-
十字花科Brassicaceae植物主产北温带,约375属3 200种。在中国主要集中于西南、西北、东北高山以及丘陵地区[1],其中萝卜属Raphanus与芸薹属Brassica植物是中国最重要的蔬菜与油料作物,该科部分种类还可作为药用、观赏用、染料用或食用[2]。植物表皮蜡质是覆盖在陆生植物地上部器官表面的脂质成分,也存在于木栓的基质、愈伤组织、花粉粒以及种皮中[3],其疏水结构在植物表面起极其重要的防卫功能,在植物与周围环境的相互作用中发挥着重要作用[4]。本研究从十字花科植物蜡质类型、结构、成分、含量、功能、遗传特性、合成与转运途径、分子机制等方面进行综述,为十字花科植物的蜡质代谢研究提供参考。
HTML
-
十字花科植物的蜡质呈片状、柱状和网状等26种形态类型,不同种类植物的蜡质形态不同[5]。徐秀萍等[6]采用扫描电镜(SEM)观察拟南芥Arabidopsis thaliana表皮蜡质,发现主要呈杆状,少量呈片状、管状、碟状和伞状。李红莲[7]发现:红菜薹Brassica campestris表皮蜡质为片状和网状构成的不规则三维结构。李帅等[8]发现:甘蓝型油菜‘中双11’Brassica napus‘Zhongshuang 11’叶表皮蜡质结构主要为杆状和颗粒状(小片状)。牟香丽等[9]发现:不同生长时期甘蓝Brassica oleracea var. capitata表皮蜡质呈现不同结构,苗期大多为颗粒状、片状和针状,结球期则较多为圆柱状和片状,成熟期以片状和针状为主。张曦[10]发现白菜Brassica pekinensis成熟叶片蜡质大多呈现出棒状且前端有圆形突起。
-
十字花科植物蜡质主要为超长链脂肪酸及其衍生物,包括烷烃、脂肪酸、醇、醛、酮的同系物,偶尔会出现环状化合物,如甾醇或三萜类化合物等[11]。蜡质通常使用三氯甲烷、正己烷等有机溶剂提取,采用气相色谱-质谱联用(GC-MS)技术鉴定成分[12]。植物表皮蜡质各成分占比不同。如白菜[10]成熟叶片蜡质的主要成分中酮类占1.02%,醇类占8.33%,烷烃占55.09%,酯类占26.34%。不同种类植物的表皮蜡质成分含量也存在差异[13]。如甘蓝型油菜‘中双11’叶[8]表皮蜡质成分中烷烃为8.24 μg·cm−2,次级醇为1.72 μg·cm−2,酮为1.62 μg·cm−2,初级醇为0.57 μg·cm−2,脂肪酸为0.10 μg·cm−2,醛为0.79 μg·cm−2,未知成分为9.33 μg·cm−2,总量为22.37 μg·cm−2;拟南芥[14]茎秆的表皮蜡质中初级醇为0.58 μg·cm−2,脂肪酸为0.10 μg·cm−2,醛为1.90 μg·cm−2,烷烃为13.13 μg·cm−2,次级醇为3.83 μg·cm−2,酮为5.95 μg·cm−2,总含量为28.99 μg·cm−2。而普通白菜自交不亲和系13S106[15]叶片的蜡质成分中烷烃为13.60 μg·cm−2,醛为0.90 μg·cm−2,醇为3.20 μg·cm−2,酮为8.30 μg·cm−2,脂肪酸为1.20 μg·cm−2,蜡酯为2.40 μg·cm−2。在上述不同十字花科物种中,蜡质主要成分均为烷烃,次要成分则有所不同。
-
十字花科植物表皮蜡质在维持水分平衡、反射紫外线、减少外来机械损伤、降低低温伤害、抵御细菌真菌入侵、防止昆虫侵食等抵抗生物与非生物胁迫中起着重要作用[16],同时兼具影响叶片和果实着色、防止果实开裂和植株育性等生理功能[17]。
-
低温胁迫易引起植物酶活性降低、细胞膜结构改变、细胞失水、代谢紊乱等,对植物生长发育造成多种负面影响[18]。倪郁等[19]发现:4 ℃低温胁迫下拟南芥生长发育缓慢、叶色变深,蜡质晶体的分布密度、大小、形态等发生改变。唐帅等[20]发现:4 ℃低温胁迫下拟南芥叶片表皮蜡质成分增加,烷烃、脂肪酸、醛、初级醇和酮相对含量分别增加54.34%、29.61%、54.40%、24.07%和137.80%;荧光定量聚合酶链式反应(PCR)检测显示蜡质相关基因的表达水平显著提高,说明拟南芥通过提高蜡质含量来缓解低温胁迫,预防低温对植物内部组织的伤害。
-
紫外线中UV-B波长为280~315 nm,可对植物表面造成损伤。PRUDNIKOVA等[21]发现:过量UV-B处理会造成植株干质量降低,叶面积减小,净光合速率下降及花期变短等。宋超[14]发现:UV-B胁迫下拟南芥蜡质相对含量明显增加,蜡质相关基因CER3、CER4、KCS1表达量显著提高,其中CER4基因的相对表达增加了13.80倍,CER1和WIN1表达量降低。此外,UV-B胁迫下拟南芥蜡质晶体结构发生熔融,晶体由杆状变成片状,蜡质覆盖面积增加,蒸腾作用减少,从而达到反射更多紫外线的效果。
-
植物蜡质作为疏水屏障,在限制非气孔水分散失中扮演重要角色[22-23]。柴凌燕[24]发现:拟南芥过量表达蜡质相关转录因子WIN1可提高蜡质合成量,调节表皮渗透性,增强植株耐旱性。LÜ等[25]发现:拟南芥CER9编码一种与植物抗旱性相关的决定因子,该因子缺失可以增加蜡质合成,阻塞更多的气孔,抑制蒸腾作用。周燕等[26]发现:甘蓝型油菜中BnWIN2C01的特异性表达影响了叶片蜡质合成,从而影响植株水分平衡。
-
花粉发育异常会导致植株减产或杂交不育,蜡质是花粉表面含油层的重要成分,在植物生殖发育方面发挥重要作用。KOCH等[27]发现:蜡质不仅影响植物叶片和果实的形态、发育,还影响植株花粉的发育情况。刘艳艳等[28]发现:FAX1基因缺失会抑制拟南芥营养生长,造成植株矮小、茎纤细、花粉稀少、角果短小等,同时还影响花粉壁的发育与花粉的育性,进而影响授粉过程。徐法青[29]发现:拟南芥CER3基因参与花粉脂质的合成或转运,该脂质的缺失会影响花粉与柱头的识别,阻断水合作用,最终导致雄性不育。
-
蜡质可以减少叶片表面水分,减少病菌停留和降低病菌入侵。JU等[30]发现:蜡质的晶体结构可促使水分形成水滴以便滑落,并带走叶片表面的灰尘、污染物和病菌等。SURVILA等[31]发现:相较于正常植株,拟南芥蜡质缺失植株表面细菌更多,更易发生病害。蜡质在植物抵抗虫害方面也起着重要作用,它可通过光的反射,改变植物表现出的颜色,影响昆虫视觉,减少昆虫取食和产卵。BOHINC等[32]以8种基因型甘蓝为对象进行田间试验,发现蜡质对甘蓝跳甲Phyllotreta spp.和菜椿Eurydema spp.的生存有抑制作用,且蜡质含量越高,植株上甘蓝跳甲和菜椿越少。
1.1. 十字花科植物蜡质的形态和成分
1.1.1. 蜡质的形态结构
1.1.2. 蜡质的化学成分
1.2. 十字花科植物蜡质的功能
1.2.1. 抗低温胁迫
1.2.2. 抗紫外线胁迫
1.2.3. 抗干旱胁迫
1.2.4. 抗败育
1.2.5. 抗病虫害
-
十字花科植物蜡质缺失表型明显,表面无蜡质覆盖的植株呈现叶色亮绿等性状,包括单基因隐性遗传、单基因显性遗传和双基因隐性遗传等3种遗传特性。
-
ANSTEY等[33]在青花菜Brassica oleracea var. italica中发现了十字花科植物中第1个符合单基因隐性遗传规律的蜡质缺失突变体。刘泽洲等[34]发现:甘蓝蜡粉缺失突变体10Q-961符合单基因隐性遗传规律。李红莲等[7]对无蜡质红菜薹与有蜡质红菜薹杂交建立6世代群体并进行研究,发现蜡质缺失突变体符合隐性遗传规律。王灿洁等[15]发现红菜薹自交系13S106蜡质缺失性状受隐性单基因控制。
-
蒲媛媛等[35]发现:甘蓝型油菜光叶突变体GL的蜡质缺失性状受单个显性基因控制。刘东明[36]发现:甘蓝10Q-974亮绿性状符合单基因显性遗传规律,突变基因 BoGL1位于8号染色体177 kb的区间内,与基因Bol018504的表达相关。
-
周熙荣等[37]发现:甘蓝型油菜杂交F2中出现少数蜡质缺失的植株,其无蜡质性状由2对隐性基因控制。莫鉴国等[38]对加拿大引进的无蜡质甘蓝型油菜种质材料‘Nilla’进行研究,发现其蜡质缺失性状也是受2对隐性基因控制。
2.1. 单基因隐性遗传
2.2. 单基因显性遗传
2.3. 双基因隐性遗传
-
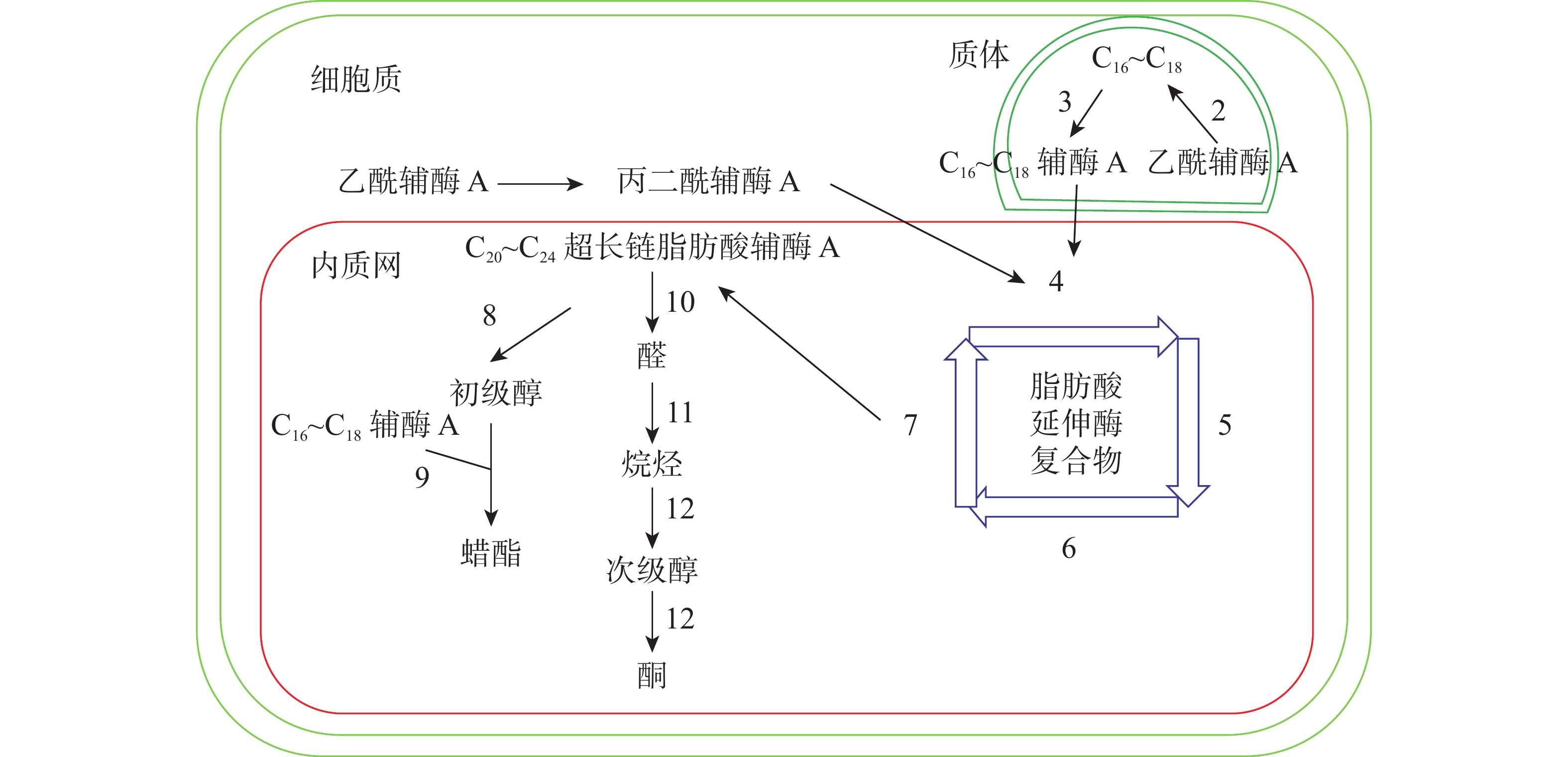
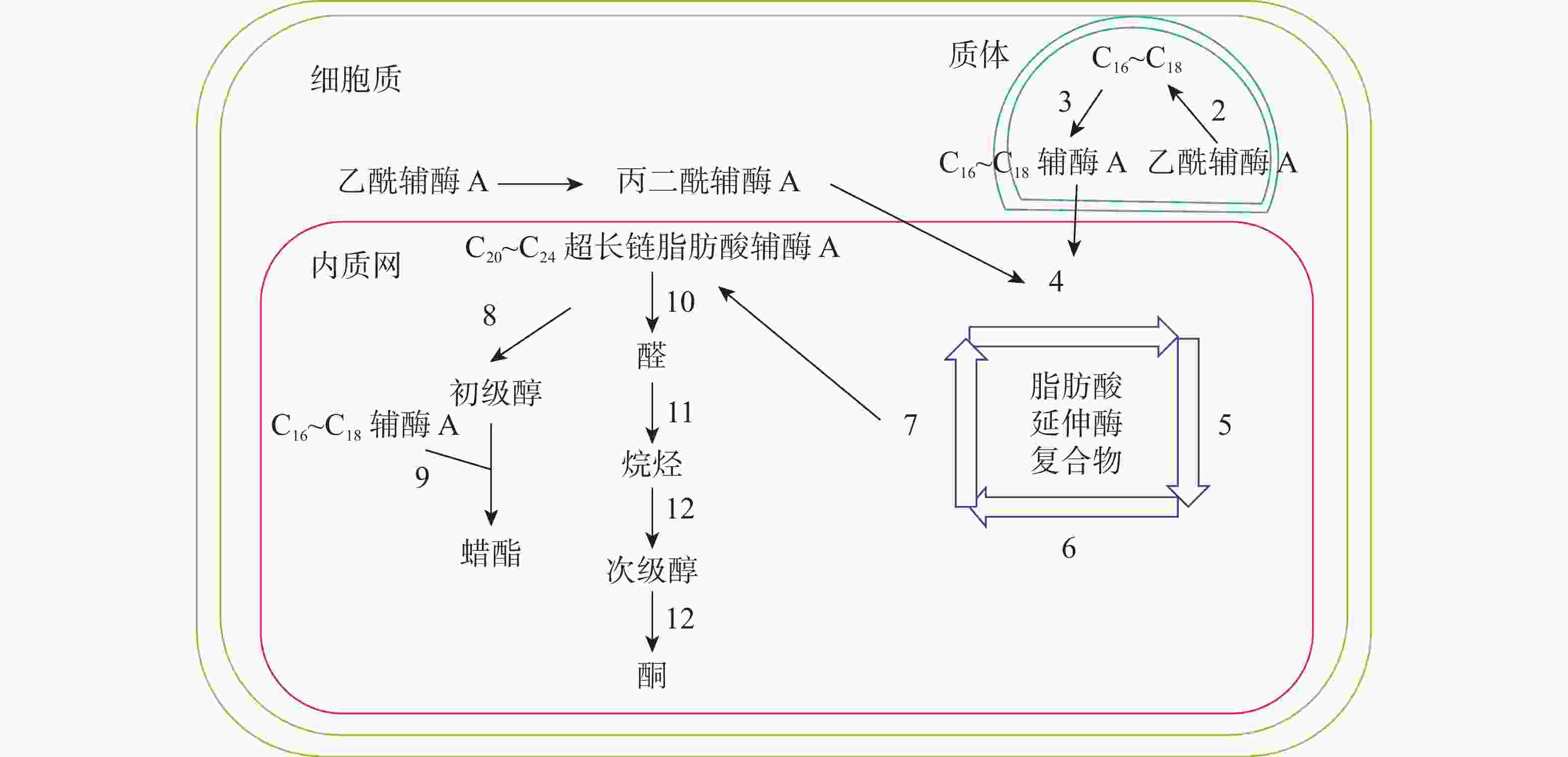
十字花科植物蜡质合成C16~C18脂肪酸合成、C20~C34超长链脂肪酸合成、超长链脂肪酸衍生物合成等3个途径(图1)。合成相关酶如表1所示。
序号 酶 缩略符 基因 参考文献 1 乙酰辅酶A羧化酶 ACC ACC1,ACC2 [39] 2 酰基载体蛋白硫解酶 FAT FATA,FATB [40] 3 长链酰基辅酶A合成酶 LACS LACS1,LACS2,LACS4 [41-44] 4 β-酮酰辅酶A合成酶 KCS FAE1,CER6,KCS1,FDH [45-46] 5 β-酮酰辅酶A还原酶 KCR KCR2 [11] 6 β-羟酰-酰基辅酶A脱水酶 HCD PAS1,PAS2 [47-48] 7 反式烯酰辅酶A还原酶 ECR CER10 [49] 8 脂肪酰辅酶A还原酶 FAR CER4 [50] 9 蜡酯合成酶 WS WSD1 [51] 10 脂肪酰辅酶A还原酶 FAR CER3 [52] 11 醛脱羰酶 AD CER1 [53-54] 12 中链烷烃羟化酶 MAH MAH1 [55] Table 1. Enzymes involved in wax biosynthesis in Arabidopsis thaliana
-
C16~C18脂肪酸在质体中合成,初始反应物乙酰辅酶A在乙酰辅酶A羧化酶(ACC)和脂肪酸合成酶的作用下,以每次增加2个碳原子的方式延长碳链,形成C16~C18的酰基载体蛋白[39],然后在酰基载体蛋白硫酯酶(FAT)作用下水解生成C16~C18脂肪酸[40]。脂肪酸经长链酰基碳烯A合成酶(LACS)催化后以脂肪酰辅酶A的形式进入内质网中[41],进行下一步反应。ZHAO等[42]发现:拟南芥的9个LACS基因中,LACS1和LACS2参与蜡质的合成。LÜ等[43]和JESSEN等[44]进一步发现:LACS1和LACS4参与花粉外被长链脂肪酸的合成。
-
C20~C34超长链脂肪酸合成场所是内质网。C16~C18脂肪酰辅酶A与丙二酰辅酶A通过脂肪酸延伸酶复合物(FAE)进行合成,每次循环增加2个碳原子,多次循环延伸碳链,最终形成C20~C34超长链脂肪酸,其中丙二酰辅酶A由乙酰辅酶A于细胞质中经过乙酰辅酶A羧化酶催化形成。该反应中的FAE属于多酶复合体,包括β-酮酰辅酶A合成酶(KCS),β-酮酰辅酶A还原酶(KCR),反式烯酰辅酶A还原酶(ECR)和β-羟酰-酰基辅酶A脱水酶(HCD)4种酶,其中KCS是该反应的关键酶,对反应底物具有特异性。QUIST等[45]发现:拟南芥KCS基因分为FAE1类和ELO类,前者包含FAE1、CER6、KCS1和FDH等4个亚组,而ELO类基因功能还未见报道。SUH等[46]发现:拟南芥与蜡质相关的基因有KCS1、KCS2、KCS13、KCS10、KCS20和CER6等;拟南芥中的KCR基因有KCR1和KCR2等2种,KCR1没有功能,KCR2参与超长链脂肪酸的合成。ZHAO等[47]发现:相比野生型,cer10突变体器官小,蜡质少;CER10基因在表皮和种子中有ECR功能活性,参与超长链脂肪酸合成。目前对HCD的研究较少,BACH等[48]发现:pas2-1突变体的蜡质含量明显少于野生型,PAS2基因功能完全丧失会最终导致胚死亡,推测PAS2在超长链脂肪酸合成和生物发育中起到非常关键的作用。ROUDIER等[49]发现:内质网中的PAS1和PAS2,KCR和ECR存在蛋白互作,并认为PAS1在多酶复合体中扮演分子构架的角色。
-
同位素示踪和气相色谱质谱技术已经验证了超长链脂肪酸通过酰基还原途径和脱羰基途径衍生出其他蜡质成分。酰基还原途径也叫醇合成途径,超长链脂肪酰辅酶A经脂肪酰辅酶A还原酶(FAR)还原产生初级醇,初级醇与C16~C18脂肪酸辅酶A经蜡酯合成酶(WS)缩合产生蜡酯。拟南芥通过酰基还原途径产生的蜡质相对含量约为20%。 CER4基因编码的酰基辅酶A还原酶在该途径中起到关键作用,主要将拟南芥表皮和根部脂肪酸还原成初级醇。ROWLAND等[50]发现:拟南芥cer4突变体茎中醇与蜡酯含量显著降低。LI等[51]发现:拟南芥wsd1突变体蜡酯含量明显少于野生型。脱羰基途径也叫烷烃合成途径,超长链脂肪酰辅酶A经脂肪酰辅酶A还原酶(FAR)还原产生的醛经醛脱羰酶脱羰产生烷烃,经中链烷烃羟化酶(MAH)1次羟化产生次级醇,再次羟化生成酮。拟南芥约80%的蜡质组分由该途径产生。BERNARD等[52]发现:拟南芥cer3突变体中醛含量减少,说明CER3基因在产生醛的过程起着重要作用。OSHIMA等[53]和刘秀林[54]发现:拟南芥cer1突变体茎表皮蜡质组分中烷烃含量减少,而醛含量增加,说明CER1编码的酶参与烷烃产生。GREER等[55]发现:MAH1是烷烃羟化酶,拟南芥mah1突变体中次级醇和酮的含量显著减少。
-
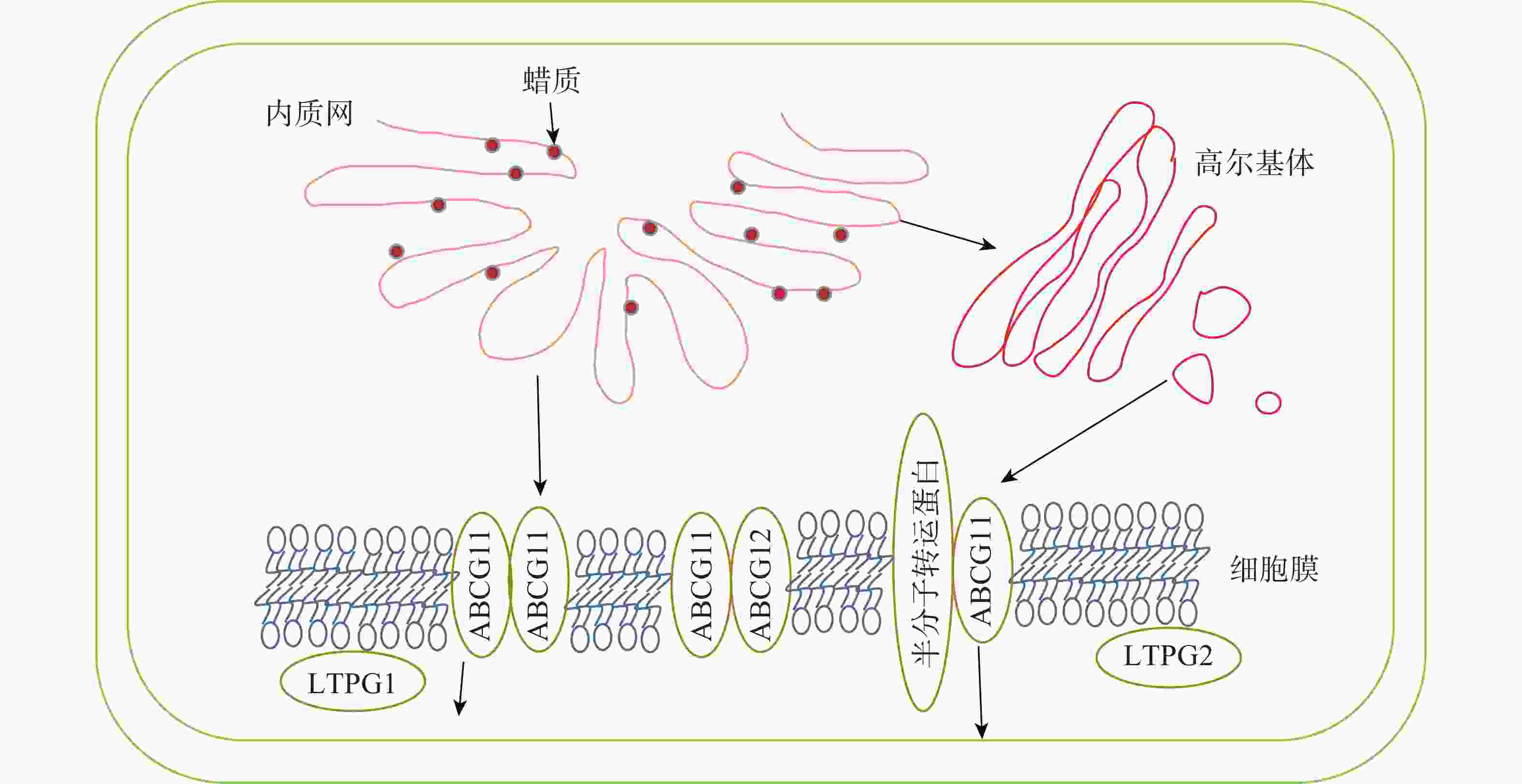
内质网上经过各种酶加工修饰合成的蜡质成分会先转运到细胞膜,再通过转运蛋白进行跨膜运输,最后经脂质转移蛋白跨细胞壁转运到角质层,转运途径及转运蛋白见图2和表2。
-
目前对蜡质从内质网转运到质膜有2种推测:①蜡质通过内质网与质膜内侧接触的部分直接进行运输;②蜡质先进入内质网分泌的囊泡,再经高尔基体转运到细胞膜内侧[56]。
-
蜡质到达质膜后利用相关转运蛋白进行跨膜运输。ABCG11和ABCG12是拟南芥中2个蜡质转运相关的半分子转运蛋白,LUO等[57]发现:ABCG11通过与另1个ABCG11结合形成同源二聚体或是与其他半分子转运蛋白结合形成异源二聚体来转运蜡质分子。BIRD等[58]发现:abcg11突变体生长速度减缓,表皮蜡质含量减少。QUILICHINI等[59]发现:ABCG12基因编码定位在质膜上的ABC转运蛋白,ABCG12基因的缺失导致拟南芥表皮部位的蜡质显著减少,而细胞内蜡质总含量并没有显著变化,说明该基因缺失只影响了质膜中的蜡质转运过程,而细胞内蜡质合成并没有受阻。
-
到达细胞膜外的蜡质由脂质转移蛋白(LTPs)转运到角质层。LTPG1是一种脂质转移蛋白,包含8个保守的半胱氨酸,形成疏水囊泡。DeBONO等[60]发现:ltpg1突变体的茎和角果表皮蜡质C29烷烃含量减少,但其他蜡质成分不存在显著差异,说明突变体缺失的蛋白可能对C29烷烃转移具有专一性。孙伟[61]发现:Th-nsLTP是一个非特异性脂转移蛋白,通过参与小盐芥表皮蜡质转移过程,使表皮蜡质含量减少,晶体结构从杆状转为柱状。
3.1. 蜡质合成途径
3.1.1. C16~C18脂肪酸合成
3.1.2. C20~C34超长链脂肪酸合成
3.1.3. 超长链脂肪酸衍生物合成
3.2. 蜡质转运途径
3.2.1. 细胞内的蜡质转运
3.2.2. 蜡质的跨膜运输
3.2.3. 蜡质转移到角质层
-
十字花科植物蜡质形成分子机制极其复杂,今后可从以下几个方面深入探究。①当前对蜡质形态结构与成分含量的研究都是独立的,若能寻找不同蜡质成分形成晶体过程中的空间折叠规律,将有助于探明蜡质成分与蜡质结构复杂多样性背后的具体对应关系。②目前在蜡质合成与转运方面研究较多,但在外界环境条件如低温、干旱或光照强度对蜡质合成影响方面的研究较少,需要更多的相关研究阐明环境影响蜡质合成的机制。③培育蜡质过量的新品种对于研究蜡质形成分子机制及抗病育种都具有重要意义。









 DownLoad:
DownLoad:
