-
随着全球工业化的推进,重金属的排放量与日俱增,治理重金属污染成为世界环境难题之一。重金属污染物主要包括铜、汞、镉、铬、砷、镍、铅、锌等具有一定毒性的物质[1],重金属致癌、致畸且具有不可降解性、生物富集性,对人类及生物链上的其他生物造成不可逆转的损害[2]。铜的过量摄入会导致肠胃疾病、肾脏衰竭、毛细血管损伤、中枢神经系统损坏等严重疾病[3]。已有膜分离、化学沉淀法、离子交换树脂法、电化学处理法和吸附等方法应用于去除水中的铜离子(Cu2+)[4]。但高成本,低效率,低选择性和制备方法复杂等缺点限制了这些方法的应用[5]。生物质炭是生物质材料在低氧或无氧条件下热解所得的富碳产物[6],因具有多孔结构、比表面积大、含氧官能团丰富、吸附能力强等特点,在众多吸附材料中脱颖而出[7]。目前,用于制备生物质炭的原料很多,如木屑、稻草、竹子、稻壳、松木等[8-12]。生物质炭是用于吸附重金属离子的有效吸附剂[5, 13-14]。生物质炭的吸附性能很大程度上取决于其理化性质,如比表面积、孔隙结构和元素组成等,理化性质受升温速率、热解温度和热解时间影响,因此,选择合适的原料和热解条件对生物质炭的吸附能力具有重要影响。报纸是一种可回收、成本低廉的生物质资源。它通常由纤维素、半纤维素、木质素和填料(碳酸钙、高岭土等)组成。废报纸常被回收用于制作纸箱、肥料、天花板纸砖等[15],但是可回收再利用纤维含量低。由于废报纸是富含碳的材料,以废报纸为原料,通过热解制备生物质炭,是一种环保且成本低廉的废报纸回收再利用方法。本研究旨在探讨热解温度对废报纸生物质炭理化性质和吸附能力的影响,以期寻找高效吸附重金属离子的新型生物质材料。
HTML
-
以废报纸为原材料,清理干净后粉碎至80~100目备用。将废报纸置于真空管式炉(BTF-1200CⅡ,贝意克,中国)中,在氮气保护下,以5 ℃·min-1的升温速率分别升至400、500、600 ℃,保温1 h,再以5 ℃·min-1的降温速率降至室温。所制生物质炭标记为WBC-400、WBC-500、WBC-600,研磨过200目筛,烘干备用。
-
生物质炭灰分使用国家标准(GB/T 17664-1999)测定。碳、氢、氮用CHN元素分析仪(Vario EL Cube,Elementar,德国)测定,氧用差量法测定。矿物质元素和溶液中Cu2+采用原子吸收光谱法[16](TAS-990,Pgeneral,中国)测定。比表面积和比孔容积通过比表面积分析仪(ASAP20,Micromeritics,美国)测定,生物质炭比表面积根据Brunauer-Emmett-Teller(BET)法计算,孔径大小采用Barrett-Joyner-Halenda(BJH)方法计算。表面形貌特征和元素能谱采用场发射扫描电镜(SEM-EDS, S-4800, Hitachi,日本)进行观察分析。表面官能团变化通过傅里叶红外光谱仪(Nicolet 6670,Thermo Fisher,美国)进行定性分析。晶型结构使用XD-3型X射线衍射仪(XD-3,Pgeneral,中国)进行测定。吸附前后的价态分布和元素含量使用XPS(ESCALAB 250Xi,Thermo Fisher,美国)测定。pH用pH计(Rex PHS-25)测定(固液比为生物质炭:去离子水=1:20)。
-
将30 mg生物质炭和25 mL Cu2+(CuSO4·5H2O,0.01 mol·L-1 NaNO3做背景电解质)溶液混合于100 mL锥形瓶中,置于恒温水浴振荡器中,振荡条件为30 ℃、180 r·min-1。吸附完成后以4 000 r·min-1离心5 min,上清液过0.45 μm水系滤膜后测定Cu2+质量浓度。3次重复,取平均值。生物质炭Cu2+吸附量Qe=(C0-Ce)V/m。其中:Qe为Cu2+吸附量(mg·g-1);C0为Cu2+初始质量浓度(mg·L-1);Ce为Cu2+平衡质量浓度(mg·L-1);V为Cu2+溶液体积(L);m为吸附剂投加量(g)。
-
Cu2+溶液初始pH用0.1 mol·L-1 HCl和0.1 mol·L-1NaOH调节至2.0、3.0、4.0、5.0。称取30 mg生物质炭于100 mL锥形瓶中,加入25 mL不同初始pH的100 mg·L-1 Cu2+溶液,在30 ℃、180 r·min-1下水浴振荡6 h后,测定上清液Cu2+质量浓度,计算吸附量。
-
称取30 mg生物质炭于100 mL锥形瓶中,加入25 mL、pH为5.0的100 mg·L-1 Cu2+溶液。在30 ℃、180 r·min-1下水浴振荡,于15、30、45、60、90、120、150、180 min后测定Cu2+质量浓度,并计算吸附量。用伪一级动力学、伪二级动力学和颗粒内扩散方程模拟吸附动力学机制[17]。伪一级动力学模型的方程式为:Qt=Qe(1-e-k1t);伪二级动力学模型的方程式为:t/Qt=1/(K2 Qe2)+ t/Qe;颗粒内扩散模型的方程式为:Qt= Kidt1/2。其中:Qe为平衡吸附量(mg·g-1);Qt为t时刻吸附量(mg·g-1);t是吸附时间(min);K1是伪一级反应速率常数(min-1);K2是伪二级反应速率常数(g·mg-1·min-1);Kid是颗粒内扩散速率常数(mg·g-1·min-0.5)。
-
取30 mg生物质炭于100 mL锥形瓶中,加入25 mL、pH 5.0的不同质量浓度(50、100、150、200、250、300、350、400 mg·L-1)Cu2+溶液,30 ℃、180 r·min-1吸附24 h至吸附平衡后测定Cu2+质量浓度,并计算吸附量。采用Langmuir和Freundlich 2种经典模型来模拟吸附等温线。Langmuir和Freundlich等温吸附模型公式如下[14]:Ce/Qe=Ce/Qmax+1/(KLQmax);lgQe=lgKF+1/n(lg Ce)。其中:Ce是吸附平衡时Cu2+质量浓度(mg·L-1);Qe是吸附平衡时Cu2+吸附量(mg·g-1);Qmax是吸附平衡时最大吸附容量(mg·g-1);KL是Langmuir吸附常数(L·mg-1);KF是Freundlich吸附常数(mg·g-1);1/n是吸附强度。
-
3次重复,使用SPSS 24.0进行单因素方差分析(one way ANOVA)和最小显著差法(LSD)进行显著性检验(0.05),利用Origin 9.1制图。
1.1. 生物质炭的制备
1.2. 生物质炭的理化性质测定
1.3. 吸附试验方法
1.3.1. 生物质炭对Cu2+的吸附
1.3.2. 不同pH条件下生物质炭对Cu2+的吸附
1.3.3. 生物质炭对Cu2+的吸附动力学
1.3.4. 生物质炭对Cu2+的等温吸附
1.4. 数据统计
-
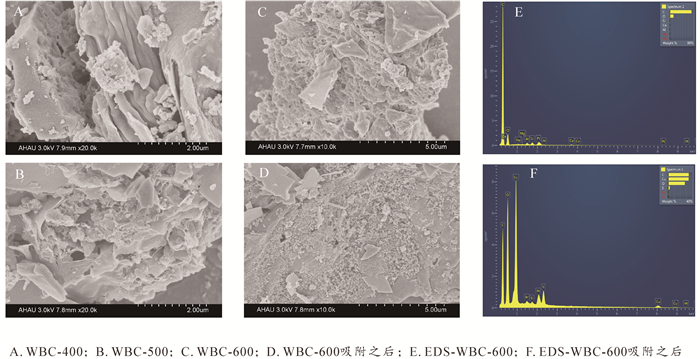
由图 1A~C可以看出:随着热解温度的增加,表面孔隙结构更加明显,孔径结构更发达。与吸附前相比,吸附后生物质炭表面存在大量的沉淀物,表明生物质炭吸附铜之后可能形成新的矿物质(图 1D)。从图 1E可以看出:生物质炭含有钙、镁、铝、硅、钠等矿物元素,这些矿物元素的存在可为Cu2+吸附提供大量的吸附位点,从而达到更高的吸附容量。图 1F可明显观察到Cu2+的存在,证明了Cu2+吸附到生物质炭上后,而其他矿物元素基本消失,可能由于钙、铝和镁等矿物元素与Cu2+发生了离子交换进入水体,表明生物质炭中的矿物元素参与了吸附。
-
由表 1可知:(N+O)/C、H/C和O/C也随着热解温度的增加而减少,说明随着热解温度的增加,生物质炭极性下降,芳香性增强,含氧官能团减少,表面亲水性降低[18]。生物质炭中钙质量分数远高于其他文献中生物质炭的钙质量分数[19-20]。
生物质炭 pH C/(mg·g-1) N/(mg·g-1) H/(mg·g-1) O/(mg·g-1) H/C O/C (N+O)/C Ca/(g-1) Mg /(g-1) Fe/(mg·g-1) Cu/(g-1) Mn/(g-1) Zn/(g-1) 灰分/(mg·g-1) SBET/(m2·g-1) WBC-600 10.01 ± 0.02 638.4 ± 1.7 1.8 ± 0.1 20.9 ± 0.1 87.7 ± 0.2 0.03 ± 0.06 0.14 ± 0.09 0.14 ± 0.12 30.05 ± 3.05 3.09 ± 1.22 2.29 ± 0.03 0.76 ± 0.44 0.61 ± 0.06 1.99 ± 0.20 251.1 ± 2.0 211.34 ± 27.66 WBC-500 9.66 ± 0.01 625.8 ± 0.8 2.3 ± 0.4 28.6 ± 0.1 114.0 ± 1.1 0.05 ± 0.01 0.18 ± 0.01 0.18 ± 0.07 22.46 ± 0.06 2.80 ± 0.07 2.28 ± 0.09 0.75 ± 0.13 0.60 ± 0.21 1.43 ± 0.02 229.3 ± 5.8 28.72 ± 12.35 WBC-400 8.30 ± 0.02 588.5 ± 0.3 2.4 ± 0.2 35.0 ± 0.2 166.3 ± 0.5 0.06 ± 0.01 0.28 ± 0.05 0.28 ± 0.05 17.63 ± 0.09 1.32 ± 0.93 2.75 ± 0.01 0.75 ± 0.03 0.59 ± 0.01 1.06 ± 0.06 207.9 ± 4.5 8.25 ± 2.67 说明:数据为平均值+标准差 Table 1. Physicochemieal characteristics of the three biochars
当热解温度从400 ℃升高到600 ℃时,生物质炭的pH值从8.30增加到10.01。在较高热解温度下得到的生物质炭具有较高的pH值[21],纤维素和半纤维素在200~300 ℃下分解产生有机酸和酚类化合物,使得pH值降低,随着热解温度的增加,碱金属盐从热解结构中释放,导致pH增加[22]。且灰分质量分数从20.78%增加到25.11%,这可能由于温度升高,有机物质量分数减少,致使灰分质量分数的相对值增加。
当热解温度从400 ℃增加到600 ℃时,生物质炭的比表面积从8.25 m2·g-1急剧增加到211.34 m2·g-1,表明随着热解温度的增加,材料的裂纹数量和孔隙率显著增加,与SEM中结果一致。因此,热解温度在孔形成过程起到重要作用。WBC-400~600的比孔容积分别为0.016、0.045、0.049 cm3·g-1,平均孔径大小分别为1.753、1.614、1.425 nm。热解温度为400 ℃时,纤维素和半纤维素逐渐热解,使得生物质炭形成一定的孔隙结构,热解温度增加到600 ℃时,木质素大量降解,导致生物质炭的孔径变小,比表面积急剧增加,这表明在较高温度下更容易形成微孔[23]。
-
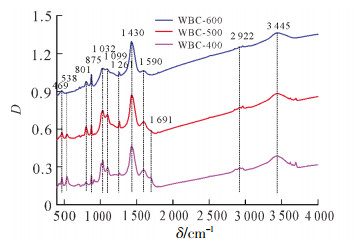
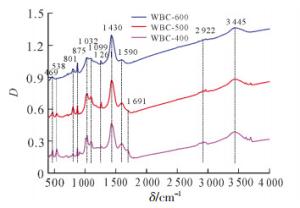
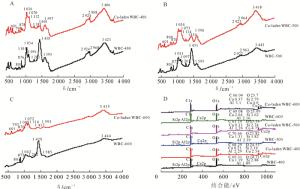
如图 2所示:3种生物质炭在3 445和1 430 cm-1处有相同的吸收峰,3 445 cm-1 归属于—OH的伸缩振动,1 430 cm-1属于CO32-吸收峰[24]。1 430 cm-1处吸收峰的强度随着热解温度的升高而增加,表明生物质炭中碳酸盐含量随着热解温度的增加而增加,这与元素分析中钙质量分数的变化结果一致。同时,1 691 cm-1处峰的吸收强度随热解温度升高越来越弱,且在600 ℃热解生物质炭中消失,这表明在较高热解温度下,C═O键容易被热解成气体或者液体副产物,导致羧基和酮类的C═O减少[25]。
1 590 cm-1处的吸收峰可归因于木质素中芳环的C═C键拉伸,或者羧基的C═O伸缩振动[4]。1 261 cm-1处的吸收峰属于酚—OH的伸缩振动[21]。当热解温度逐渐增加,脂肪族—CH2吸收峰(2 922 cm-1)逐渐消失,芳香族—CH(875,801 cm-1)的振动峰逐渐明显,这表明生物质炭中非极性脂肪族官能团减少,芳香结构增加,与元素分析中H/C变化一致[26]。在1 099 cm-1处存在纤维素物质的C—O—C不对称吸收峰[27],1 032 cm-1处存在含氧官能团的吸收峰[18],且随着热解温度的增加,吸收强度逐渐变弱,表面含氧官能团数量减少,这与元素分析中O/C的变化一致。此外,3种生物质炭在469 cm-1处存在强烈的Si—O—Si吸收峰[28],表明生物质炭中含有硅酸盐,与EDS谱图中硅(Si)的存在一致。
-
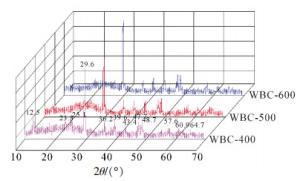
由图 3可以看出:3种生物质炭表现出相似的石墨结构和无机结构。在2θ = 29.6°处均存在强烈的结晶峰,归属于CaCO3的结晶峰[24],吸收峰的强度随着热解温度的升高而增加,与红外光谱中1 430 cm-1和元素分析的结果一致。此外,生物质炭中还出现一些结晶物质,如MgCO3、CaMg(CO3)2、NaAlSiO4、ZnO、SiO2、Al2O3等,进一步证实了废报纸基生物质炭材料中含有丰富的无机元素。
-
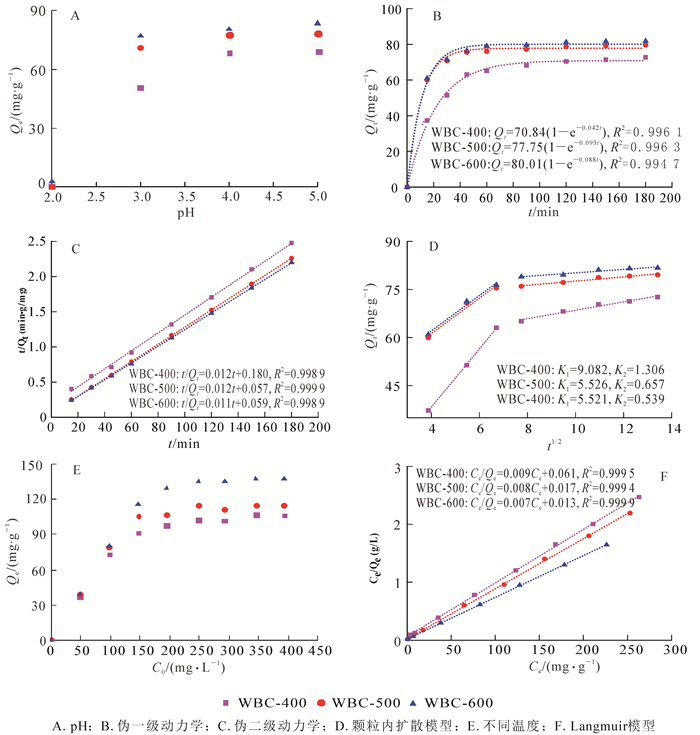
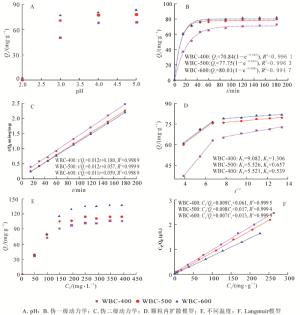
pH会影响吸附剂的表面电荷、金属离子的形态以及溶液中的电离程度[13]。在pH高于5.6时,会形成铜的氢氧化物沉淀。为了避免沉淀物的形成,在pH为2.0~5.0之间研究初始pH对Cu2+吸附的影响。由图 4A所示:pH从2.0增加到5.0时,3种生物质炭的吸附量迅速增加,且pH为5.0时吸附量达最大。pH为2.0时,生物质炭的吸附能力较差,这是因为pH较低时,溶液中大量的氢离子(H+)和Cu2+竞争生物质炭上的吸附位点。随着pH的增加,溶液中H+浓度降低,竞争吸附减弱,有利于生物质炭对Cu2+的吸附。不同pH条件下,3种热解温度生物质炭的吸附性能差异不显著(P>0.05)。所以,在以下吸附试验中,选取pH 5作为最佳pH值。
-
结果表明:3种生物质炭在前40 min的吸附速率较快,60 min内均达到吸附平衡,之后趋于平缓。在初始吸附阶段,这种快速吸附可能是因为生物质炭具有较高的比表面积和较多可用的吸附位点(丰富的含氧官能团和矿物元素),后期由于吸附位点饱和,吸附速率逐渐达到平衡。图 4C可知,伪二级动力学模型能较好的拟合整个吸附过程(R2分别为0.998 9、0.999 9、0.999 9),且拟合值和实测值相差不大。生物质炭吸附更符合伪二级动力学模型,说明Cu2+的吸附速率与炭表面的活性位点呈正比关系,控速步骤主要是生物质炭与Cu2+之间的化学吸附过程,且通过活性位点表面上的离子交换吸附[29]。不同时间条件下,WBC-500和WBC-600吸附性能差异不显著(P>0.05)。
图 4D可知:吸附可分为快吸附和慢吸附2个阶段,并且能很好地拟合颗粒内扩散模型(R2>0.99)。第1阶段可能是因为外部表面吸附,Cu2+通过水相扩散到生物质炭表面,吸附率很高。第2阶段是颗粒内扩散,即Cu2+扩散到生物质炭的孔中。拟合曲线没有穿过原点,说明颗粒内扩散不是唯一的速率控制步骤,内扩散和液膜扩散同时控制吸附速率,而且Ki1明显大于Ki2,说明液膜扩散是主要的控速步骤。WBC-400快吸附一级反应速率大于WBC-500和WBC-600,表明低热解温度制备的生物质炭的快吸附速率更大。这可能因为该生物质炭表面含氧官能团含量较多,能够提供更多的吸附位点,从而具有较大的K值。而随着热解温度的升高,生物质炭表面含氧官能团减少,一些矿物元素形成难溶的碳酸盐晶体,减缓了CO32-的释放速率,降低了与Cu2+的反应速度[26]。
-
由图 4E可知:随着Cu2+初始质量浓度的增加,吸附容量不断增加而后趋于平衡。Cu2+质量浓度在200 mg·L-1以下时,吸附容量随着Cu2+质量浓度的增加而增加。因为初始浓度越高,生物质炭与Cu2+表面的质量浓度差越大,为其克服溶液传质阻力提供了更高的驱动力。当Cu2+初始质量浓度高于200 mg·L-1时,生物质炭表面的有效活性位点达到饱和,Cu2+吸附量趋于平衡[30]。WBC-400具有较多的含氧官能团和更高的O/C比,但是对Cu2+的吸附能力却远远低于WBC-600,这表明含氧官能团(酚羟基和羧基)在吸附中所占的比例很小,而比表面积和灰分含量在吸附中起到决定性作用。不同初始质量浓度时,WBC-400和WBC-500之间,WBC-500和WBC-600之间的吸附能力差异不显著(P>0.05)。由图 4F可知:3种生物质炭的等温吸附模型都符合Langmuir模型(R2>0.99 9),且根据Langmuir模型计算所得吸附量与实测值相差不大,表明废报纸生物质炭对Cu2+的吸附是单分子层吸附。根据生物质炭的比表面积和元素分析结果表明:随着热解温度的增加,生物质炭的比表面积和矿物元素含量逐渐增加。吸附容量的变化也遵循类似的趋势,这表明比表面积和矿物元素含量是去除Cu2+的主要驱动力。
3种生物质炭WBC-600、WBC-500和WBC-400的最大吸附量分别为138、115和107 mg·g-1,远大于蚯蚓粪(24.27 mg·g-1)[5]、牛粪(44.5 mg·g-1)[13]、甘蔗渣(63.8 mg·g-1)[31]、玉米秸秆(12.5 mg·g-1)[32]等生物质炭的吸附量。
-
由图 5A~C可以看出:废报纸生物质炭吸附前后官能团发生明显的变化。3 444~3 423 cm-1归属于—OH的伸缩振动吸收峰,转移到3 418~3 406 cm-1附近。1 595~1 585 cm-1附近归属于—COOH和C═C的吸收峰转移到1 597~1 593 cm-1附近。这些吸收峰的变化表明羧基和酚羟基与Cu2+反应形成表面络合物,C═C与Cu2+之间存在π—π相互作用[5]。吸附Cu2+之后,生物质炭中属于碳酸钙的1 430 cm-1附近的强烈吸收峰减弱甚至消失,与XPS谱图中348 eV处Ca2p的峰变化一致[33],表明碳酸钙参与了吸附过程,可能是CO32-与Cu2+形成沉淀吸附。1 091 cm-1附近的吸收峰转移到1 112和1 070 cm-1附近,可能由于C—N还原Cu2+形成氰化物[13]。此外,吸附后的生物质炭在601 cm-1出现新的吸收峰,归属于Cu—O伸缩振动,这表明生物质炭表面形成了稳定的铜氧化物沉淀,吸附之后XPS中933 eV处出现Cu2p峰值,也证明了形成了稳定的Cu—O[13]。由XPS谱图(图 5D)可以看出,吸附后铜质量分数增加而Ca、Si质量分数减少,与EDS结果相一致,进一步证实生物质炭的沉淀和离子交换吸附机制。
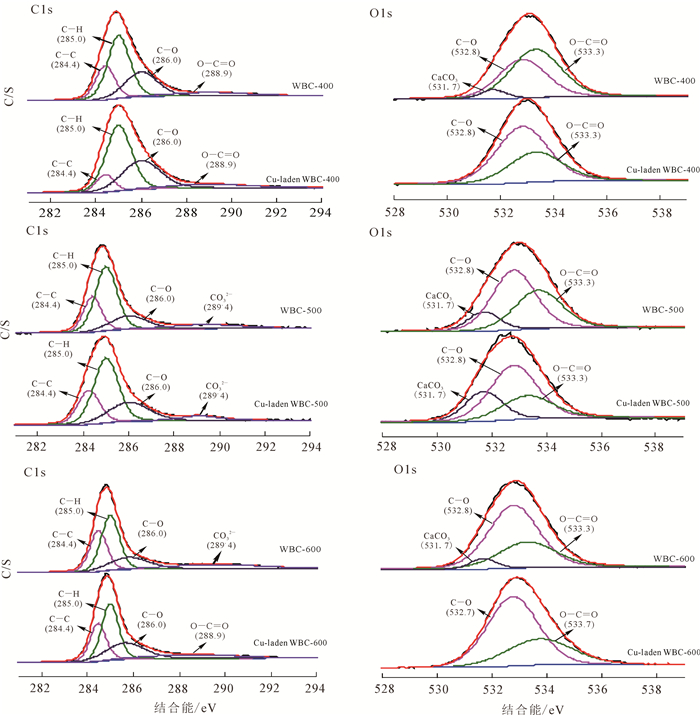
高分辨率XPS进一步证实了沉淀吸附机理(图 6)。C1s在(284.4 ± 0.1)eV处的峰归属于石墨碳,(285.0 ± 0.1)eV处是C—H键的峰,(286.0 ± 0.1)eV处是C—O键的峰,(288.9 ± 0.1)eV处是O—C═O键的峰,289.4 eV归属于CO32-的峰[34]。O1s区域在(532.8 ± 0.1)eV和(533.3 ± 0.1)eV分别为C—O和O—C═C键的峰[34]。观察WBC-600吸附前后XPS图谱可以发现:O1s区域在(531.7 ± 0.1)eV的CaCO3峰于吸附后消失,与前文元素分析和EDS结果一致,进一步证实CaCO3参与了Cu2+去除(沉淀吸附)。根据XPS图谱中元素所占的百分比可知:热解温度的增加只改变了表面碳和氧元素的质量分数,并未改变元素的种类。
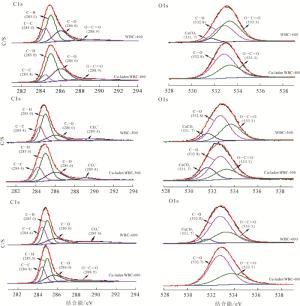
Figure 6. High-resolution XPS spectra of C1s, O1s in biochars (WBC) before and after Cu2+ adsorption
综上所述,生物质炭的吸附机制有络合反应(螯合和配位)、表面沉淀作用、离子交换作用、π—π作用和物理吸附等。络合作用主要为表面含氧官能团—OH、—COOH、—CO—、—O—等与Cu2+形成稳定性不同的金属-有机配合物。沉淀作用主要是Cu2+的氧化物沉淀,如Cu(OH)2、Cu(OH)、CuO等沉淀物。离子交换作用是生物质炭表面的质子与重金属离子发生离子交换反应,从而吸附Cu2+,可发生离子交换作用的质子主要为Ca2+、Mg2+、Si4+、Mn2+、Fe2+和Al3+等。物理吸附主要是生物质炭表面的孔径吸附。
2.1. 生物质炭表面形貌分析
2.2. 生物质炭的理化性质分析
2.3. 生物质炭红外光谱分析
2.4. 生物质炭晶体结构分析
2.5. 生物质炭对Cu2+吸附的研究
2.5.1. 不同初始pH对Cu2+吸附的影响
2.5.2. 吸附动力学
2.5.3. 吸附等温线
2.6. 吸附机制
-
本研究采用简单、低能耗和环保的方法(热解)制备废报纸生物质炭。并探讨了热解温度对生物质炭的理化性质和吸附性能的影响。600 ℃热解的生物质炭具有最大的比表面积和最多的矿物元素,且吸附性能最好,最大吸附量为138 mg·g-1。伪二级动力学模型和Langmuir等温吸附模型能较好地模拟生物质炭的吸附过程,表明废报纸生物质炭是单分子层吸附,吸附速率受化学吸附控制。该生物质炭的吸附机制有沉淀吸附、离子交换吸附、络合吸附和π—π键作用,多种吸附机制的融合为提高吸附量起到了互相促进的作用。所制备的生物质炭是具有一定应用前景的、环境友好的、高效的Cu2+吸附材料。










 DownLoad:
DownLoad: