-
换锦花Lycoris sprengeri为石蒜科Amaryllidaceae石蒜属Lycoris球根植物,多为红色与蓝紫色的复色,具有较高的观赏价值,常用作园林造景花卉。植物花色呈色的主要成分由花色苷、类胡萝卜素和甜菜色素组成,其中花色苷是最重要的一种类黄酮化合物[1]。花色苷以2-苯基苯并吡喃型阳离子为苷元,沿类黄酮修饰途径衍生出3种类型,即天竺葵素(橙色)、矢车菊素(红色)和飞燕草素(蓝紫色),其他花色苷均由此3种类型衍生而来[2-3]。不同种类与数量的花色苷在细胞液泡中的积累与储藏使植物组织表现出不同的颜色,而哪种色素含量越高,花瓣的颜色越偏向该色素,其他色素则起辅色作用[4]。研究发现可采用二极管阵列-高效液相色谱-电喷雾离子阱飞行时间串联多级质谱仪(HPLC-DAD-ESI/IT-TOF/MSn)、液质联用仪(HPLC-MS)和高效液相色谱仪(HPLC-DAD)等能够准确测定植物花色苷的主要成分,加快植物花色苷成分对花色呈色的研究。韩雪等[5]利用(HPLC-DAD-ESI/IT-TOF/MSn)从6种不同蓝莓Vaccinium品种鉴定出13种花青素类化合物。刘雨佳等[6]应用HPLC-MS和HPLC-DAD鉴定出矢车菊素-3-葡萄糖苷、天竺葵素-3-葡萄糖苷、天竺葵素-3-芸香糖苷和天竺葵素-3-丙二酰葡萄糖苷为“法兰地”草莓Fragaria ananassa ‘Falandi’果实中主要花色苷成分,并认为温度、光照和pH值均会对草莓果实花色素苷有一定的影响。HU等[7]比较超声处理和常规溶剂萃取处理蓝莓果渣中主要花色苷为锦葵色素-3-半乳糖苷。近年来对铁皮石斛Dendrobium officinale[7-8]、风信子Hyacinthus orientalis[4]、红色菊苣Cichorium intybus[8-9]、蓝莓[9-11]、玫瑰Rosa rugosa[12]等观赏或药用植物的研究发现:花色苷具不稳定性,花朵颜色除了受内部组成色素的影响外,可能还受到光、热、pH及金属离子等理化因子的影响[13-14]。换锦花花色丰富,变化多样,但鲜有对换锦花花色呈色的花色苷成分和花色变化的影响因素的研究报道。为了进一步探索换锦花花色苷主要成分及花色变化的影响因素,本研究利用紫外分光光度法和HPLC-DAD技术分析换锦花花色苷的主要成分,并分析温度、光照、pH和金属离子等理化因素对换锦花花色苷稳定性和主要成分的影响,旨在为研究换锦花花色变化的机制和换锦花花色品种选育奠定基础。
HTML
-
换锦花种植于浙江农林大学遗传学科石蒜属植物种质资源圃,于2018年8月中下旬采集换锦花盛花期花瓣。
-
将换锦花花瓣于避光冻干7 d后,研磨成粉末,干燥器避光保存备用。精密称量换锦花花瓣干粉末0.040 0 g,加入2 mL体积分数为1%甲醇-盐酸提取液摇匀,密塞,20 ℃超声波提取30 min,12 000 r·min−1离心15 min,收集上清液,0.45 μm微孔滤膜过滤,滤液于−20 ℃冰箱保存备用,重复3次。
-
HPLC分析:使用Agilent1 200高效液相色谱系统,色谱柱Agilent Eclipse-plus C18柱(150 mm×3.0 mm,1.8 μm),柱温40 ℃。流动相(体积分数)由A(0.1%甲酸水溶液)和B(100%乙腈)组成,流动相洗脱梯度:0 min,98.0% A;1.0 min,98% A;5.0 min,60% A;12.0 min,30% A;15.0 min,5% A;20.0 min,5% A。流动相流速为0.40 mL·min−1,样品上样量为1.00 μL。在520 nm处检测换锦花花瓣总花色苷质量分数,并以标准品半定量法进行定量。质谱检测:使用安捷伦6545 Q-TOF质谱检测器全扫描模式记录质荷比为50~1 000的数据,扫描谱图速度为2张·s−1。电喷雾(ESI)离子源使用正极和负极2种离子化模式,毛细管电压为3.5 kV,喷头电压正离子模式时为500 V,负离子模式时为1 500 V,碰撞电压110 V,喷雾器压强310 kPa,鞘气流速为8 L·min−1。采集二级质谱时的碰撞电压(CID)分别为10、20和40 V。
-
利用紫外分光光度计对换锦花花色苷粗提液在波长为400~600 nm范围内扫描,确定花色苷的最大吸收波长。采用pH示差法测定换锦花提取液中的花色苷含量[15]。分别用pH 1.0的盐酸-氯化钠缓冲液和pH 4.5的乙酸-乙酸钠缓冲液将换锦花提取液稀释2倍,在510和700 nm处测定吸光度[D(510)和D(700)],根据公式D=[D(510)−D(700)]pH 1.0−[D(510)−D(700)]pH 4.5,C=(A×M×DF×Ve)/(ε×0.576 793)和花色苷主要成分质量分数(mg·g−1)=C×Ve×n×M/(ε×m)分别计算吸光度D、提取液中总花色苷质量分数C[用矢车菊素-3-葡萄糖苷(CGE)质量分数表示(mg·g−1)]和花色苷主要成分质量分数。其中:M为矢车菊素-3-葡萄糖苷的相对分子质量(449.2 mg·mol−1);DF为换锦花花瓣提取液稀释倍数;Ve为换锦花提取液总体积(L);ε为摩尔吸光系数(29 600 L·mol−1);0.576 793为比色皿光路直径(cm);m为样品质量(g)。
-
采用Eclipse-plus-C18 column (4.6 mm×250.0 mm,5 µm),流动相为甲醇(A)-1%甲酸水(B),梯度洗脱:0~20 min(A:5%~60%),20~25 min(A:60%~100%),25~30 min(A:100%~100%),流速1 mL·min−1,检测波长280 nm,柱温30 ℃,进样量10 µL。以质量浓度为0.1 g·L−1的矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷标准液做为标准品。
-
将换锦花花色苷提取液配成适当比例的溶液,通过紫外分光光度法分别检测温度和光照对换锦花花色苷溶液稳定性的影响。测定配好的换锦花花色苷提取液在各自检测波长下的吸光度,作为初始吸光度D0。恒温培养箱设定30、40、50、60、70 ℃共计5个温度梯度作单因素处理。待测样品提取液于避光环境在各温度条件下隔1 h测定1次,连续监测6 h,取出后快速恢复至室温,测定并且记录吸光度(D)的变化情况,重复3次。
-
测定配好的花色苷提取液在各检测波长下的吸光度,作为初始吸光度D0。设置3种光源:①自然光[室内向阳窗台温度(29.5±0.5) ℃];②日光灯光照度(3 680±81) lx,温度(24.5±0.5) ℃;③避光处理,温度(24.5±0.5) ℃;隔1 h取样1次,连续监测6 h,测定记录吸光度(D)的变化情况,重复3次。
-
取1.8 mL换锦花花色苷提取液分别加入氢氧化钠或盐酸溶液,使花色苷溶液形成不同pH梯度:pH为1.0~13.0,梯度间隔为1。观察花色苷提取液颜色的变化,记录吸光度,重复3次。
-
取1.8 mL换锦花花色苷提取液分别加入不同浓度金属离子缓冲液至2.0 mL,混匀,观察反应液颜色变化后避光平衡2 h。在400~600 nm内,采样间隔2 nm扫描,确定最大吸收峰以及对应的吸光度。所用金属离子分别为Fe3+、Fe2+、Ca2+、Al3+、Zn2+、Mg2+、Cu2+、K+,各溶液的离子最终浓度为0、0.001 0、0.005 0、0.010 0、0.050 0、0.100 0 mol·L−1,重复3次。
-
在不同的理化因素(温度、光照、pH、金属离子浓度)处理下的溶液按照1.3.4的条件进行HPLC分析,重复3次。
-
采用Origin 8.0软件进行数据整理和绘图,采用中药色谱指纹图谱软件进行HPLC数据整理和分析。
1.1. 材料
1.2. 方法
1.2.1. 换锦花花色苷提取液制备
1.2.2. 换锦花花色苷组分分析
1.2.3. 换锦花总花色苷质量分数测定
1.2.4. 花色苷组成成分HPLC分析
1.2.5. 温度对换锦花花色苷稳定性的影响
1.2.6. 光照对换锦花花色苷稳定性的影响
1.2.7. pH对换锦花花色苷稳定性的影响
1.2.8. 金属离子对换锦花花色苷稳定性的影响
1.2.9. 理化因子处理下花色苷组成变化分析
1.3. 数据处理
-
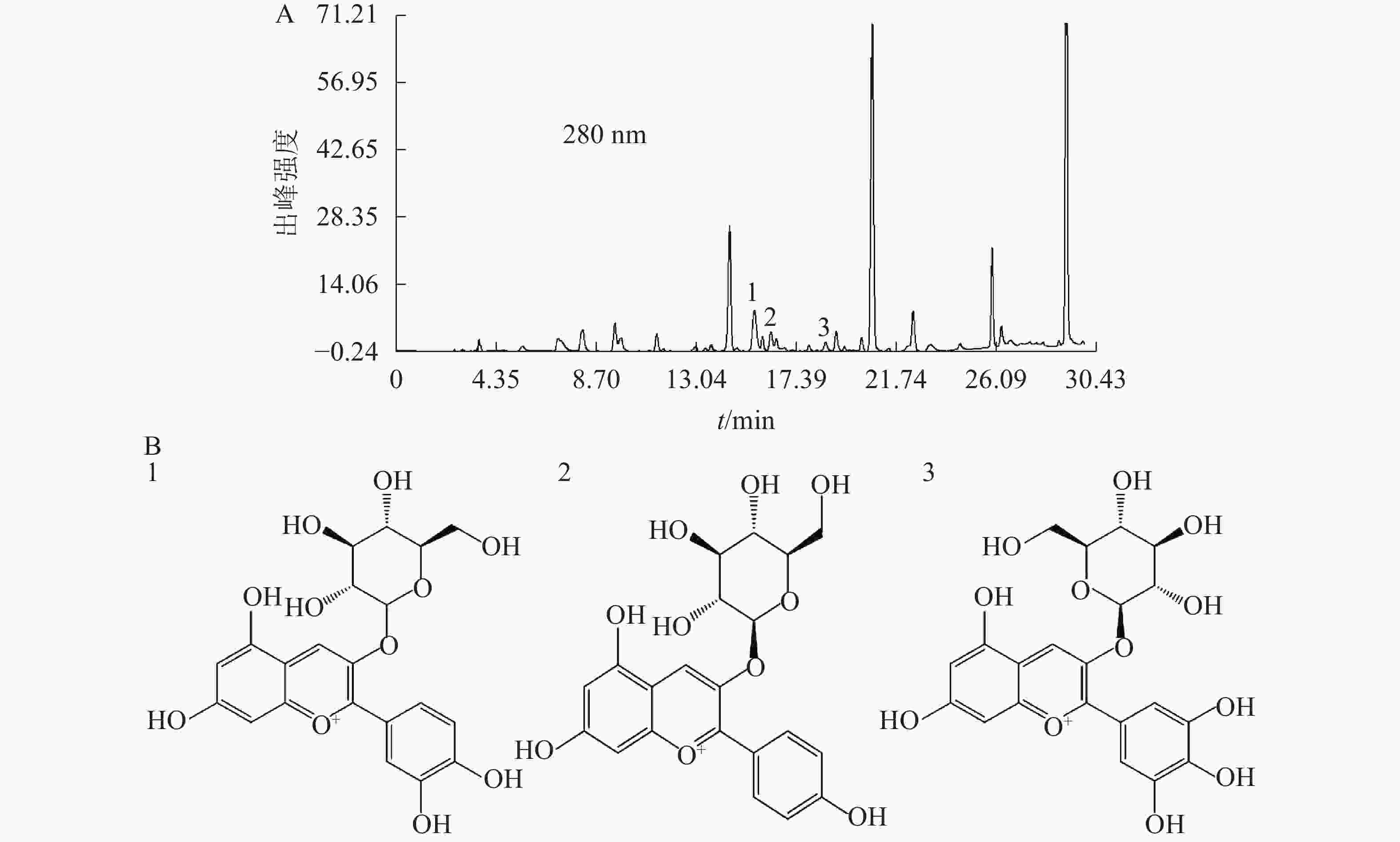
对换锦花花瓣代谢组分析可知:换锦花花瓣中花色苷元成分的种类及其具体含量(表1),其中主要为矢车菊素(cyanidin)、飞燕草素(delphinidin)和天竺葵素(pelargonidin)三大类,而葡萄糖苷是主要的花色苷苷元,因此选择矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷为标准品,利用HPLC检测换锦花花色苷在不同理化因素下3种成分及质量浓度的变化规律。
编号 化合物名称 峰面积 苷元类型 1 矢车菊素3-O-葡萄糖苷 cyanidin-3-O-glucoside 64.32 矢车菊素cyanidin 2 矢车菊素双葡萄糖苷 cyanidin3, 5-diglucoside 22.92 3 矢车菊素-3-芸香糖苷 cyanidin-3-rutinoside 16.50 4 矢车菊素-3-桑布双糖苷 cyanidin-3-sambubioside 14.05 5 矢车菊素-3-鼠李糖苷 cyanidin-3-rhamnoside 11.74 6 矢车菊素-3-阿拉伯糖苷 cyanidin-3-arabinoside 6.62 7 矢车菊素-3-苦甙-5-鼠李糖苷 cyanidin-3-sophroside-5-rhamnoside 4.07 8 矢车菊素-3-O-[(4-羟基-E-肉桂酰)-(6)-D-吡喃葡萄糖苷] cyanidin-3-O-
[(4-hydroxy-E-cinnamoyl)-(6)-D-glucopyranoside]1.34 9 飞燕草素-3-O-葡萄糖苷 delphinidin-3-O-glucoside 33.17 飞燕草素delphinidin 10 飞燕草素-3-O-(6''-O-α-鼠李吡喃糖基-β-吡喃葡萄糖苷delphinidin-3-O-
(6''-O-alpha-rhamnopyranosyl-beta-glucopyranoside)16.19 11 飞燕草素-3-桑布双糖苷 delphinidin-3-sambubioside 9.35 12 飞燕草素双葡萄糖苷 delphinidin-3, 5-diglucoside 3.66 13 飞燕草素-3-O-芸香糖苷-5-O-葡萄糖苷 delphinidin-3-O-rutinoside-5-O-glucoside 0.12 14 天竺葵素 pelargonidin 15.55 天竺葵素pelargonidin 15 芍药花素双葡萄糖苷 peonidin-3, 5-diglucoside 11.08 芍药花素 peonidin 16 矮牵牛素-3-O-葡萄糖苷 petunidin-3-O-glucoside 3.29 矮牵牛素 etunidin Table 1. Anthocyans composition of L. sprengeri petals
-
根据pH式差法计算换锦花花色苷质量分数为3.56 mg·g−1。经扫描换锦花花色苷提取液的吸收波长为400~600 nm,最大吸收峰为530 nm,因此,选用530 nm为换锦花花色苷的最大吸收波长(λvis max)。
-
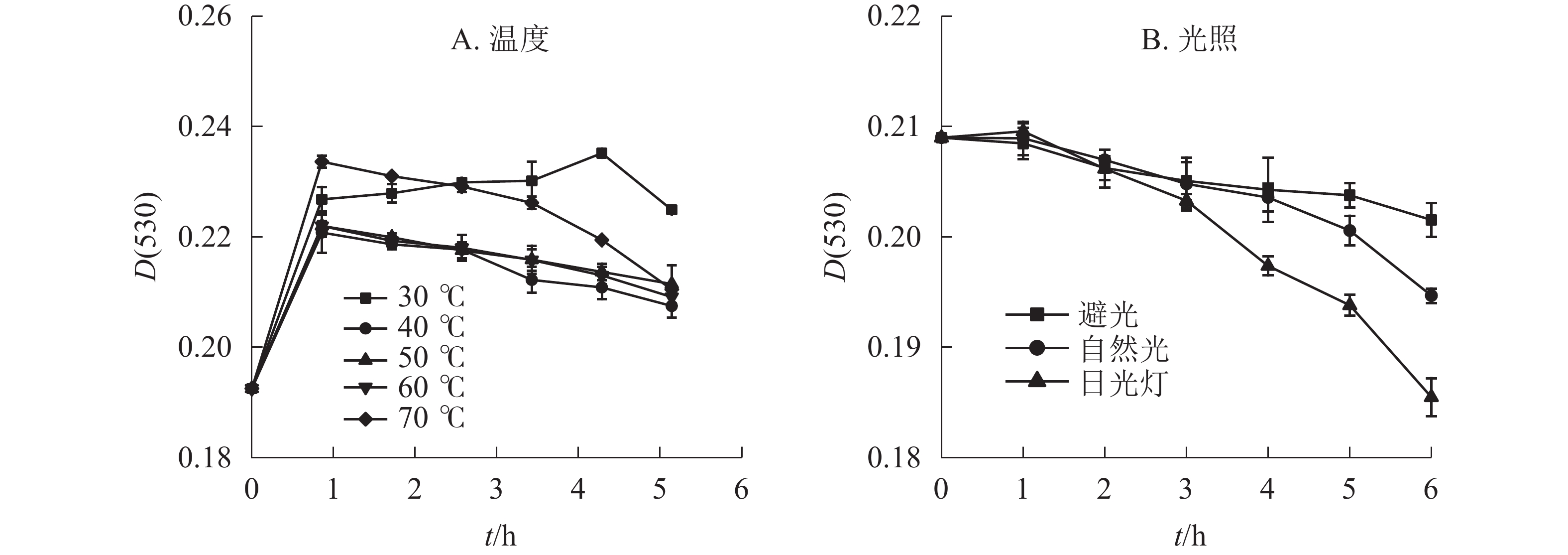
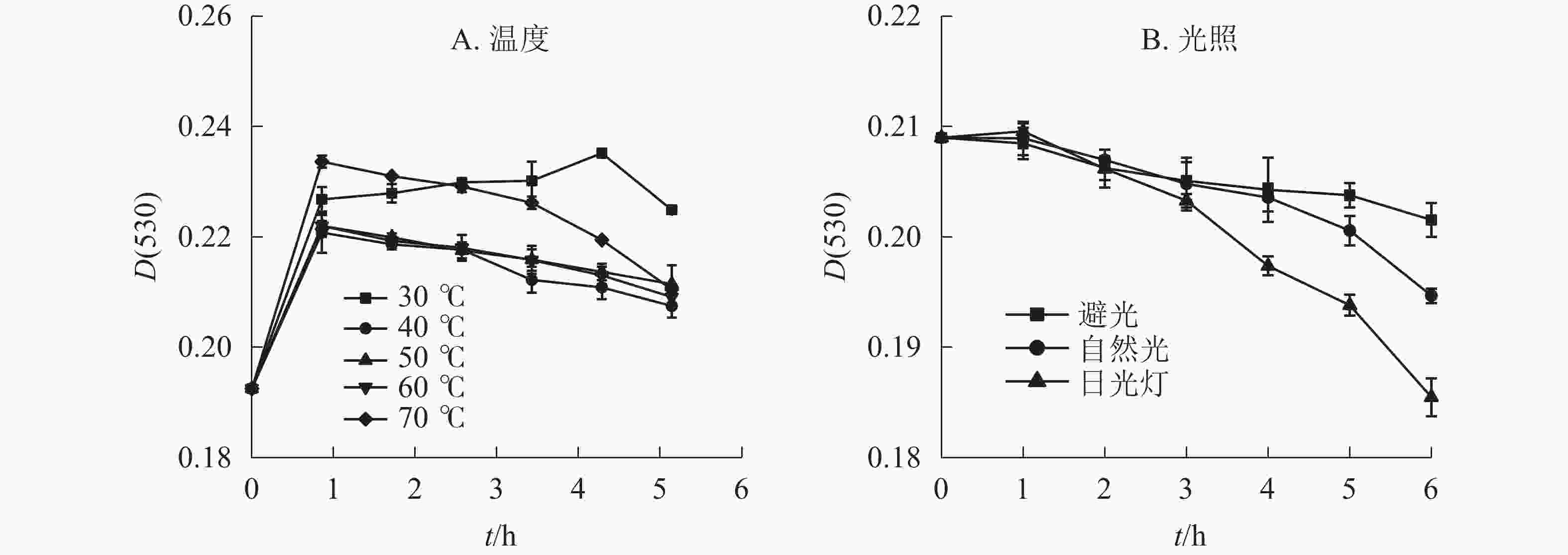
在不同温度下,换锦花花色苷提取液吸光度随时间的变化呈现先上升后下降的趋势(图1A)。在30~70 ℃中水浴1 h后,换锦花花色苷吸光度均显著上升,且温度为30 ℃时,随着加热时间的延长,花色苷吸光度继续上升到5 h以后开始降解,比原花色苷溶液吸光度提升22.17%,而40~70 ℃则是随着温度的升高以及加热时间的延长,花色苷的降解速率逐渐加快,提取液的吸光度[D(530)]比处理1 h时的吸光度分别降低了6.02%、4.75%、5.80%、9.91%。因此,不同的温度对换锦花花色苷稳定性的影响呈现一定变化规律,低温下且升温时间短时,花色苷比较稳定,且有提高花色苷质量浓度的趋势,可能是花色苷溶液在低温条件下稳定,且对花色苷有一定的增色作用。随着温度的升高和加热时间的延长,其吸光度显著下降,砖红色溶液逐渐变淡,可能是高温破坏花色苷结构引起花色苷物质褪色的原因[16]。
-
光照方式和光照时间长短对换锦花花色苷稳定性有一定影响。不同光照处理的换锦花花色苷的吸光度随着光照时间的延长均呈下降趋势(图1B),日光灯照射下换锦花花色苷提取液吸光度下降最快,花色苷提取液颜色变淡。日光灯和自然光照相比,避光保存花色苷提取液的吸光度变化不显著。避光、自然光、日光灯条件下换锦花花色苷的吸光度比原提取液吸光度分别下降3.57%、6.85%、11.25%。换锦花花色苷提取液在3种光照下的稳定性从强到弱依次为避光、自然光、日光灯,符合花色苷类色素对光敏感且稳定性差的特征[17]。
-
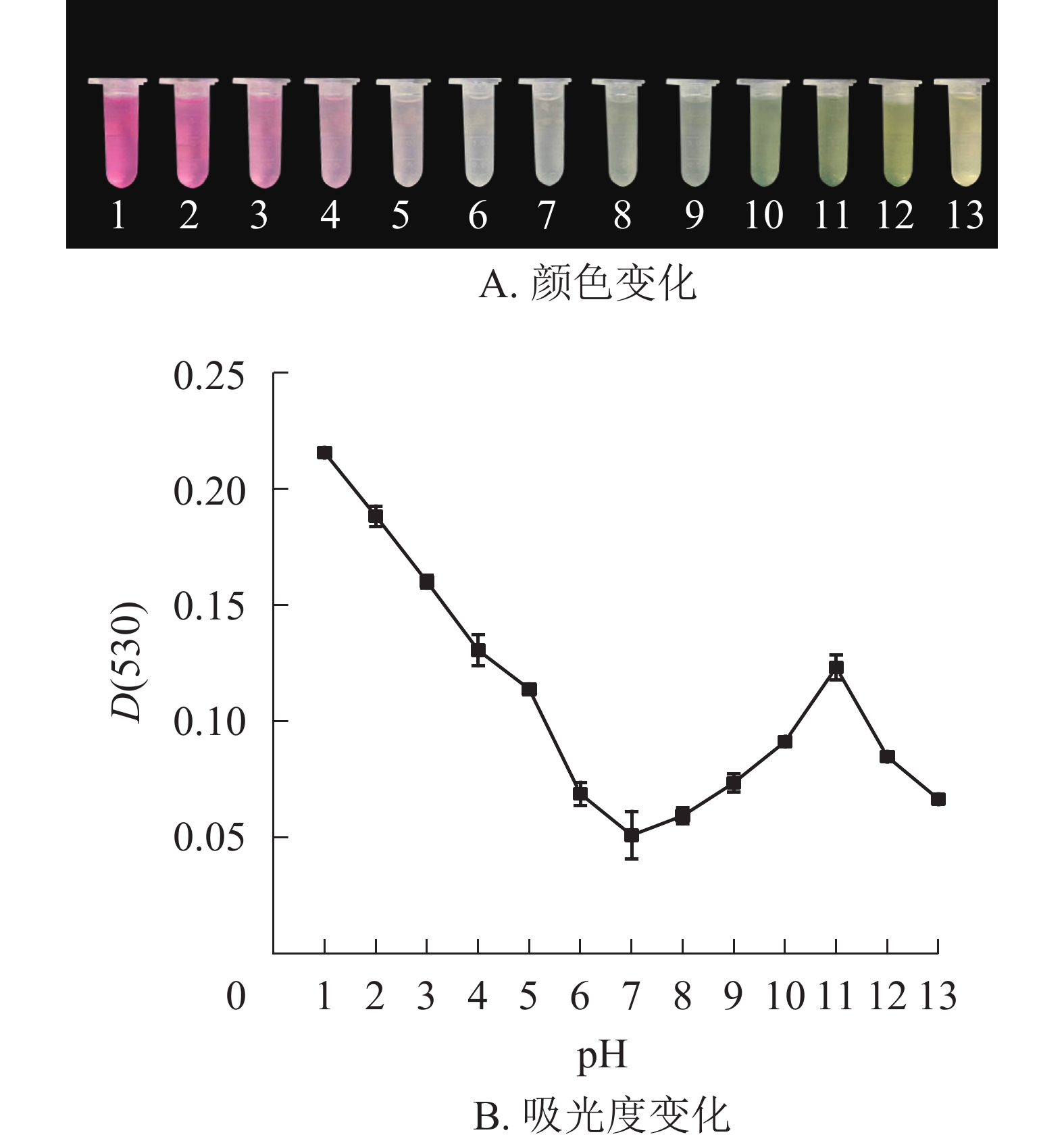
研究结果表明:换锦花花色苷提取液的颜色和吸光度受pH的影响较大。pH≤3.0时,换锦花花色苷提取液为红色,溶液吸光度较高。花色苷主要以二苯基苯并吡喃阳离子的结构存在。当pH近中性时,溶液颜色接近无色,花色苷提取液的吸光度逐渐降低,这时花色苷主要以无色的查尔酮结构存在。在碱性条件下(pH≥7.0),花色苷提取液颜色逐渐变成蓝色,最后变为黄色,提取液的吸光度随着pH增大而逐渐升高。当pH≥11.0后,提取液吸光度又逐渐下降(图2A和图2B),花色苷中的酚类物质生成性质活泼的醌,主要以蓝色醌式结构存在[18-19],所以提取液颜色开始向蓝黄色转变。
-
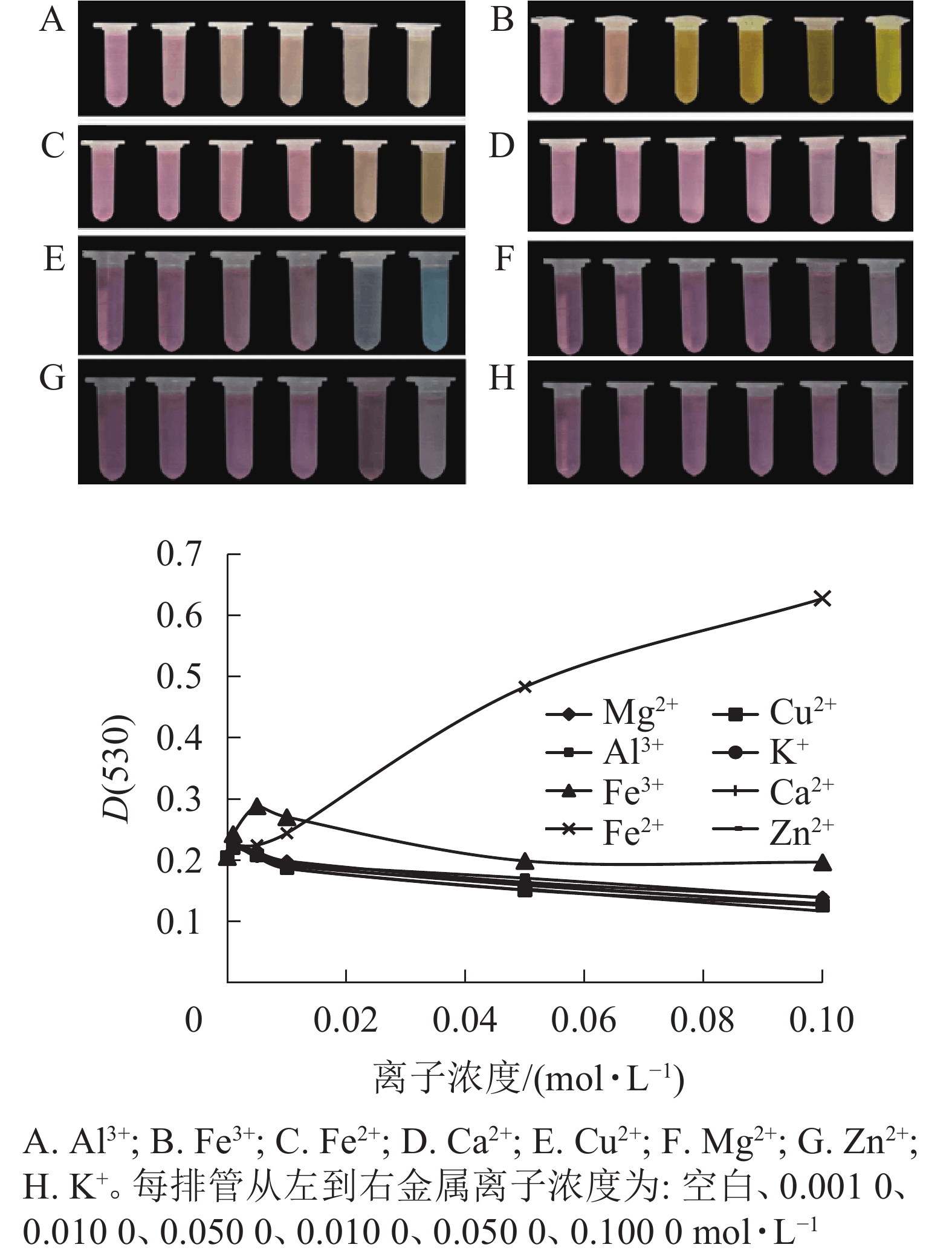
不同金属离子对花色苷的稳定性影响不同(图3)。大部分高浓度金属离子可破坏花色苷的结构。Fe3+、Ca2+、Al3+、Zn2+、Mg2+、Cu2+、K+ 等7种金属离子对花色苷稳定性的影响趋势基本一致,当浓度<0.001 mol·L−1时,花色苷吸光度随金属离子浓度的增大而增大,当浓度>0.001 mol·L−1后,其吸光度逐渐下降且逐渐稳定;但Al3+、Fe3+、Cu2+均可引起换锦花花色苷溶液的变色现象。随着Al3+浓度增加,花色苷溶液颜色由粉红色变为淡橙色;随着Fe3+浓度增加,花色苷溶液颜色逐渐向橙黄色过渡,最终转为黄褐色;随着Cu2+浓度增加,花色苷溶液颜色由红色逐渐转为亮蓝色。Ca2+、Zn2+、Mg2+和K+使花色苷提取液颜色保持不变,但随着金属离子浓度增高,花色苷溶液颜色变浅。换锦花花色苷吸光度则随着Fe2+浓度的增加不断增加,且花色苷溶液的颜色随着Fe2+浓度增加颜色逐渐加深变为橙黄色。
8种金属离子在0~0.01 mol·L−1浓度范围内对换锦花花色苷增色效应明显,能够帮助稳定花色苷的构型[20-21]。图3中能明显能看出Fe2+离子对花色苷提取液的影响最大,对花色苷的增色作用随着离子浓度的增加而加强,可能是因为花色苷具有邻位羟基结构,能够和金属离子形成螯合物[22],且这种螯合物的稳定性高于花色苷,一旦生成不易逆转,从而影响花色苷的稳定性。其他几种金属离子随着浓度的增加,对换锦花花色苷皆有一定的破坏作用。所以,一定浓度金属离子对花色苷有正向增色作用,但仍应尽量避免花色苷与高浓度金属离子接触。
-
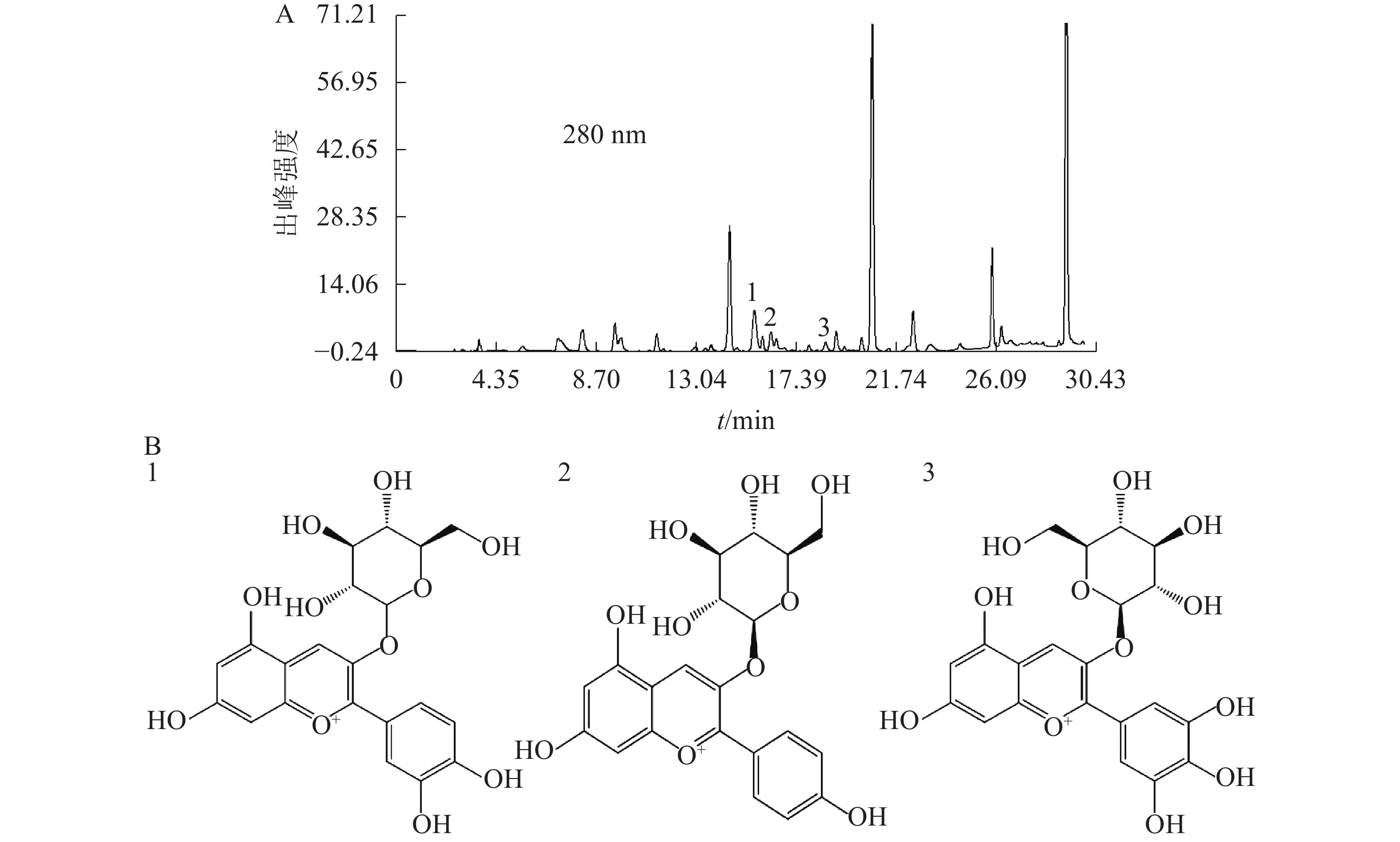
基于代谢组分析结果,选择矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷为标准品,换锦花花色苷中这3种成分的质量分数分别为2.95、0.41和0.42 mg·g−1。图4A为换锦花花色苷提取液在280 nm处的HPLC色谱图,得到矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷峰面积及其具体出峰时间。利用Formula Predictor 4.2软件对3种不同的花色苷结构进行检索,得到矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷苷元结构(图4B)。
-
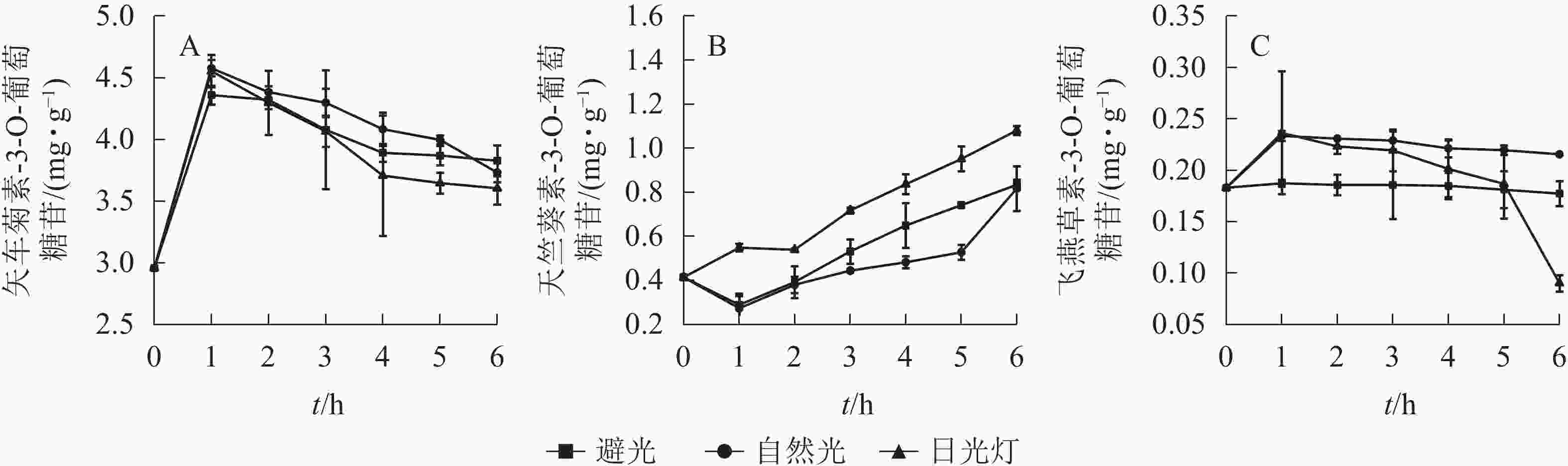
换锦花3种花色苷随着温度的升高和时间的延长呈现不同的变化趋势(图5),矢车菊素-3-O-葡萄糖苷质量分数逐渐降低、天竺葵素-3-O-葡萄糖苷质量分数逐渐上升、飞燕草素-3-O-葡萄糖苷质量分数变化不明显,与提取液颜色变化趋势一致。花色苷提取液于30~70 ℃处理6 h后,矢车菊素-3-O-葡萄糖苷质量分数分别降低了20.11%、43.99%、41.65%、56.57%、71.85%。相同条件下,30~60 ℃时天竺葵素-3-O-葡萄糖苷质量分数比对照分别提高了150.42%、352.80%、502.74%、393.02%。花色苷提取液颜色由砖红色逐渐变为橙红色,可能是由于温度升高导致矢车菊素-3-O-葡萄糖苷失去1个羟基基团而转变为1个羟基基团的天竺葵素-3-O-葡萄糖苷,在70 ℃ 2 h后天竺葵素-3-O-葡萄糖苷质量分数急剧下降,可能是高温破坏了天竺葵素-3-O-葡萄糖苷结构;飞燕草素-3-O-葡萄糖苷质量分数变化不明显,可能由于飞燕草素-3-O-葡萄糖苷的结构中有较稳定的3个羟基基团。
-
换锦花不同花色苷质量分数随着光照和时间的变化而变化(图6),在避光、自然光和日光灯条件下,矢车菊素-3-O-葡萄糖苷质量分数均呈下降趋势,与原提取液相比分别下降了26.75%、28.58%、31.02%;天竺葵素-3-O-葡萄糖苷质量分数则分别升高了232.63%、225.75%、330.90%。在避光和自然光照条件下飞燕草素-3-O-葡萄糖苷变化趋势平缓,但在日光灯光照1 h后呈现下降趋势,5 h后飞燕草素-3-O-葡萄糖苷质量分数呈直线下降,到6 h时仅为原提取液质量分数的4.35%。日光灯下的矢车菊素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷质量分数减少最快,天竺葵素-3-O-葡萄糖苷质量分数上升最快。可见在3种不同光源中,日光灯对花色苷具体成分影响很大。这一结果和不同温度条件下花色苷质量分数变化趋势一致,随着光照强度和时间的增加,矢车菊素-3-O-葡萄糖苷逐渐降低、天竺葵素-3-O-葡萄糖苷逐渐上升、飞燕草素-3-O-葡萄糖苷除了日光灯下过长时间照射外急剧下降,短时间内没有明显的变化。可能是由于飞燕草素-3-O-葡萄糖苷B环结构中有3个较为稳定羟基基团[23]。长时间强光照射破坏了飞燕草素-3-O-葡萄糖苷B环结构的羟基基团,导致其质量分数骤然下降。
-
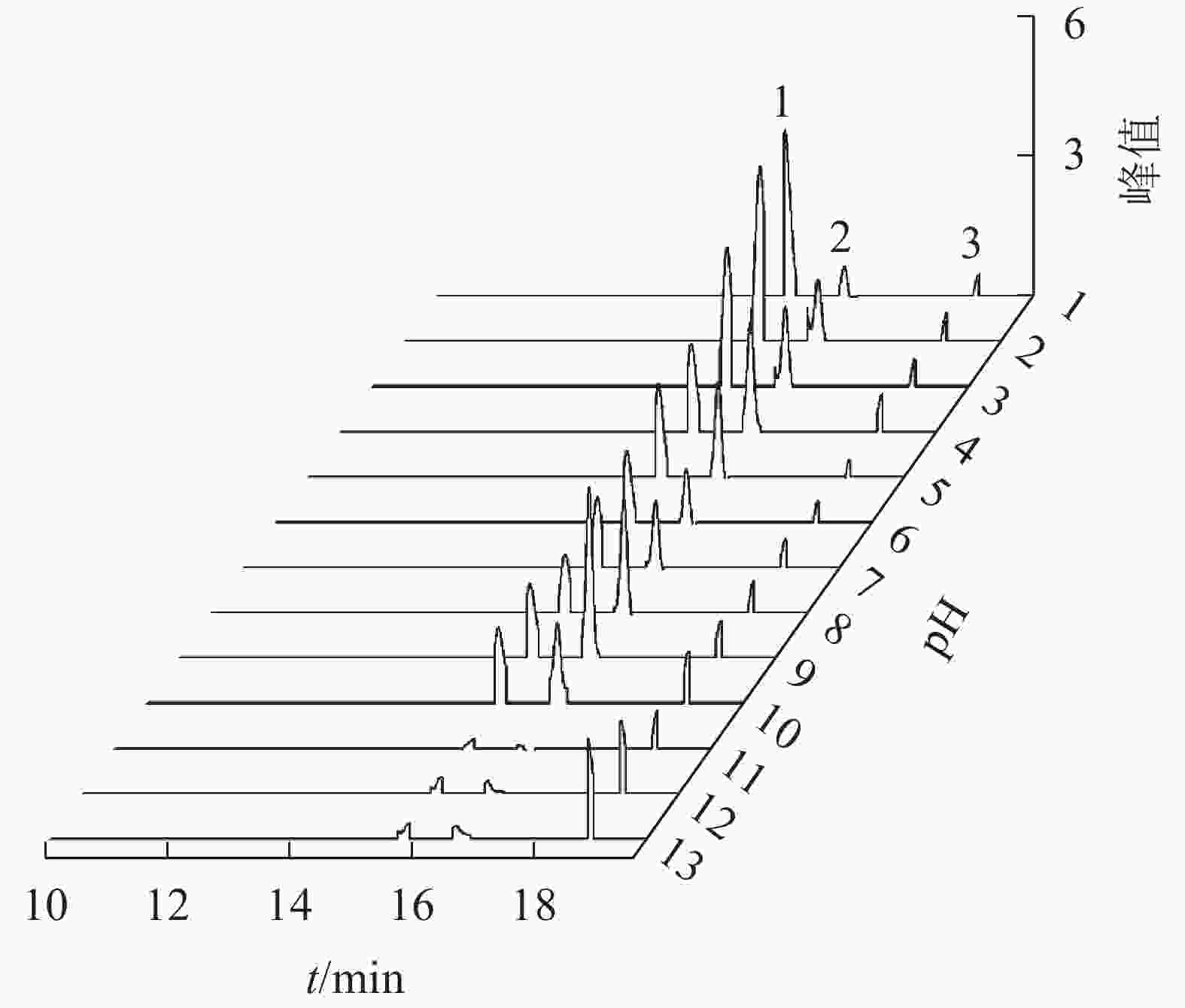
换锦花矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷和飞燕草素-3-O-葡萄糖苷的峰值随着提取液酸碱度的变化而变化。花色苷提取液pH为1.0~13.0时,随着pH的增加,矢车菊素-3-O-葡萄糖苷峰值逐渐降低,飞燕草素-3-O-葡萄糖苷峰值逐渐升高,而天竺葵素-3-O-葡萄糖苷峰值变化不明显(图7)。由此可见,酸碱度对天竺葵素-3-O-葡萄糖苷结构的稳定性影响较大,矢车菊素-3-O-葡萄糖苷结构在酸性条件下较稳定,而飞燕草素-3-O-葡萄糖苷的结构则在碱性条件下比较稳定。
2.1. 换锦花花色苷组分分析
2.2. 理化因素对换锦花总花色苷的影响
2.2.1. 换锦花总花色苷质量分数及其最大吸收波长
2.2.2. 温度对总花色苷稳定性的影响
2.2.3. 光照对总花色苷稳定性的影响
2.2.4. pH对总花色苷稳定性的影响
2.2.5. 金属离子对总花色苷稳定性的影响
2.3. 不同处理下换锦花花色苷主要成分质量分数的变化
2.3.1. 温度对换锦花花色苷3种主要成分稳定性的影响
2.3.2. 光照对换锦花花色苷3种主要成分稳定性的影响
2.3.3. pH对花色苷提取液中3种主要成分稳定性的影响
-
花色苷是重要的功能性天然色素,可使植物各器官部位呈红色和蓝色的酚类物质[24],但性质极不稳定,易受到内部和外部环境条件的影响。本研究通过不同光照、温度、pH及金属离子浓度等外部理化因素对换锦花总花色苷稳定性进行试验,并利用HPLC分析了花色苷的主要成分、质量分数及稳定性。
在温度≤30 ℃条件下,换锦花花色苷较为稳定,且对花色苷具有一定的辅色效应,高温可加快花色苷的分解。可以认为:2-苯基苯并吡喃环阳离子和查尔酮之间的可逆转化导致花色苷的热降解;高温条件下平衡是向着查尔酮方向移动的,而有色的2-苯基苯并吡喃环阳离子和醌型碱形式明显减少[25]。与温度相比,光照对换锦花花色苷稳定性的影响不明显。避光条件下花色苷的稳定性几乎不受影响,自然光次之,日光灯影响最大。换锦花花色苷溶液在酸性条件下比较稳定,pH为1.0时,换锦花花色苷溶液吸光度最高,溶液为红色,且比较稳定性。pH≥4.0后,花色苷溶液逐渐由红色变为无色,最终随着pH的增加溶液颜色转向蓝黄色,吸光度随之先降低后增加。Fe3+、Ca2+、Al3+、Zn2+、Mg2+、Cu2+、K+、Fe2+等8种金属离子在0~0.01 mol·L−1浓度下,对换锦花花色苷溶液均有一定的增色效应,能够帮助稳定花色苷的构型,但随着金属离子浓度的继续增加,只有Fe2+对花色苷有正向增色作用,其他金属离子均对花色苷起破坏作用。所以换锦花在温度低于30 ℃,避光且pH≤3.0的条件下能较好保存,同时尽量避免接触高浓度金属离子。
换锦花花瓣中花色苷主要成分为矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷、飞燕草素-3-O-葡萄糖苷,其中矢车菊素产生红色,天竺葵素产生橙色,飞燕草素产生蓝紫色[1]。花色苷具有很强抗氧化作用的主要活性基团是分子中的酚羟基[26]。GARCIA-ALONSO等[23]认为:葡萄酒中飞燕草-3-O-葡萄糖苷B环上具有3个羟基,具有最强的抗氧化活性,矢车菊素-3-O-葡萄糖苷次之,天竺葵素-3-O-葡萄糖苷最弱。在不同的温度和光照条件下,飞燕草素-3-O-葡萄糖苷是最为稳定的,矢车菊素-3-O-葡萄糖苷和天竺葵素-3-O-葡萄糖苷对光照和温度比较敏感,且天竺葵素-3-O-葡萄糖苷质量分数变化更大。随着时间的延长和温度、光照强度的增加,矢车菊素-3-O-葡萄糖苷质量分数显著下降。天竺葵素-3-O-葡萄糖苷则恰恰相反,其质量分数逐渐升高,超过一定限度才开始下降,说明适当的温度和光照对天竺葵素-3-O-葡萄糖苷有正向稳定作用[27],与换锦花在高温和强光条件下提取液颜色由深红转变为橙红相一致。花色苷的含氧杂环上是氧正离子,以正阳盐形式存在,其结构在不同pH下会发生变化[28-29],在pH为1.0~13.0时,换锦花提取液颜色由红色变为无色,最终随着pH的增加,颜色又向蓝色转变,矢车菊素-3-O-葡萄糖苷质量浓度也随着pH的增加呈明显下降趋势,飞燕草素-3-O-葡萄糖苷峰值也随着pH的增加呈明显上升趋势。推测在酸性条件下,飞燕草素-3-O-葡萄糖苷中的醌型碱结构被破坏;在碱性条件下,矢车菊素-3-O-葡萄糖苷中的2-苯基苯并吡喃环阳离子遭破坏。天竺葵素-3-O-葡萄糖苷的变化规律有待进一步研究。
-
换锦花花色苷的主要成分是矢车菊素、天竺葵素和飞燕草素;换锦花花色苷稳定性受温度、光照、pH和金属离子浓度等理化因素的影响。高温、强光和高pH可降解花色苷,导致花色苷结构之间的转变。矢车菊素-3-O-葡萄糖苷质量分数随着时间延长和光照、温度强度的增加而降低,天竺葵素-3-O-葡萄糖苷则呈相反趋势,飞燕草素-3-O-葡萄糖苷在光照和温度不同条件下较稳定;随着pH增加,矢车菊素-3-O-葡萄糖苷含量逐渐降低,飞燕草素-3-O-葡萄糖苷逐渐增加。Al3+、Fe2+、Cu2+、Fe3+均使花色苷溶液有变色现象,Ca2+、Zn2+、Mg2+和K+对花色苷提取液影响不大,但金属离子在浓度0~0.01 mol·L−1时对换锦花花色苷均有一定的增色效应。随着Fe2+浓度的增加增色作用明显。












 DownLoad:
DownLoad: