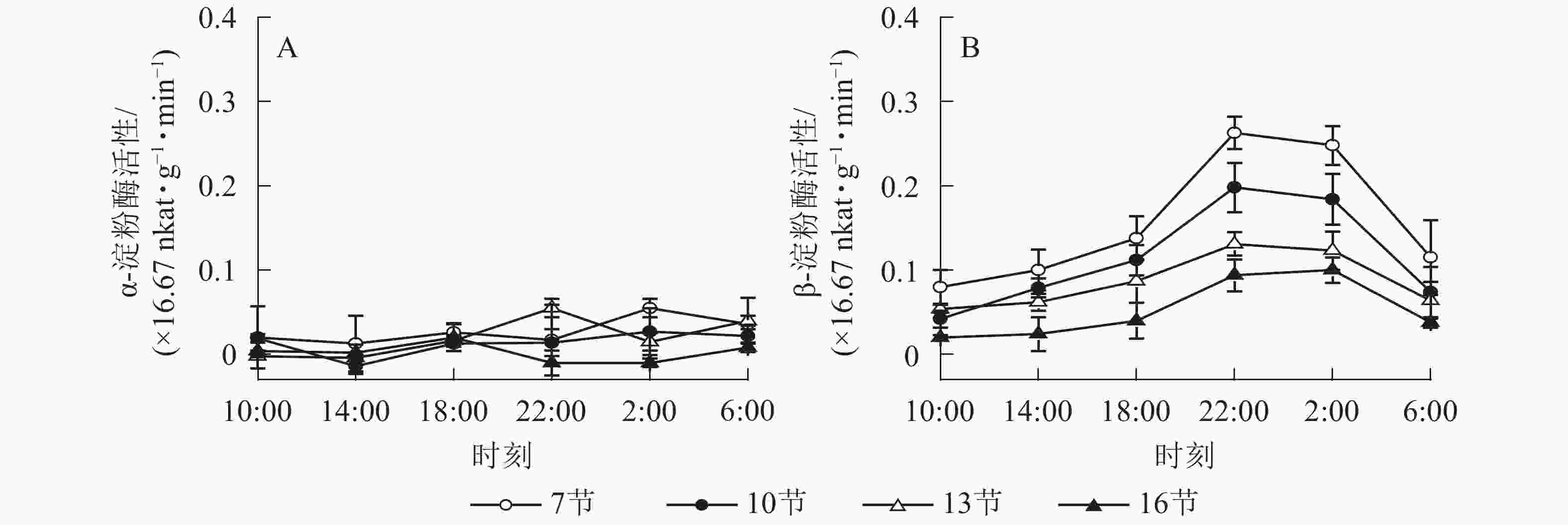
如图2A所示:AMY活性差异不显著,整体无明显变化规律。如图2B所示:BAM活性第7、10和13节较高,第16节活性低且变化不显著。白天BAM活性较稳定,18:00后活性较高且在22:00达最高,第7、10和13节22:00活性分别比18:00高90.5%、76.7%和50.5%(P<0.01),之后活性极显著下降,6:00与22:00 相比分别降低了56.2%和62.6%和51.1%(P<0.01)。
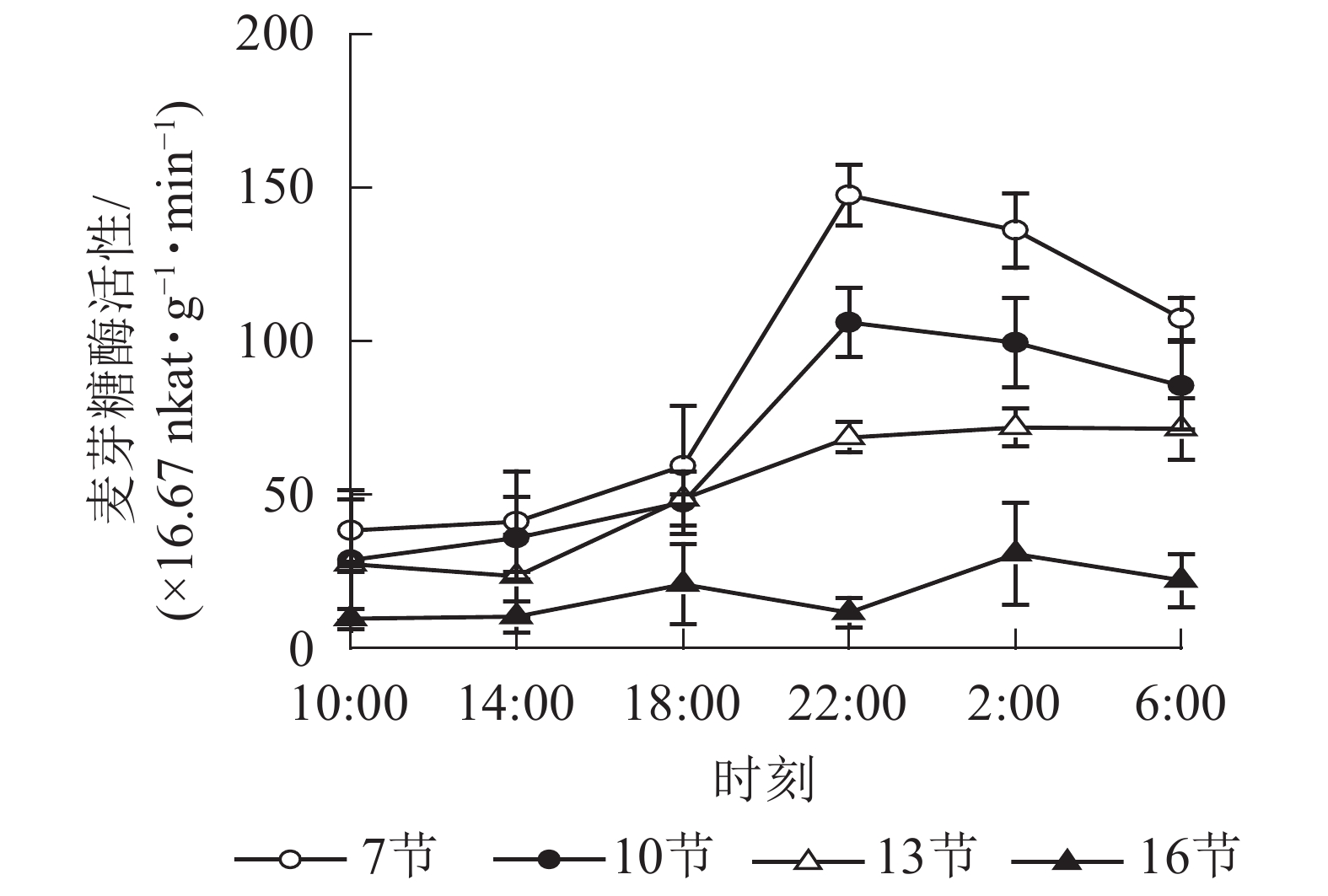
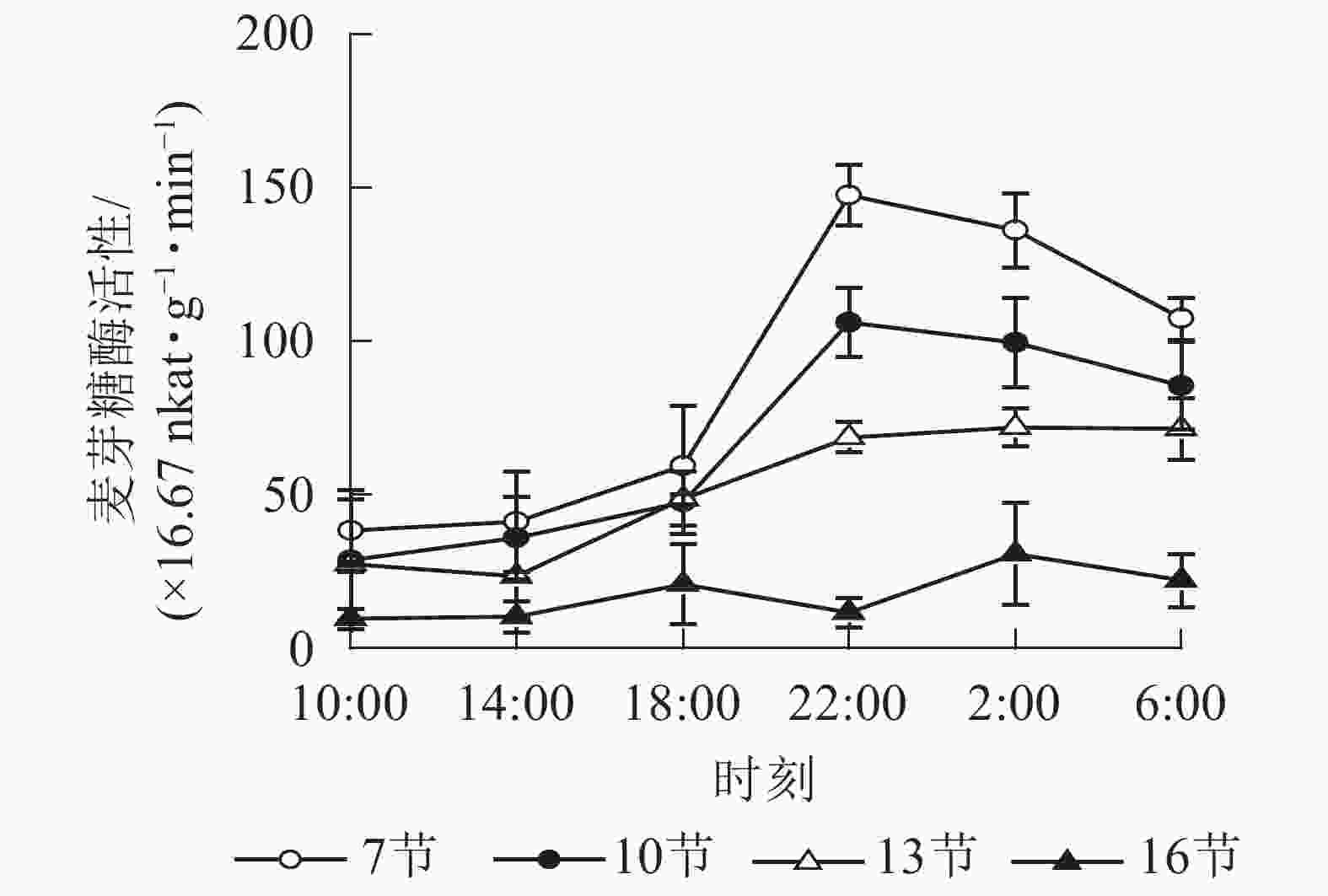
如图3所示:麦芽糖酶的活性在第7、10和13节较高,第16节活性最低且变化不显著。白天麦芽糖酶的活性较稳定,18:00后活性较高且在22:00达最高,第7、10和13节22:00分别比18:00高2.5,2.2和1.4倍(P<0.01),之后第7节和第10节极显著下降,6:00分别比22:00降低了27.2%(P<0.01)和19.4%(P<0.05)。
-
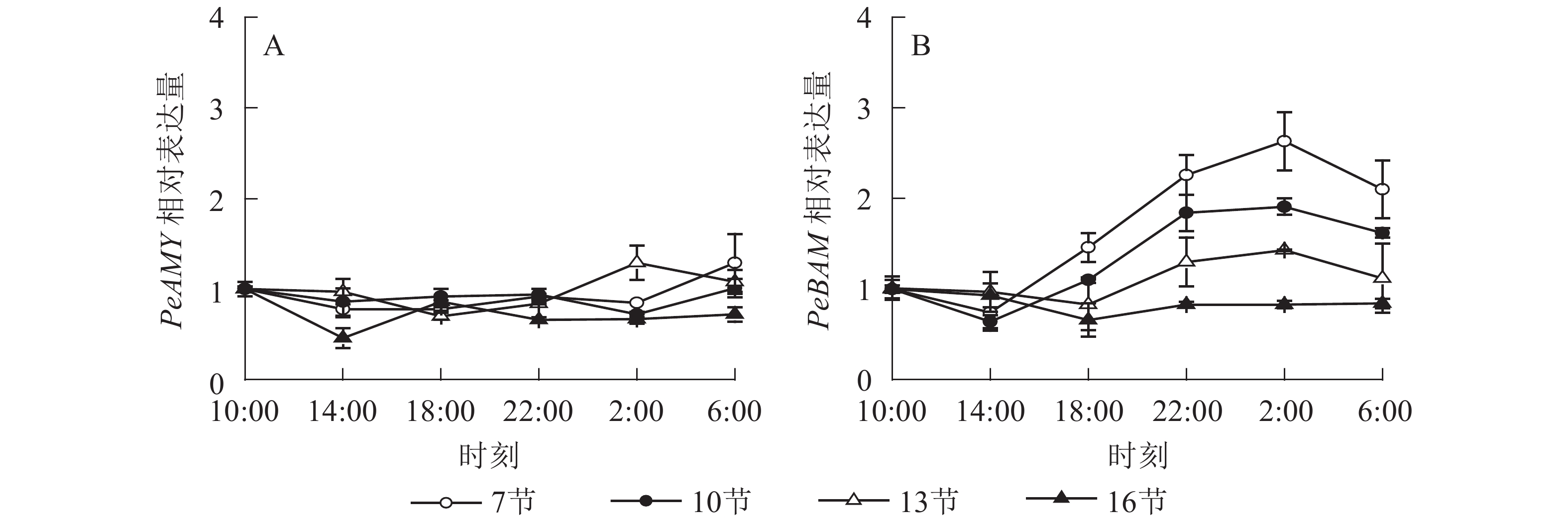
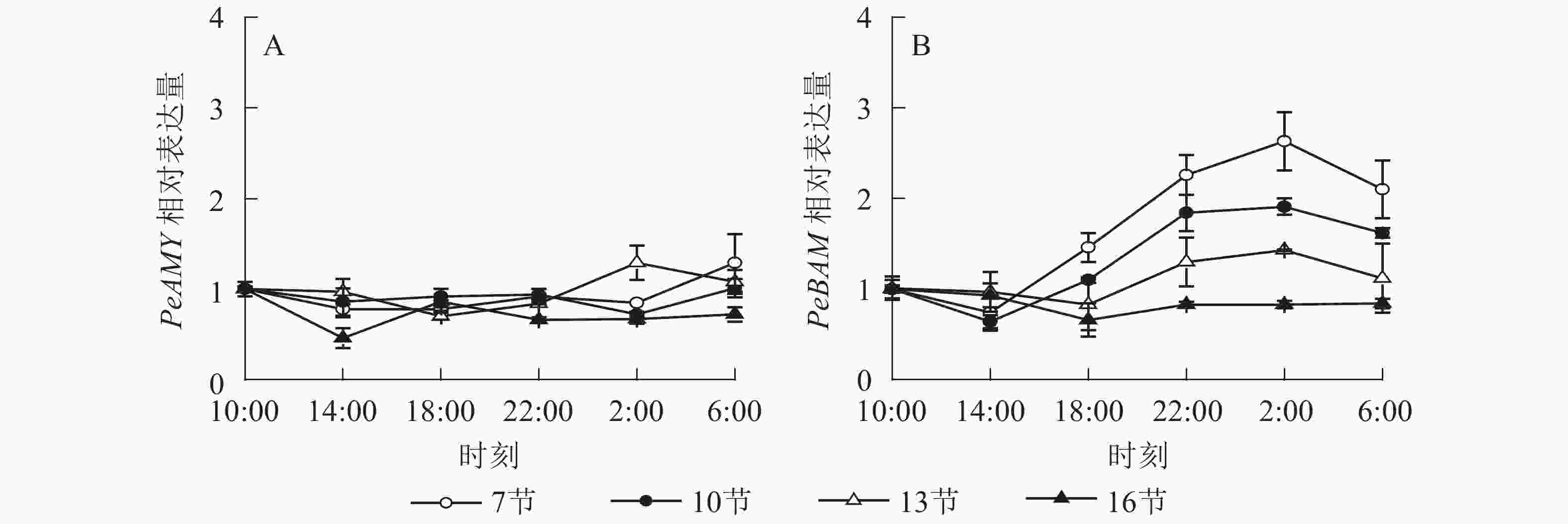
如图4A所示:PeAMY基因表达不显著,整体无明显变化规律。如图4B所示:PeBAM基因在第7节和第10节表达量较高,第13节表达量相对较低,第16节表达量最低且变化不显著。白天基因表达量低,18:00后表达量极显著上升,2:00达最高峰。第7、10和13节2:00分别比18:00高1.8、1.8和1.7倍(P<0.01),之后表达量下降,6:00分别比2:00降低了20.1%、15.2%和21.7%(P<0.05)。
2.1. NSC质量分数变化
2.2. 酶活性的变化
2.3. 基因表达模式
-
碳水化合物是参与植物代谢的主要能量物质,在调节植物生长发育中起关键性作用[24]。GRAF等[7]研究玫瑰Rosa rugosa花中碳水化合物昼夜节律控制发现:淀粉含量在光照下增加,黑暗中逐渐下降。IZUMI等[25]研究拟南芥叶片发现:白天积累的淀粉在夜间逐渐减少,夜间几乎完全消耗,蔗糖、葡萄糖和果糖在夜间整体水平较低。本研究表明:毛竹茎秆在白天具有较高的可溶性糖和淀粉质量分数,而在夜间则极显著下降。可能是因为白天生长慢,可溶性糖利用少,不断合成淀粉储存在茎秆中,夜间生长加快,可溶性糖消耗快,淀粉大量降解成可溶性糖为黑暗中继续生长提供能量[26]。推测毛竹快速生长时期糖和淀粉代谢可能受到光周期和生物钟调控[27]。LINGLE等[28]研究生长期甘蔗Saccharum officinarum茎秆发现:蔗糖和总糖质量分数由下往上逐渐降低。翟建云等[29]研究毛竹茎秆碳水化合物代谢发现:自下而上淀粉质量分数逐节上升,葡萄糖、果糖和蔗糖逐渐下降。本研究中,茎秆中下部节间可溶性糖质量分数高,淀粉质量分数低,上部节间NSC质量分数水平与中下部相反。可能是因为毛竹茎秆中下部生长速度快,淀粉大量转化成可溶性糖为快速生长提供碳源,茎秆上部生长较慢,可溶性糖需求少,淀粉降解慢[30],表明茎秆发育和成熟是从下往上顺次推进的[29]。
淀粉酶是淀粉水解反应过程中的一种重要催化剂,酶活性的高低影响淀粉的降解速率[31]。PONGRATZ等[32]研究菠菜Spinacia oleracea叶绿体发现:夜间淀粉酶活性比白天高。郑德森[33]研究甘蔗Saccharum officinarum叶片淀粉酶活性变化发现:BAM活性通常高于AMY。本研究中,茎秆中下部节间BAM活性比上部节间高。中下部生长速度快,淀粉在BAM催化作用下大量降解为麦芽糖,此时麦芽糖酶活性较高,加速麦芽糖分解成单糖;上部生长缓慢,淀粉利用较少,酶活性相应较低。以上研究结果表明:毛竹茎秆内AMY在淀粉降解中不起作用,BAM在毛竹茎秆内淀粉转化成可溶性糖的过程中起着关键性作用[13, 34]。
在茎秆生长的不同时间、不同节间,淀粉酶基因表达量存在较大差异。HARMER等[35]研究拟南芥叶片发现:BAM具有昼夜生理周期变化。刘美等[15]研究发现:小麦Triticum aestivum种子萌发时AMY基因相对表达量呈上升趋势,且与酶活性呈极显著相关。阳江华等[36]研究发现:白天橡胶树Hevea brasiliensis HbBAM1基因表达量显著高于夜间。本研究中,PeAMY基因表达变化不显著,PeBAM夜间表达量显著高于白天,说明PeBAM主要在夜间发挥其生物学功能。毛竹茎秆夜间生长速度快,此时非结构性碳水化合物含量低,PeBAM基因表达量高,PeBAM基因可能在夜间调控淀粉的降解。茎秆中下部节间PeBAM基因表达量比上部节间高,可能是因为茎秆中下部节间生长速度快,淀粉快速转化成可溶性糖为快速生长提供碳源,此时,PeBAM基因大量表达加速淀粉降解;上部生长速度慢,可溶性糖利用较少,淀粉降解较少,基因表达量低。与酶活性变化趋势相同,PeBAM基因表达与酶活性之间可能有密切联系。LLOYD等[16]研究小麦淀粉降解过程发现:AMY在胚乳中过表达,导致淀粉降解率升高,可溶性糖增加。MITA等[37]研究拟南芥莲座叶发现:随着可溶性糖含量升高,淀粉含量显著增加,BAM基因转录水平和其活性都显著提高。本研究中,PeBAM基因夜间表达量极显著高于白天,非结构性碳水化合物含量也有明显的昼夜变化规律,表明PeBAM的基因表达可能被糖信号所调控,从而在毛竹茎秆的快速生长中起重要作用[38]。
综上所述,毛竹在茎秆快速生长期将白天光合作用形成的可溶性糖转化成淀粉储藏起来,生长速度慢;夜间PeBAM基因表达增强使BAM活性上升,促进淀粉快速转化成可溶性糖提供碳源,生长速度快。茎秆发育和成熟是从下往上顺次推进的,NSC质量分数和PeBAM基因表达在茎秆上部节间变化不显著;中下部PeBAM基因表达达到最高,淀粉降解最快。表明毛竹茎秆快速生长与PeBAM基因的表达密切相关,BAM在毛竹茎秆淀粉降解中可能起主要作用。










 DownLoad:
DownLoad: