-
彩叶桂品种群Osmanthus fragrans Colour Group是近年来新培育的桂花新类型,其营养体部分(即枝条和叶片)有着完全不同于绿色的明显色彩变异,并能够维持较长时间[1]。彩叶桂观赏期更长,园林用途更广泛,未来前景十分广阔。然而,由于低温的限制,目前彩叶桂的栽培主要局限于长江流域及以南地区,尚未得到有效的开发应用[2]。另外,迄今尚未有关于彩叶桂抗寒相关的系统研究,其抗寒品种的筛选和适宜种植的范围尚不明确,这也极大地限制了彩叶桂的推广和应用。低温是影响自然界植物正常生长和分布的一项关键因素[3],因此,研究低温对植物的影响具有重要的价值。低温环境中,植物细胞膜、渗透调节物质、抗氧化酶等都会产生一系列变化以抵御低温伤害。因此,通常采用相对电导率、丙二醛、可溶性糖、可溶性蛋白质、超氧化物歧化酶、过氧化物酶等生理指标作为评价植物抗寒性的参考依据[4-5]。本研究以6个不同彩叶桂品种为试验材料,通过测定其相对电导率、丙二醛质量摩尔浓度、可溶性糖质量分数、可溶性蛋白质质量分数、超氧化物歧化酶活性、过氧化物酶活性等6项生理指标的变化,探讨6种彩叶桂对低温胁迫的响应,完成部分彩叶桂的抗寒性评价。据此筛选适应北方地区露地栽培的优良彩叶桂品种,对彩叶桂的推广和应用具有一定的参考意义。
HTML
-
材料均取自山东农业大学林学试验站。6个彩叶桂品种为‘傲霜’‘Aoshuang’、‘冬荣’‘Dongrong’、‘罗彩1号’‘Luocai No.1’、‘罗彩2号’‘Luocai No.2’、‘永福幻彩’‘Yongfu Huancai’、‘银碧双辉’‘Yinbi Shuanghui’。2019年11月中旬,选择各品种长势良好一致、无病虫害的4年生扦插苗,剪取1年生枝上第3~5节位大小均匀的叶片,用塑封袋装好置于冰盒带回实验室。
-
将采回的叶片混匀,每个品种分成均匀的9份,设3次重复,洗净并吸干水分,将1组放置在25 ℃室温中进行对照处理,另外8组放置在可控温冰箱中,按照预设的8个温度梯度(即0、−4、−8、−12、−16、−20、−24、−28 ℃)给予人工低温处理。以隔10 min下降1 ℃的速度降温,降到目的温度后放置24 h,取出1组叶片在4 ℃冰箱中解冻8 h后测定。其余叶片继续按设定梯度处理。
-
相对电导率的测定采用电导法[6],将各处理温度下的相对电导率和各处理温度的关系用Logistic方程进行拟合[7],当拟合度显著时计算拐点温度即半致死温度(TL50),可溶性糖质量分数的测定采用蒽酮法,可溶性蛋白质质量分数采用考马斯亮蓝比色法[8],丙二醛质量摩尔浓度采用硫代巴比妥酸法,超氧化物歧化酶活性采用NBT法,过氧化物酶活性采用愈创木酚法[9]。
-
应用Excel 2016、SPSS 20.0软件进行数据分析,利用单因素方差分析(one-way ANOVA)和Duncan新复极差法(Duncan’s multiple-range)进行差异显著性分析,图表中数据为平均值±标准差;不同小写字母表示各品种内不同低温间在 P<0.05水平上具有显著性差异。
通过隶属函数法对6个彩叶桂品种的抗寒性进行综合性评价。隶属函数值计算公式:
式(1)~(2)中:U(xij)为i品种j指标的隶属函数值;xij为测定值;xjmax和xjmin分别为j指标测定的最大值和最小值。式(1)用于计算指标与抗寒性呈正相关的情况,式(2)用于计算呈负相关的情况[10]。
1.1. 试验材料
1.2. 低温处理
1.3. 生理指标测定
1.4. 数据分析和综合评价
-
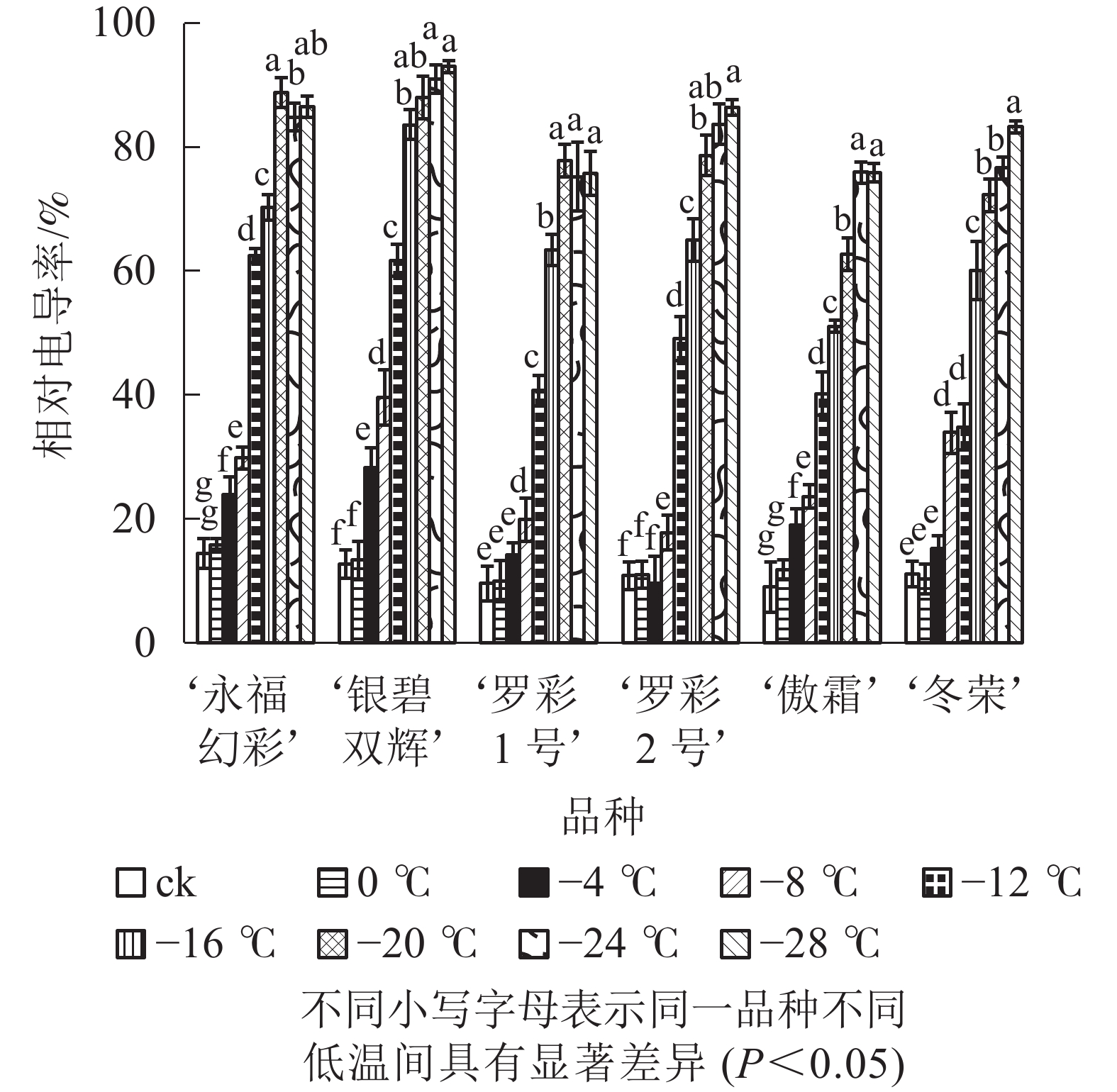
从图1可见:不同低温胁迫下6个彩叶桂品种相对电导率的变化趋势较为相似,都随温度的下降逐渐升高。在轻度低温胁迫时(0~−4 ℃),相对电导率升高较为缓慢,处于较低水平;随着温度的进一步下降(−4~−20 ℃),电导率表现快速增加的趋势。品种不同增幅不同。‘永福幻彩’幅度最小,是对照的6.16倍,‘傲霜’增加幅度最大,是对照的8.42倍,且各品种的相对电导率都已大于60%,说明此时已经出现了严重的离子渗透;降温后期(−20~−28 ℃),各品种相对电导率增加幅度很小,可见胁迫后期植物叶片细胞膜已受到相当严重破坏,几乎全部的离子都已渗透到细胞外,植株接近死亡。
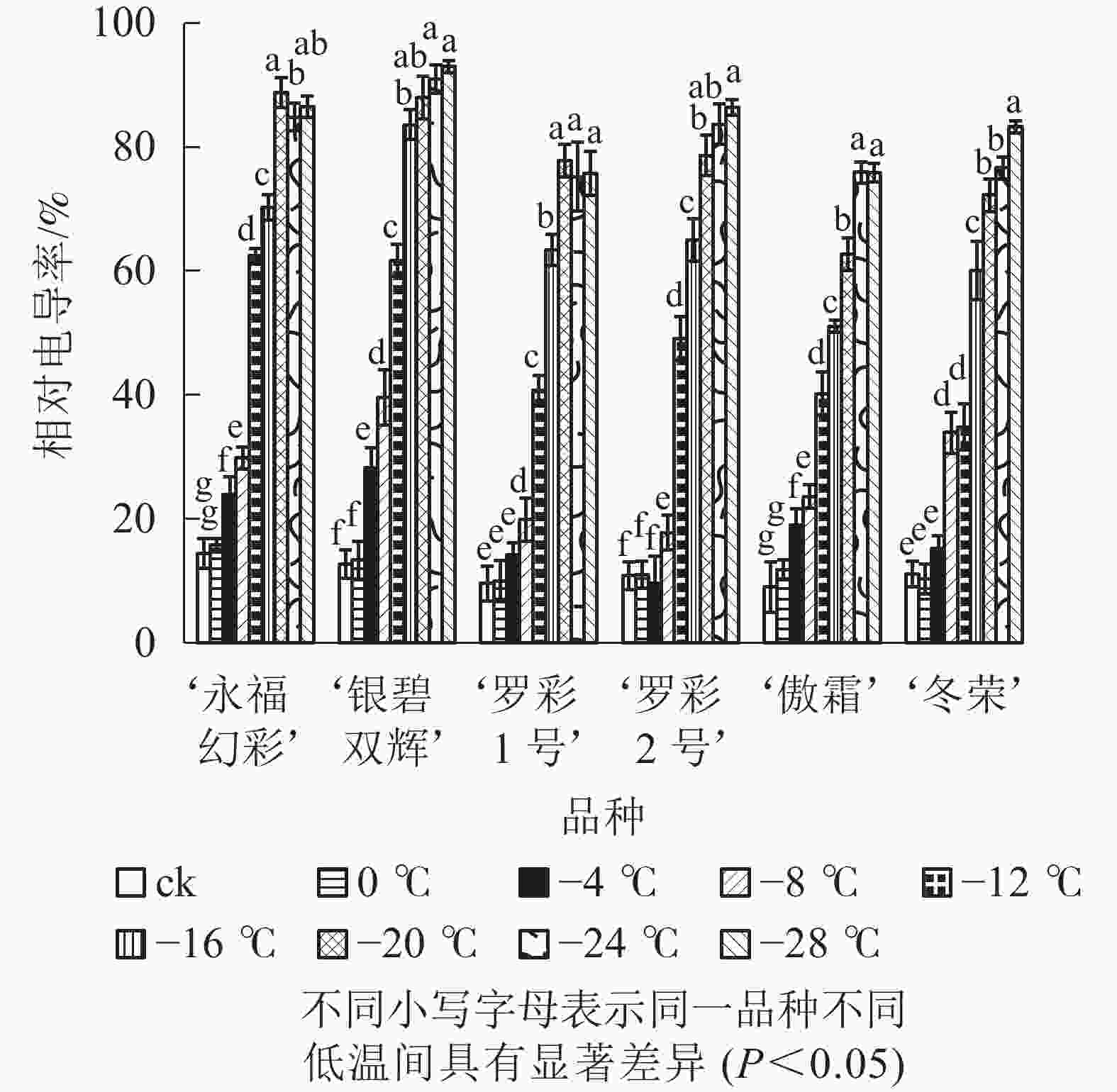
Figure 1. Changes of relative conducitivity in leaves of six cultivars of O. fragrans under cold stress
半致死温度(TL50)在一定程度上能反映植物不同品种的耐寒能力。对相对电导率数据进行 Logistic 回归分析(表1),相关系数均大于0.8,拟合度良好,结果可信。通过公式TL50=lna/b(a、b为回归方程的参数)计算半致死温度,不同品种彩叶桂抗寒性从大到小依次为‘傲霜’ ‘冬荣’ ‘罗彩2号’ ‘罗彩1号’ ‘永福幻彩’ ‘银碧双辉’。
品种 Logistic方程 半致死温度/℃ 相关系数 ‘永福幻彩’ y=0.89/(1+5.80e0.18x) −9.94 0.831 9** ‘银碧双辉’ y =0.94/(1+7.15e0.24x) −8.15 0.989 0** ‘罗彩1号’ y =0.76/(1+12.33e0.23x) −10.77 0.844 0** ‘罗彩2号’ y =0.88/(1+14.55e0.24x) −11.24 0.972 3** ‘傲霜’ y =0.86/(1+6.86e0.15x) −12.95 0.981 9** ‘冬荣’ y =0.87/(1+8.70e0.18x) −11.80 0.982 7** 说明:x表示处理温度,y表示相对电导率。**表示在 0.01 水平上极显著相关 Table 1. Regression equation of relative conductivity and TL50 in leaves of six cultivars of O. fragrans under cold stress
-
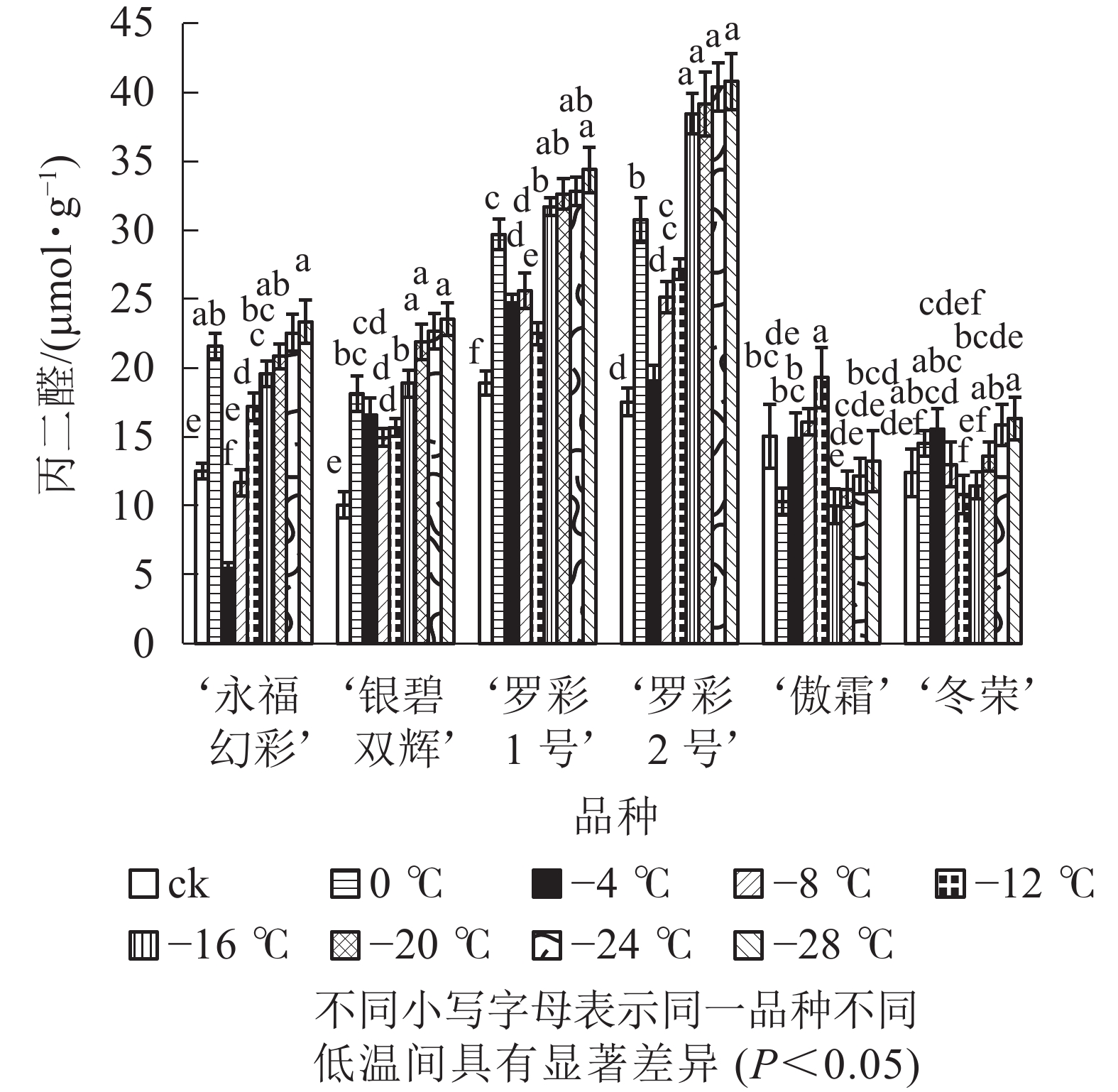
低温胁迫下,彩叶桂叶片丙二醛质量摩尔浓度随温度降低,总体上呈先增加后减少再增加的变化规律,并且各品种的变化幅度有所不同(图2)。0 ℃时叶片中丙二醛与对照相比显著上升,随即出现下降,仅‘傲霜’在0 ℃时出现短暂下降,随后又迅速上升,直到−12 ℃才又出现下降。随着胁迫的加深,各品种在降温后期丙二醛质量摩尔浓度再次呈上升趋势。上升幅度最大的为‘银碧双辉’,是对照的2.34倍,上升幅度最小的是‘傲霜’,是对照的0.88倍。
-
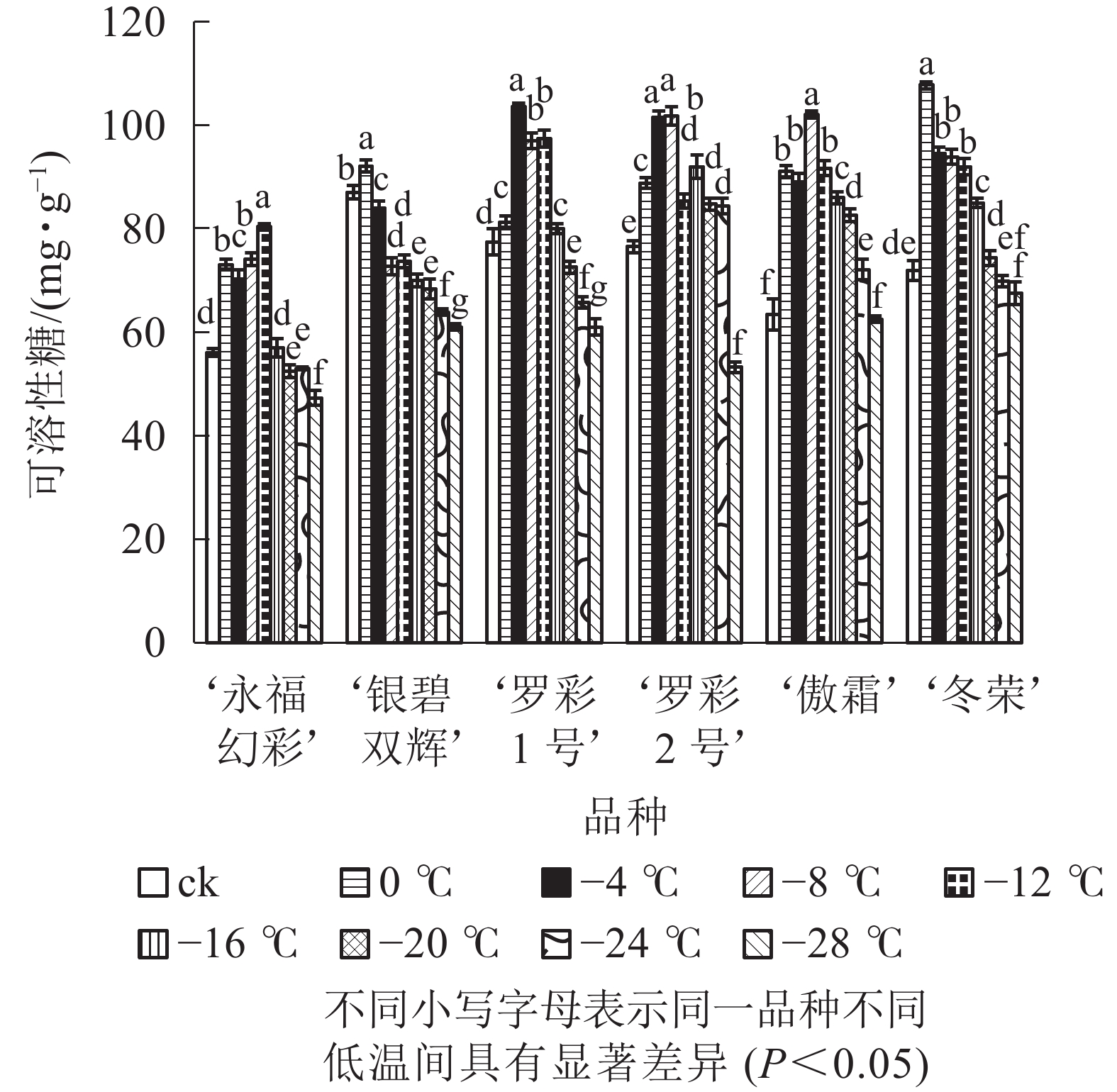
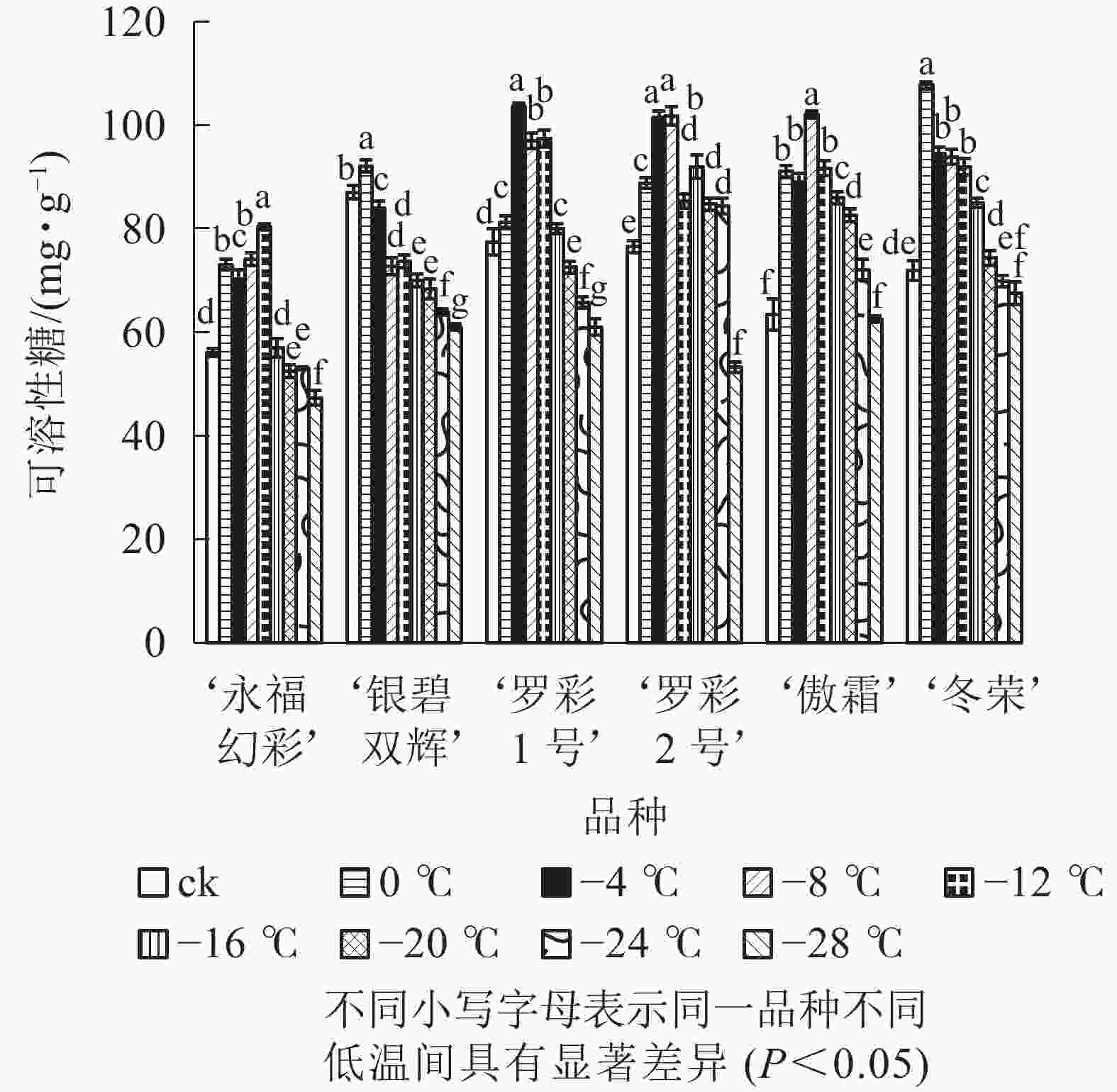
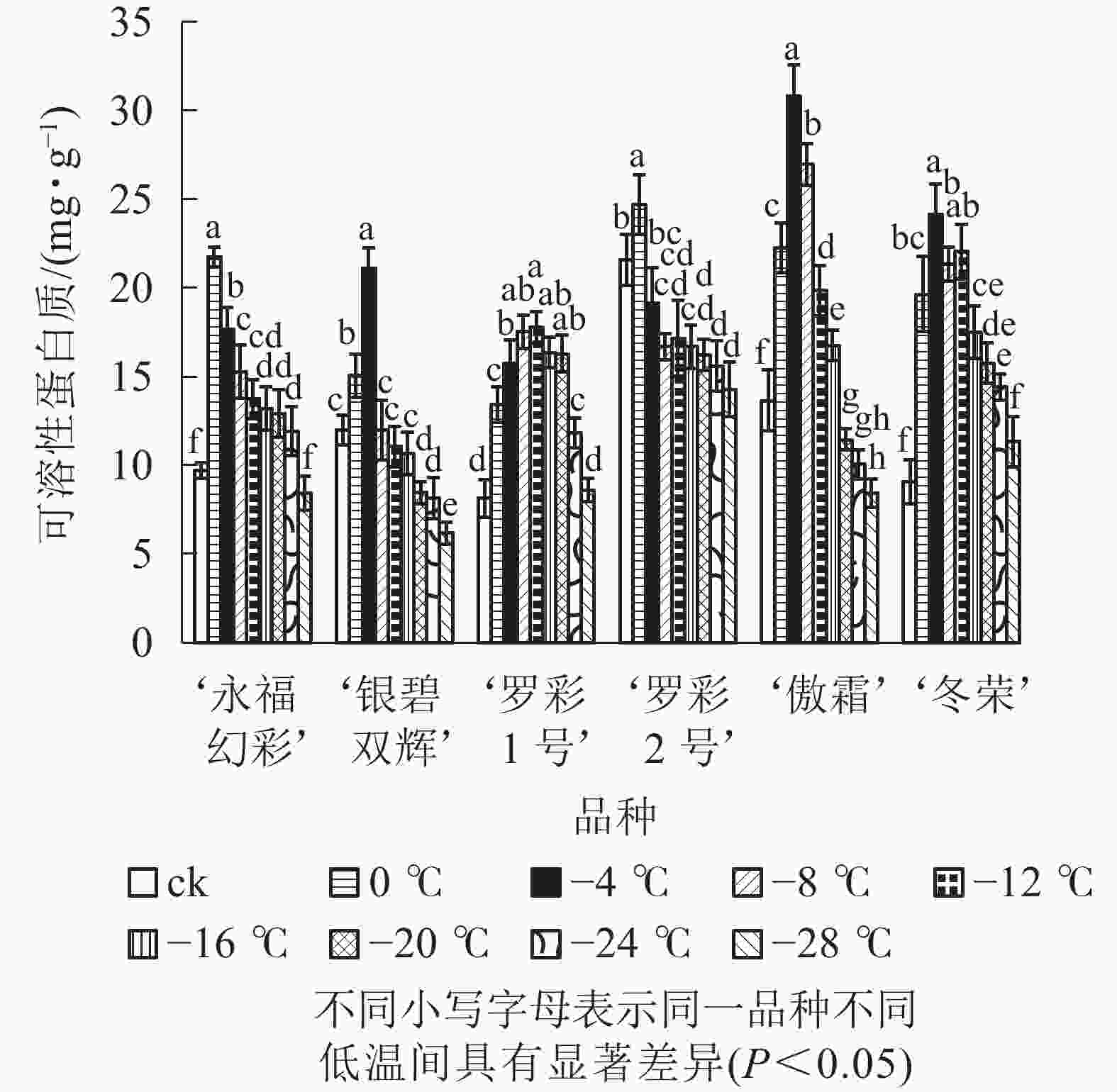
在对彩叶桂叶片进行人工低温处理时,各品种可溶性糖、可溶性蛋白质质量分数总体上均表现出“升高—降低”的变化趋势,但增加幅度和拐点温度并不相同(图3和图4)。‘永福幻彩’可溶性糖拐点温度最低,为−12 ℃,‘银碧双辉’和‘冬荣’拐点温度最高,为0 ℃;可溶性糖增幅最大的是‘傲霜’,是对照的1.61倍,增幅最小的为‘银碧双辉’,是对照的1.06倍。‘罗彩1号’可溶性蛋白质拐点温度最低,为−12 ℃,‘永福幻彩’ ‘罗彩2号’拐点温度最高,为0 ℃;可溶性蛋白质增幅最大的是‘冬荣’,是对照的2.66倍,增幅最小的是‘罗彩2号’,是对照的1.15倍。总的来说,当彩叶桂受到一定范围的低温胁迫时,可溶性糖和可溶性蛋白质质量分数表现为增加,即2个指标和植物的耐寒能力存在一定的正相关。
-
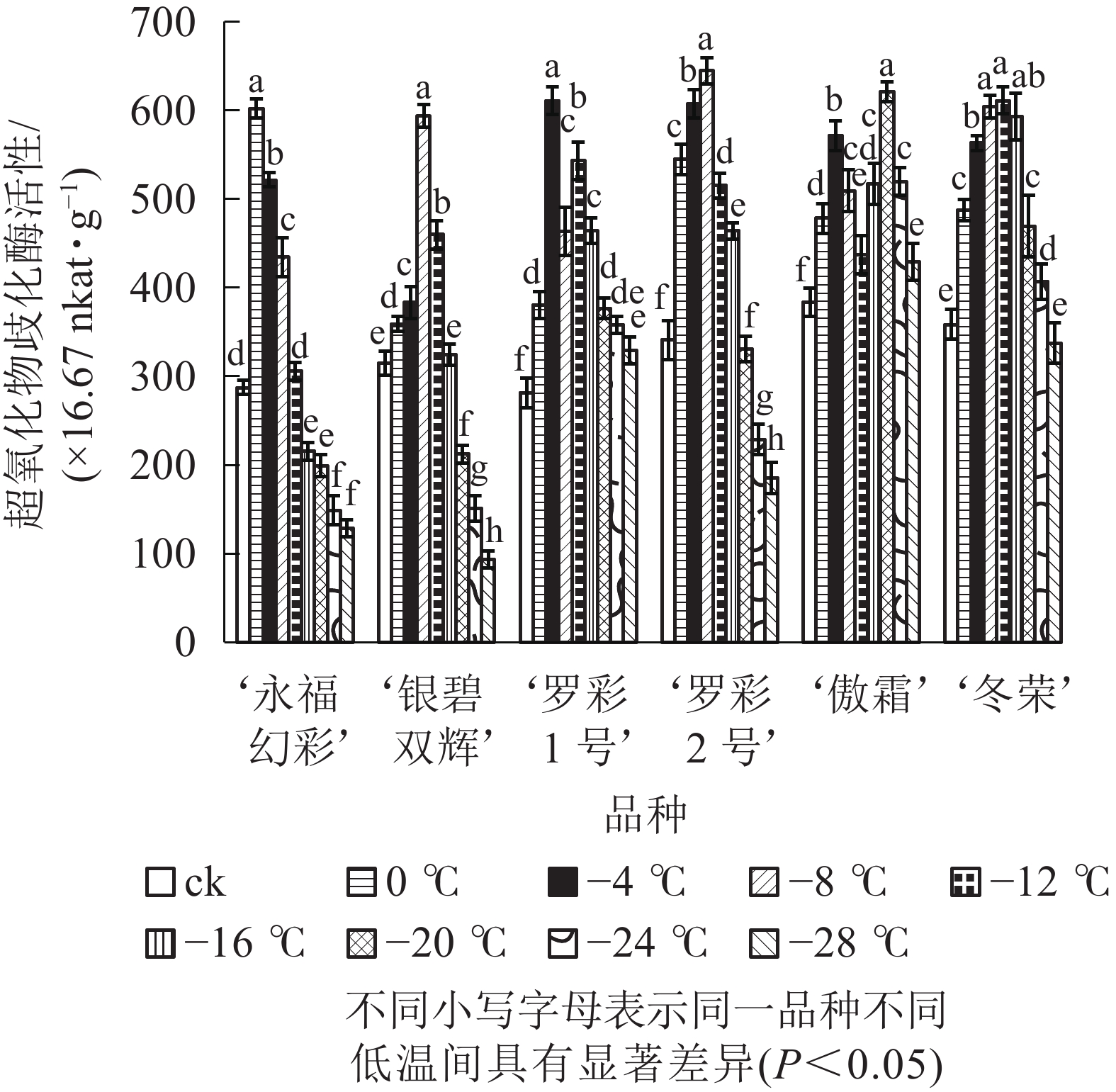
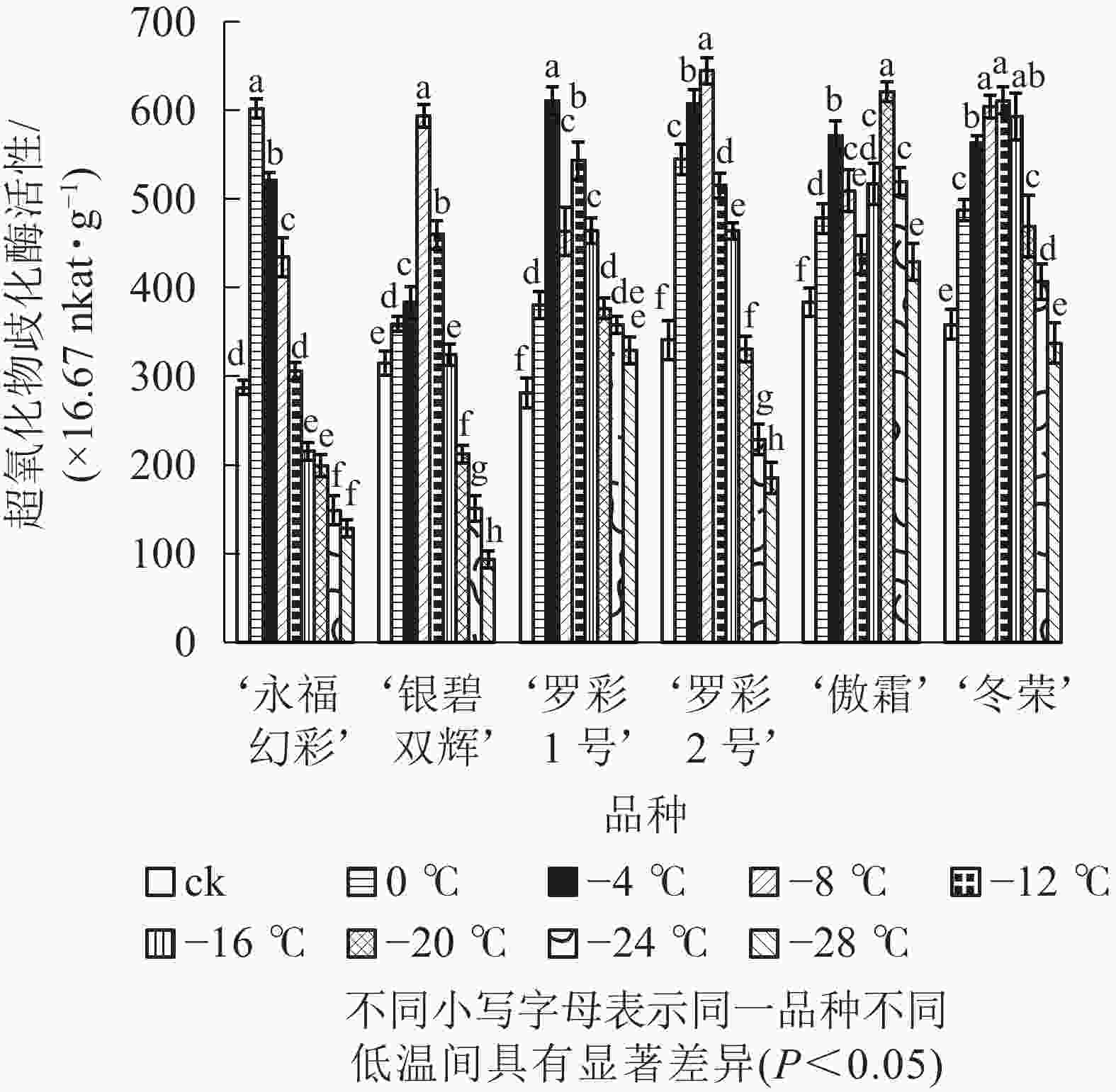
图5显示:随着低温胁迫强度增加,各品种超氧化物歧化酶活性变化趋势大体分“单峰”和“双峰”2种形式,但不同品种的峰值所对应的温度和增长幅度不同。‘罗彩1号’ ‘傲霜’叶片中超氧化物歧化酶活性呈现出“升—降—升—降”的双峰曲线。这2个品种第1个峰值所对应的温度均为−4 ℃,第2个峰值对应的温度分别为−12和−20 ℃;其余各品种均呈先上升后下降的单峰曲线,其中‘冬荣’峰值所对应的温度最低,为−12 ℃,‘永福幻彩’峰值所对应的温度较高,为0 ℃。各品种中,增幅最大的是‘罗彩1号’,是对照的2.17倍,增幅最小的是‘傲霜’,是对照的1.62倍。一般来说,低温胁迫会使植株超氧化物歧化酶活性提高,其增幅与植物自身耐寒能力高低具有正相关性。
图6显示:不同品种过氧化物酶活性随温度变化趋势也大致分为2种类型。‘永福幻彩’ ‘银碧双辉’大致呈“上升—下降”的趋势,两者达到最大值时临界温度均为−8 ℃,但增幅各不相同,峰值时的过氧化物酶活性分别是对照的3.03、4.69倍;‘罗彩1号’ ‘罗彩2号’ ‘傲霜’ ‘冬荣’过氧化物酶活性呈“下降—上升—下降”的趋势,在0 ℃时过氧化物酶活性均出现短暂下降,随后立即上升,分别在−4、−8、−4、−12 ℃时达到最大值,此时过氧化物酶活性分别是对照的1.12、1.42、1.31、1.19、1.15倍;当温度进一步降低时(−12~−28 ℃),各品种过氧化物酶活性又出现大幅度下降。一般来说,过氧化物酶活性的上升幅度与植物的耐寒能力正相关。
-
采用Excel 2016,依据隶属函数计算公式得出6个品种各指标的隶属函数值,并取平均值,通过比较隶属函数平均值大小得出6个品种的抗寒性从强到弱依次为 ‘傲霜’ ‘冬荣’ ‘罗彩2号’ ‘罗彩1号’ ‘永福幻彩’ ‘银碧双辉’(表2)。
品种 隶属函数值 平均值 排序 电导率 超氧化物歧化酶 过氧化物酶 丙二醛 可溶性蛋白质 可溶性糖 ‘永福幻彩’ 0.461 6 0.401 8 0.285 2 0.607 8 0.288 8 0.316 2 0.393 6 5 ‘银碧双辉’ 0.417 5 0.410 7 0.156 9 0.586 0 0.222 7 0.487 1 0.380 2 6 ‘罗彩1号’ 0.577 2 0.575 3 0.539 3 0.326 5 0.292 5 0.586 1 0.482 8 4 ‘罗彩2号’ 0.544 4 0.585 1 0.579 4 0.362 4 0.412 6 0.636 4 0.520 1 3 ‘傲霜’ 0.599 3 0.693 6 0.537 3 0.700 8 0.406 7 0.593 1 0.588 5 1 ‘冬荣’ 0.563 2 0.687 3 0.558 2 0.697 0 0.390 3 0.618 5 0.585 8 2 Table 2. Comprehensive evaluation of cold resistance of six cultivars of O. fragrans
2.1. 低温胁迫对 6个彩叶桂品种叶片相对电导率的影响
2.2. 低温胁迫对 6个彩叶桂品种叶片丙二醛质量摩尔浓度的影响
2.3. 低温胁迫对 6个彩叶桂品种叶片渗透调节物质的影响
2.4. 低温胁迫对6个彩叶桂品种叶片保护酶活性的影响
2.5. 隶属函数法综合评价6个彩叶桂品种的抗寒性
-
植物组织的相对电导率变化能够在一定程度上反映细胞膜的受损情况,是检测植物耐寒性的较为快速和准确的指标[11]。在植物抗寒性评价中常采用电导法,并与 Logistic 方程结合即能够较简便地筛选出抗性较强的品种[10]。本研究得出:各品种叶片相对电导率随温度的降低而不断增大,总体呈“S”曲线增长,不同品种的变化幅度有所差异,与桂花[12]、苹果Malus pumila[13]、柑橘Citrus reticulata[14]、绣线菊Spiraea salicifolia[15]、桢楠Phoebe zhennan[16]等植物的结果一致。将6个彩叶桂品种相对电导率拟合Logistic方程计算得到各品种的半致死温度并进行抗寒性排序,结果与隶属函数法进行抗寒性综合评价的结果相同,可见通过半致死温度来判断此6个彩叶桂品种抗寒性是可靠的。这在木瓜属Pseudocydonia[17]、卫矛属Euonymus[11]等植物中也得到了验证。
逆境胁迫下,丙二醛质量摩尔浓度明显升高[18]。本研究发现:随着低温胁迫程度的加重,6个彩叶桂品种叶片丙二醛质量摩尔浓度呈现先上升后下降再上升的趋势,并且在降温初始阶段就开始迅速上升,这与相思树Acacia[19]的结果一致。说明突然低温使膜脂发生过氧化,膜系统受到损伤。随着温度进一步降低,丙二醛质量摩尔浓度出现一定的下降。可见适宜的低温锻炼能够维持丙二醛质量摩尔浓度的相对稳定,缓解膜系统遭受的损害,推测此时植物体通过调动自身的调节机制如增加抗氧化酶的活性等来缓解损伤。对照超氧化物歧化酶活性变化情况来看,此时叶片的超氧化物歧化酶活性处于较高水平,这也可以证明此推测。随着低温胁迫的加深,叶片内丙二醛质量摩尔浓度再次上升,表明彩叶桂叶片细胞膜脂过氧化程度随温度的进一步降低而逐渐加深,这时植株的受损程度已经较为严重,仅依靠自身已经很难调节。
低温环境中植物细胞会大量积累各类渗透调节物质(可溶性糖、可溶性蛋白质等),其增加幅度和积累速度与自身抗寒性密切相关,抗寒性越强的植物增幅越大[20-21]。在对彩叶桂叶片进行人工低温处理时,各品种可溶性糖、可溶性蛋白质质量分数总体上均表现出“升高—降低”的变化趋势,但增加幅度和拐点温度并不相同,这与北美冬青Ilex verticillata[18]、8种常绿阔叶树[22]的研究结果类似。推测在降温初期,植物体的渗透调节机制被诱发,植物能够合成更多的可溶性糖、可溶性蛋白质,以降低细胞渗透势,改善自身抗寒能力;温度进一步降低时,该防御机制受到影响,叶片渗透调节物质含量开始减少。总的来说,各彩叶桂品种对于低温胁迫造成的伤害均具备一定的防御能力,它们在低温胁迫下可以合成更多的可溶性糖和可溶性蛋白质等渗透调节物质,通过提高细胞液浓度,维持细胞内外渗透压的平衡,以应对低温胁迫造成的伤害,但在不同品种间这种防御能力有所差异。
超氧化物歧化酶与过氧化物酶是植物体内重要的抗氧化酶。植物遭受低温胁迫时,抗氧化酶活性上升[23-25]。本研究中随着低温胁迫强度的增加,各品种间超氧化物歧化酶和过氧化物酶活性的变化有所不同。‘罗彩2号’ ‘冬荣’的超氧化物歧化酶活性及‘永福幻彩’ ‘银碧双辉’超氧化物歧化酶与过氧化物酶活性均呈先上升后下降的趋势,但不同彩叶桂品种超氧化物歧化酶、过氧化物酶活性最大值所对应的温度和增长的幅度各不相同。在降温初期,2种酶活性均有一定程度的增加趋势,可见植株对初期微弱的低温即产生了一定的防御能力来缓解损伤的速度和程度,证明了超氧化物歧化酶、过氧化物酶具有保护植物抵御低温胁迫的功能。随着温度的继续降低,胁迫进一步加深,即超氧阴离子自由基大量累积,超出了植物自身防御系统的清除能力,而且保护酶系统被破坏,超氧化物歧化酶、过氧化物酶活性急剧下降。本研究结果与苹果砧木[26]、野生柑橘[27]、黄连木Pistacia chinensis和黄山栾树Koelreuteria integrifoliola[28]的结果一致。‘罗彩1号’ ‘傲霜’叶片中超氧化物歧化酶活性表现出“上升—下降—再上升—再下降”的趋势,中间出现波动的原因可能是随着温度的进一步降低,植物的防御机制受到轻微破坏,体内超氧化物歧化酶活性出现降低,自由基大量积累,叶片受害加深。随着胁迫的加深,超氧化物歧化酶活性有回升的趋势,但很快到达保护极限,之后再次下降。本研究结果与葡萄Vitis vinifera[29]、杨桃Averrhoa carambola[30]的研究结果一致。‘罗彩1号’‘罗彩2号’‘傲霜’‘冬荣’过氧化物酶活性呈“下降—上升—下降”的趋势,在降温初期过氧化物酶活性即出现降低的原因可能是低温胁迫已经对植株产生了轻微的损伤,当温度继续下降时(0~−12 ℃),植株过氧化物酶活性有一定回升,说明植物体能够借助自身的调节来减轻逆境所造成的伤害。随着温度的进一步下降(−12~−28 ℃),低温胁迫对植物的损伤已经超越了植物体自我调节能力的限度,过氧化物酶活性便再次下降。本研究结果与葡萄[31]、多脉含笑Michelia coriacea[32]的结果一致。
综上所述,低温胁迫条件下,6个彩叶桂品种叶片生理生化指标发生了明显的变化。据其变化趋势推测彩叶桂应对低温胁迫的作用机制主要为:一是通过合成和积累渗透调节物质来提高细胞内的渗透势,二是通过抗氧化酶清除逆境下过多的活性氧自由基。隶属函数法[10]可以克服单一生理指标的片面性,能够更加全面地反映植物的实际抗寒性,得到更为准确可靠的评价结果。目前,隶属函数法已经在毛桃Amygdalus persica[33]、枇杷Eriobotrya japonica[34]、非洲菊Gerbera jamesonii[35]等植物的抗寒性研究上广泛应用。采用隶属函数法综合评价6个彩叶桂品种的抗寒能力,强弱顺序依次为‘傲霜’ ‘冬荣’ ‘罗彩2号’ ‘罗彩1号’ ‘永福幻彩’ ‘银碧双辉’。这与各品种田间越冬表现观察结果较为一致,其中‘傲霜’‘冬荣’‘罗彩2号’这3个品种较能适应北方气候。











 DownLoad:
DownLoad: