-
气候变暖加剧了全球范围内干旱程度[1]。CHOAT等[2]基于全球尺度对植物水力性状进行的Meta分析表明:所有的森林群落都易受到水力破坏的影响。然而,以往的研究主要集中于干旱区和半干旱区,对湿润地区植物的研究较少。不同环境中植物木质部性状的变异和性状权衡与组合的不同,反映植物个体及其群落获取环境资源方式的不同,因此植物采取的生活策略也存在差异[3]。植物生理生态功能,如光合速率、木质部输水效率和栓塞抗性,与木质部结构密切相关[4-9]。对植物生理生态变化与木质部解剖结构在不同环境的变异规律进行研究,有助于理解植物响应气候变化的机制和物种分布的限制。植物的生长受木质部水力效率与栓塞抗性权衡的制约[10]。对于裸子植物来说,木质部管胞很大程度上决定了水力效率[11],但是单位面积木质部管胞直径越大,数量越少,就更易发生水力障碍。此外,管胞壁上的纹孔膜面积也会随着管胞直径的增加而增加,进一步降低植物的栓塞抗性[12]。因此“安全”的水力系统通常以降低水力效率为代价[13]。然而,关于植物对水力效率和栓塞抗性适应机制的研究仍存在争议。早期的植物干旱敏感性研究发现:木质部性状反映了植物水分利用上的效率-安全权衡[14]。木本植物不存在效率-安全权衡或存在较弱的权衡[15],大多数物种在不同生境和生理状态下表现出低效率和低安全性,这与木质部性状间的权衡相矛盾[15-17]。木质部效率-安全权衡可能与一系列甚至单个解剖性状的权衡有关[18]。然而,木质部在不考虑效率-安全权衡情况下的结构-功能关系尚未得到研究。权衡可能存在与木质部安全、水力效率和机械强度之间[19-20]。LENS等[18]研究表明:许多物种的平均栓塞抗性与木质部密度(Dw)和管道厚度跨度比(Ttob)密切相关[21-22]。这3种特性之间似乎存在复杂的权衡[23],而且没有一个有说服力的结果来解释相当数量的低效率和低安全性物种,是理解植物木质部进化时的挑战[15]。此外,在干旱胁迫下,植物的枝和根都会采取各自的适应策略。有研究发现:植物的叶比枝条更容易发生栓塞现象[24-25]。然而,一些对潮湿生境中生长的木本植物的研究表明:叶和枝的栓塞抗性几乎没有差别[26],甚至叶片栓塞抗性比枝更强[27-28]。然而,WU等[29]研究发现:欧亚槭Acer pseudoplatanus、栓皮槭A. campestre、欧榛Corylus avellana根系的栓塞抗性大于枝条。枝的栓塞抗性低于根可能是植物对干旱胁迫的适应策略,通过保留根部的完整性来优化复水后的栓塞修复能力。相较于生长在干旱环境中的植物,北美红杉Sequoia sempervirens和落羽杉属Taxodium植物的木质部栓塞抗性可能较弱,因此在全球变暖导致干旱强度和频度增加的背景下,湿润地区植物可能会面临更大的威胁。本研究对杉科Taxodiaceae北美红杉、落羽杉Taxodium distichum、池杉T. distichum var. imbricatum的木质部水力功能、解剖结构性状和机械强度进行了测定,比较了3种植物枝/根木质部水力功能和解剖结构的差异,探讨了木质部水分运输、解剖结构和机械强度的定量关系,以期在器官水平和种内水平上,为评估木质部解剖学基础与木质部功能的权衡是否存在因果关系提供依据。
-
研究区位于浙江省西北部的天目山(30°18′30″~30°24′55″N,119°24′47″~119°28′27″E)。该区位于东南沿海丘陵山区,气候具有中亚热带向北亚热带过渡的特征,受海洋暖湿气流的影响,形成四季分明、雨水充沛、光照适宜且复杂多变的气候。年均气温为15.6 ℃,年均降水量为1 420 mm,月平均气温最高为7月(24 ℃),最低为1月(3 ℃),无霜期为209~235 d,年雨日为159.2~183.1 d,年均相对湿度为76%~81%,年日照时数为1 550~2 000 h,年太阳辐射为3 270~4 460 MJ·m−2。
-
于2021年2月,选取生长状况良好的北美红杉、落羽杉和池杉,各选择树高、胸径和冠幅相近的15株植物,具体见表1。8:00—11:00采集目标植株向阳部分健康生长且有充分光照的枝条2~3根,枝条长度大于1 m,长枝条上有1、2年生的小枝可用于测量导水率,小枝长为10~20 cm,直径为4~6 mm。在对应方位树冠下,10~20 cm土层小心挖取直径为4~6 mm、长为20~40 cm的根1~2根,尽量不损伤根尖和根毛。将已取的枝条和根迅速放入装有湿纸巾的黑色塑料袋中,防止水分流失和外部空气进入已经切开的管胞,立即带回实验室。木质部水力功能、解剖结构性状和机械强度指标测定来自于同一物种15个不同的个体,共45株植物。
物种 海拔/m 坡度/(°) 树龄/a 树高/m 胸径/cm 冠幅/m 北美红杉 40.43±0.14 0.62±0.07 20.79±0.64 17.21±0.46 18.14±0.31 2.70±0.09 落羽杉 40.42±0.12 0.48±0.03 16.89±0.31 23.26±0.48 16.73±0.47 2.45±0.09 池杉 42.33±0.51 1.83±0.06 17.28±0.54 23.78±0.34 17.44±0.46 2.61±0.09 说明:数值为平均值±标准误 Table 1. Overview of trees and their habitat
-
了解植物水力功能性状的变化,有助于探索不同植物在气候变化背景下的适应性与竞争力[30]。比导率(Ks)和栓塞抗性(P50)是评价木质部水分运输的2个重要水力功能性状。Ks为木质部单位边材面积导水率,最直接地反映物种的输水效率,P50为导水损失率达50%时的水势,代表着物种的栓塞抗性。脆弱性曲线描述了不同水势梯度下导水率损失比例。不同的植物栓塞抗性差异很大,即使在相同环境下生长的近缘物种,栓塞抗性也存在很大差异。
首先将采回的样品放入水中暗适应60 min,在水下再次剪取,将切口修平,用电子游标卡尺测量所剪枝条或者根段的长度和直径。茎段平均长度为162.43 mm (标准误为2.94 mm),平均直径为3.57 mm (标准误为0.06 mm);根段平均长度为170.03 mm (标准误为3.80 mm),平均直径为3.79 mm (标准误为0.09 mm)。然后将样品置于压力腔中且两端露出,压力腔上连接压力表,样品近轴端与木质部导水率与栓塞测量仪(XYL’EM-Plus, Bronkhorst, Montigny-les-Cormeilles, 法国)连接[31]。在高压(120 kPa)冲刷20 min,以确保消除原位栓塞,直至达到最大液压导水率(Khmax,kg·m·s−1·MPa−1)。冲刷溶液为20 mmol·L−1氯化钾+1 mmol·L−1氯化钙[32]。随后在低压(6 kPa)下测量枝条和根段的Khmax[33]。通过最大导水率除以边材横截面积再乘以样本长度,得到比边材导水率(简称比导率,Ks,kg·s−1·m−1·MPa−1),边材面积用显微镜(Leica DM3000, Leica, Wetzlar,德国)和图像分析软件(Image J)测量。采用空气注入法测量脆弱性曲线[34],在给定压力下施压5 min,以诱导栓塞的形成,然后测量相应的导水率(
$ {K}_{\mathrm{h}} $ , kg·m·s−1·MPa−1)。接下来以0.2或0.3 MPa(取决于不同的植物或器官)的增量重复此过程,直至导水损失率达90%以上。导水损失率(PLC)的计算公式为:所用压力与相应导水损失率之间的曲线即为样本的脆弱性曲线。使用R中fitplc包拟合导水损失率与水势之间的脆弱性曲线[35-36],并从曲线中提取相应的P50。
-
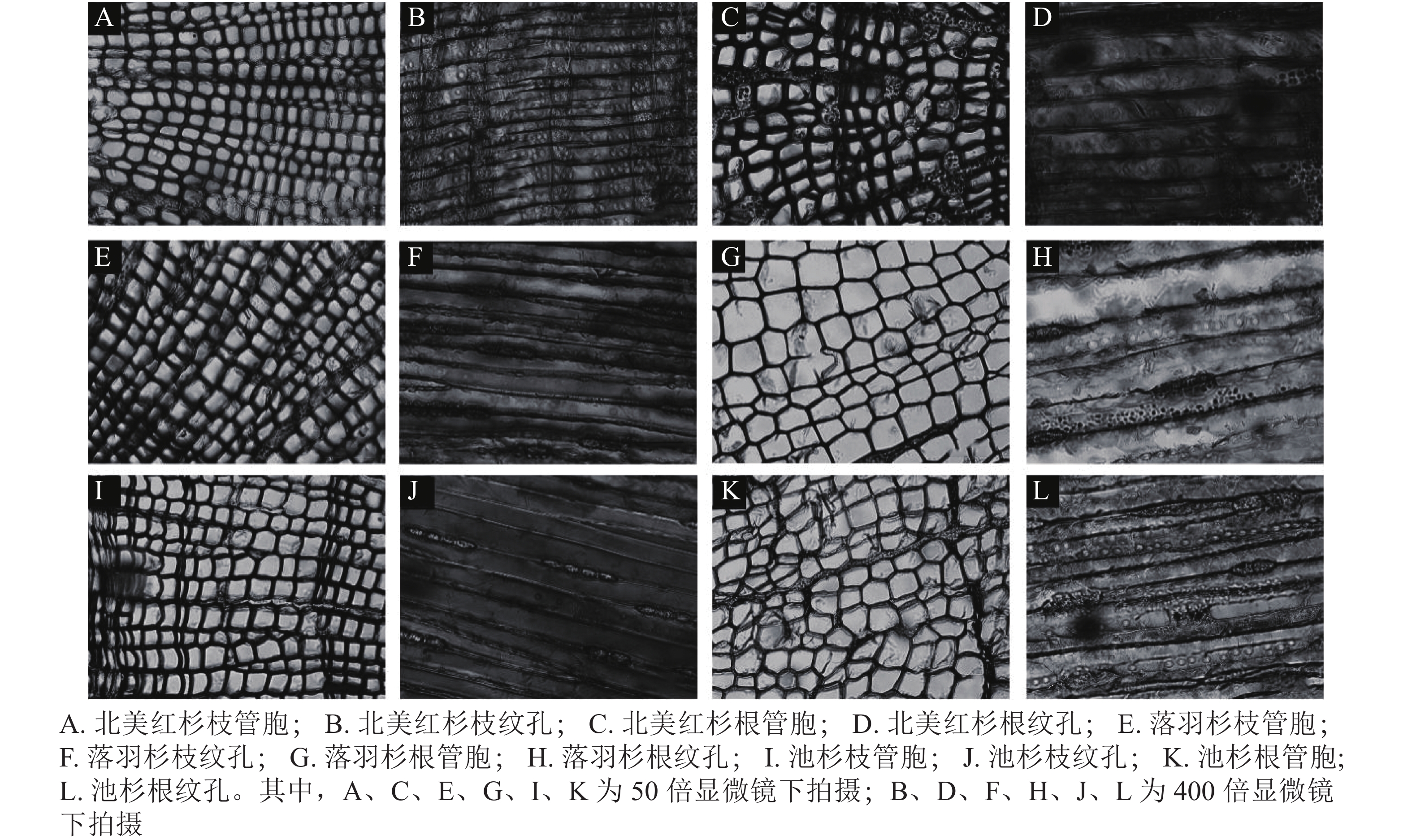
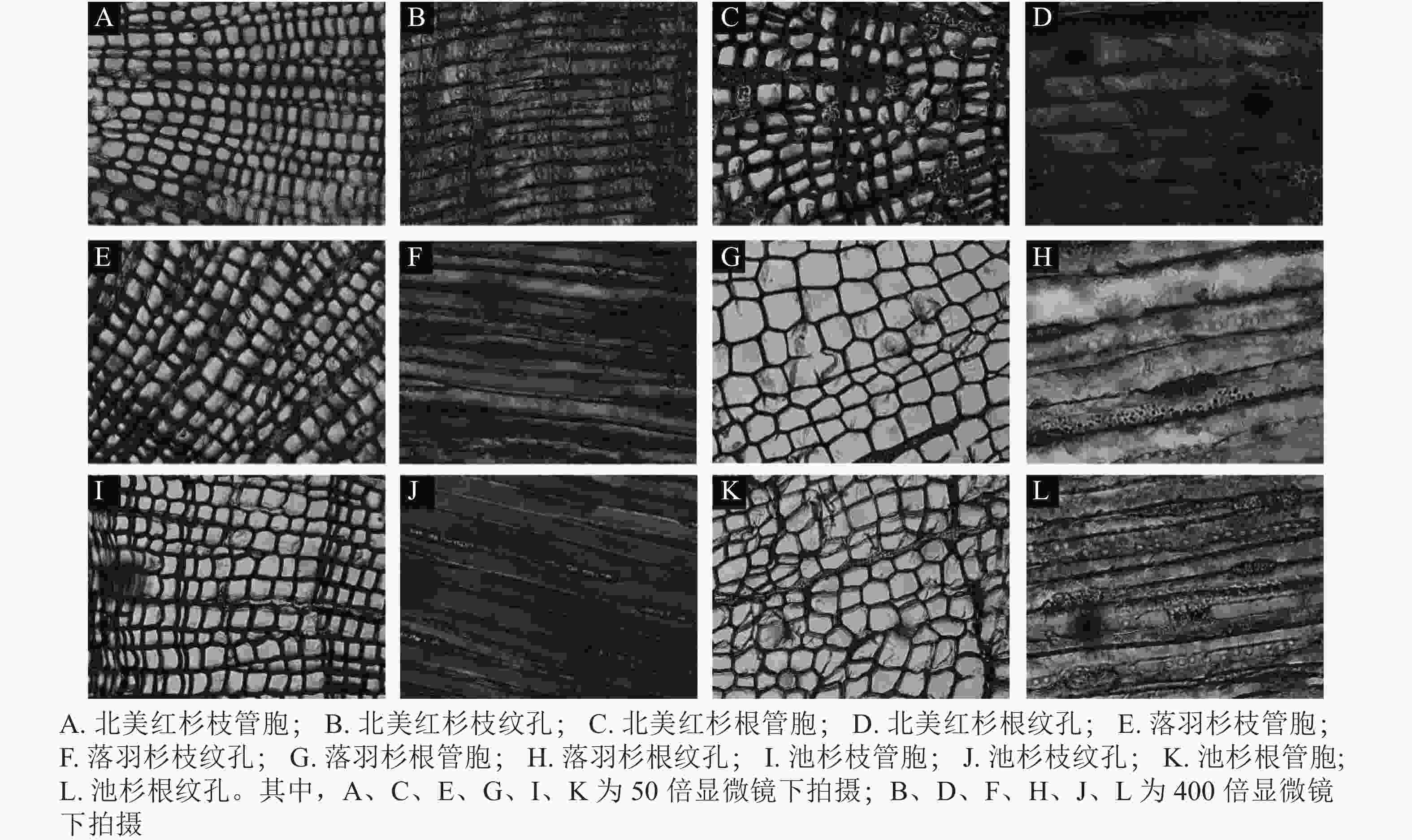
从用于测量脆弱性曲线的枝段或根段剪下2 cm长的木段,接着将其切成4小段(约0.5 cm),根段木质部解剖结构测定方法与枝条相同。将剪下的样品放入FAA固定液保存48 h,再将植物样品从固定液中取出制作成永久装片,使用Leica DM 3000显微镜拍照,分别在50和400倍镜下拍摄管胞结构和纹孔结构照片(图1)。使用Image J软件测量管胞直径(D,μm)[21]、管胞壁厚度(Tt,μm)、纹孔膜直径(Pd,μm)、纹孔口直径(Ad,μm)。计算水力直径(Dh,μm)、管胞密度(Nt,个·mm−2)、纹孔开口比(Rap)、纹孔密度(Npm,个·mm−2)[37]。水力直径计算公式为:
管胞密度计算公式:
式(3)中:nt为横切面上的管胞数量(个);St为横切面面积(mm2)。
纹孔开口比计算公式为:
纹孔密度计算公式为:
式(5)中:np为纵切面上的纹孔数量(个);Sv为纵切面面积(mm2)。
-
从用于测量脆弱性曲线的枝条上剪下3段3~5 cm长的木段,用于测量木质部密度。首先用排水法测量所剪木段的体积(V,cm3),接着将木段放入信封中并置于烘箱中,在75 ℃温度下烘干72 h,烘干后利用天平(精度0.000 1 g)称量木段干质量(M,g),最后计算木质部密度(Dw,g·cm−3)以及厚度跨度比(Ttob)。木质部密度计算公式为:
厚度跨度比计算公式为:
-
所有数据均在Excel 2019汇总整理。统计分析、图片制作在R 3.5 (version 3.5.3,R Core Team 2019)和Origin 2018中完成。在分析之前,取同株树个体的子样本平均值,在树的水平上进行重复。然后对这些数据进行Shapiro-Wilk正态性检验,必要时对其进行对数转换。
-
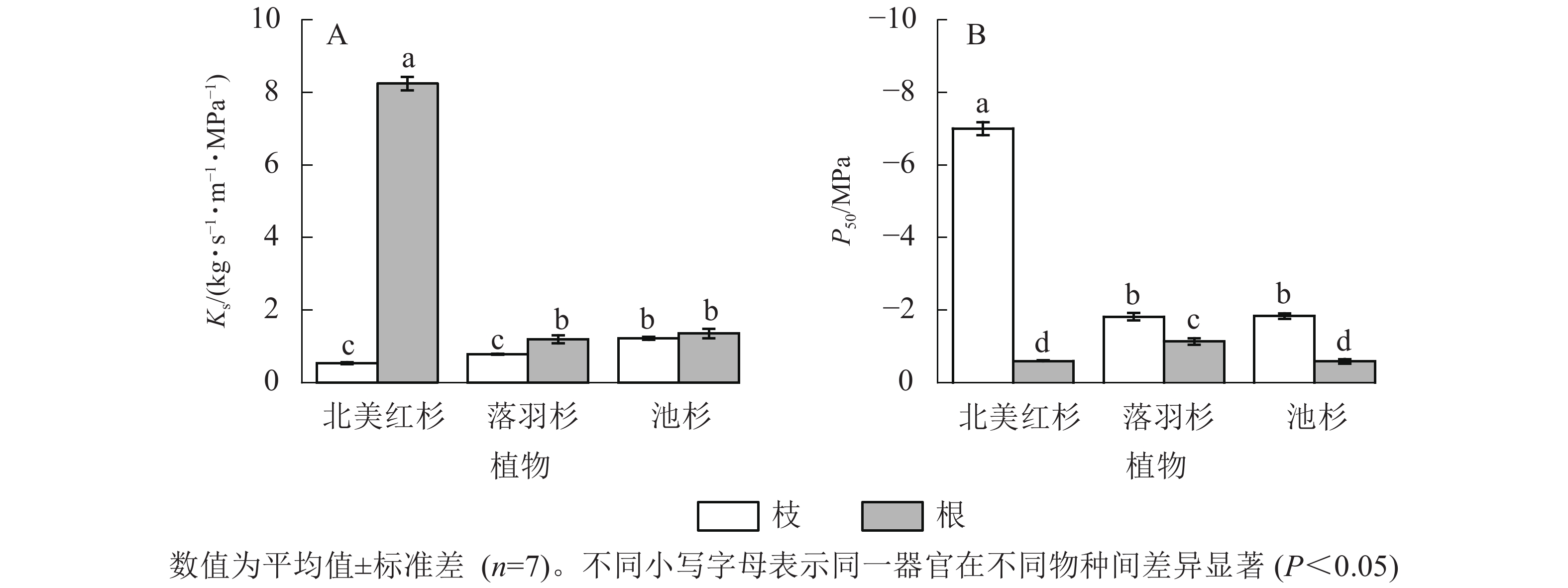
北美红杉、落羽杉和池杉枝的Ks分别为0.54、0.79和1.22 kg·s−1·m−1·MPa−1,根的Ks分别为8.25、1.19、1.36 kg·s−1·m−1·MPa−1,3种植物根的Ks均高于枝(图2A)。北美红杉、落羽杉和池杉枝的P50分别为−7.01、−1.82和−1.84 MPa,根的P50分别为−0.60、−0.14和−0.59 MPa,3种植物枝的P50均小于根(图2B)。
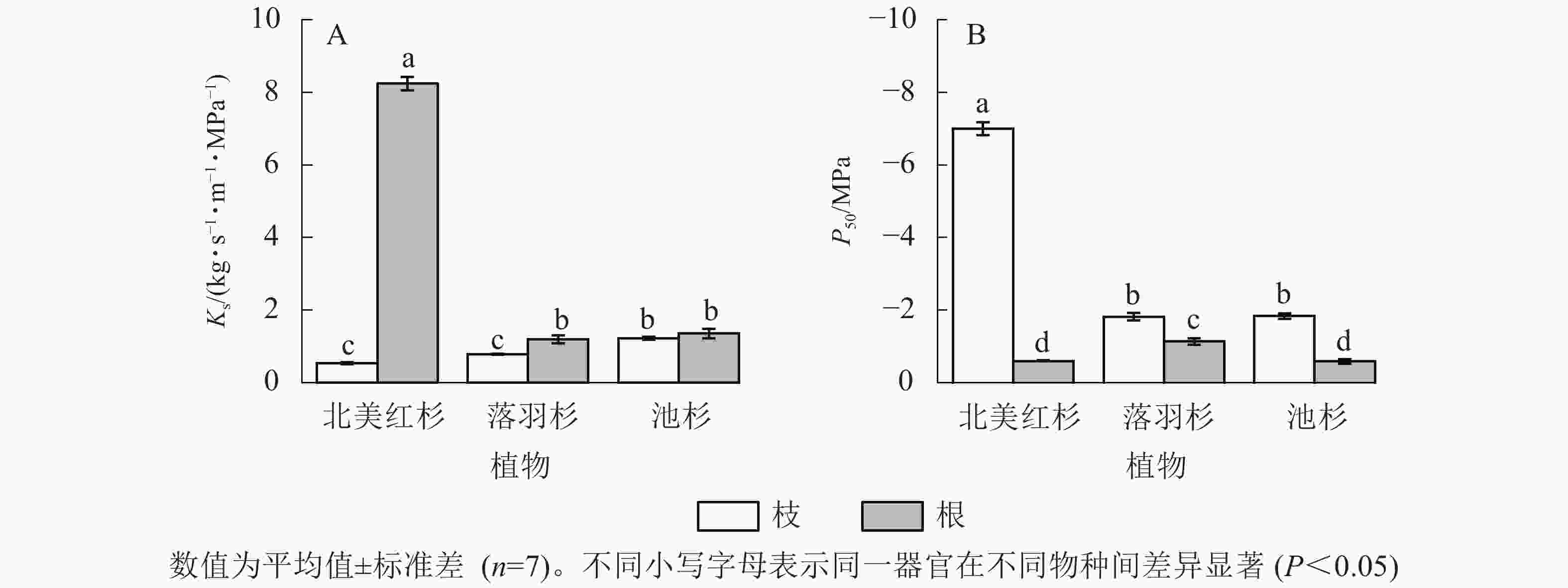
Figure 2. Specific sapwood conductivity (Ks) and cavitation resistance (P50) in the branches and roots
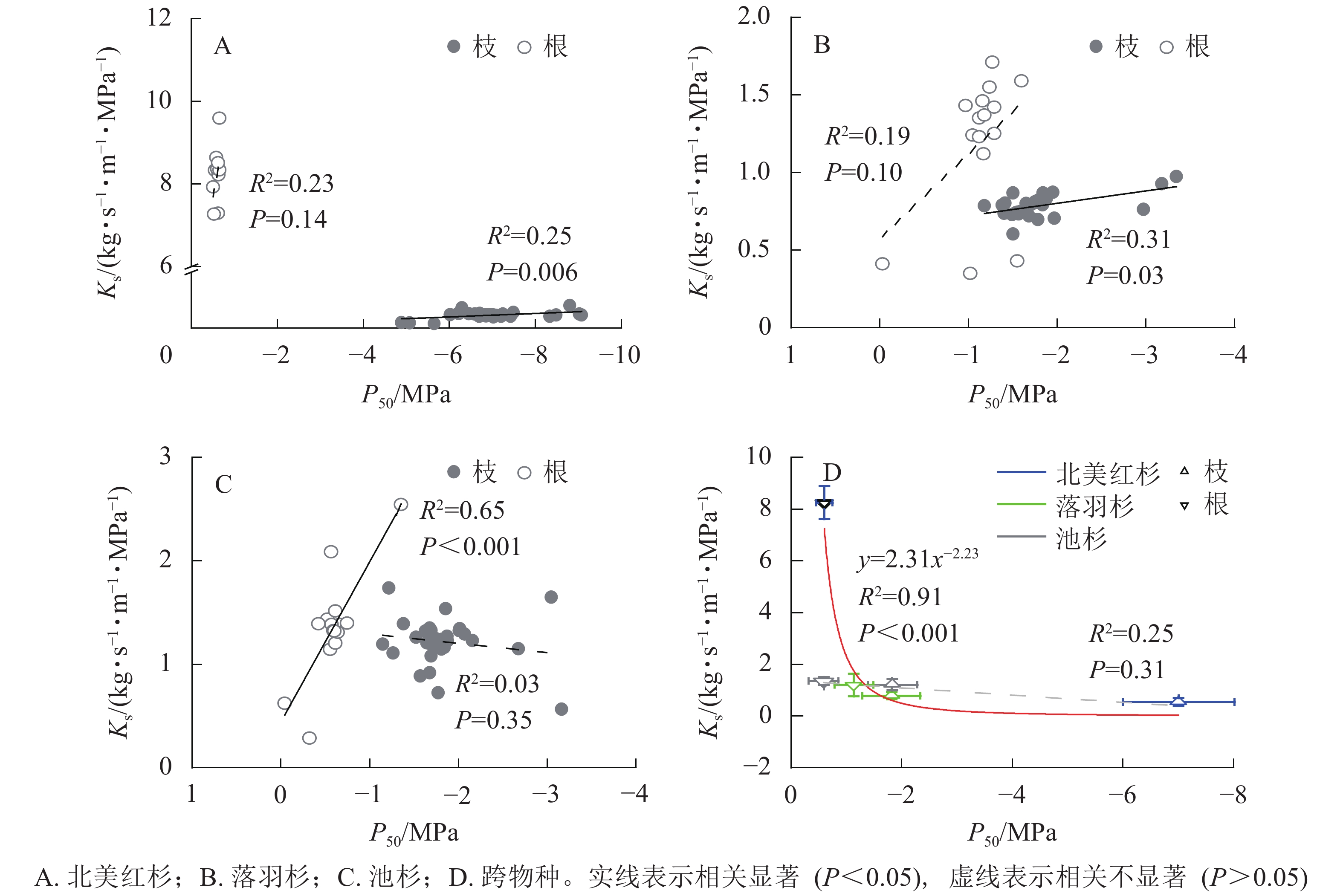
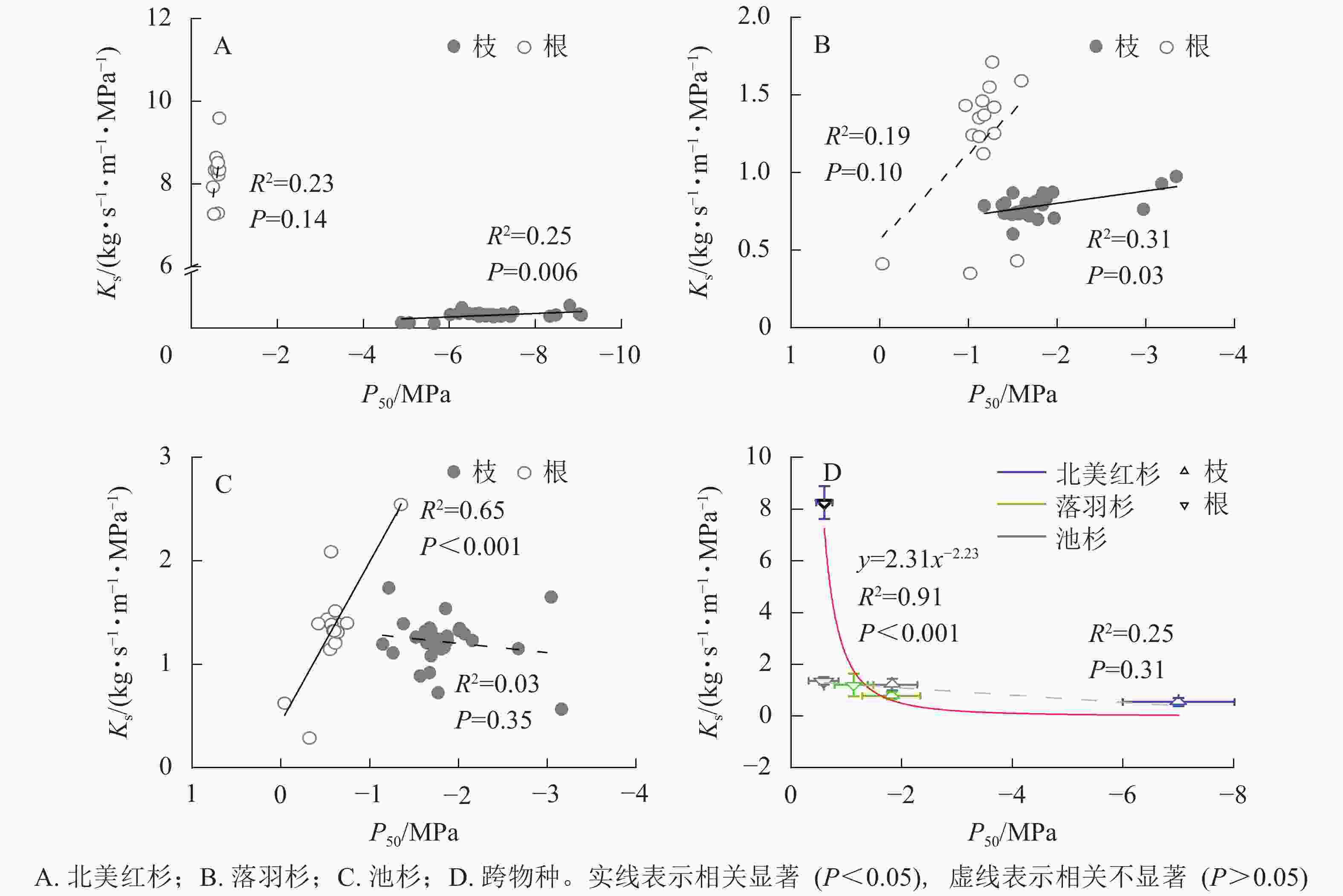
种内水平上,北美红杉枝的Ks和P50呈显著正相关(R2=0.25,P<0.01),根的Ks和P50呈正相关但不显著(R2=0.23,P=0.14,图3A);落羽杉枝的Ks和P50呈显著正相关(R2=0.31,P<0.01),根的Ks和P50呈正相关但不显著(R2=0.19,P=0.10,图3B);池杉枝的Ks和P50呈负相关但不显著(R2=0.03,P=0.35),根的Ks和P50呈显著正相关(R2=0.65,P<0.001,图3C)。结果表明:3种植物枝和根的木质部不存在效率-安全权衡。跨物种Ks与P50呈负相关,但差异不显著(R2=0.25,P=0.31,图3D)。通过幂函数拟合发现:两者存在显著的幂函数关系(R2=0.91,P<0.001),幂指数为−2.23 (95%的置信区间为−3.33~−1.14),说明在跨物种水平上,3种植物存在效率-安全权衡。
-
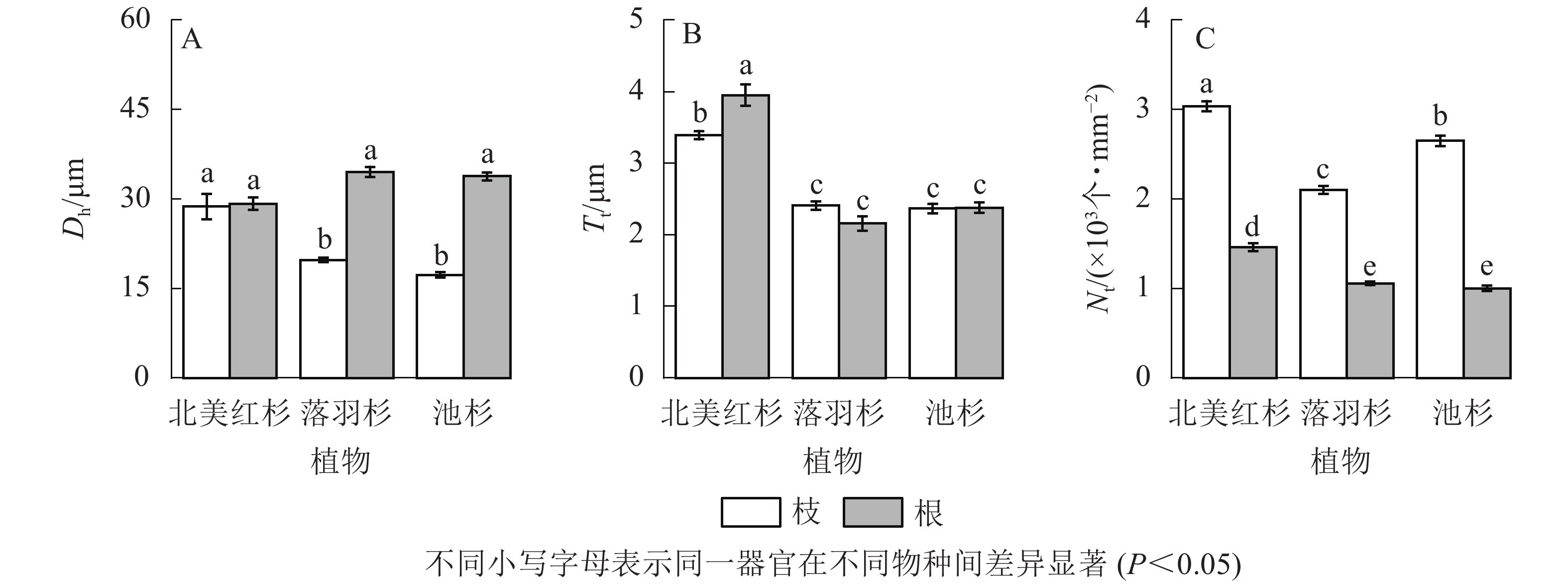
落羽杉和池杉根的水力直径(Dh)显著大于枝(P<0.05,图4A)。北美红杉根的管胞壁厚度(Tt)显著大于枝(P<0.05,图4B),北美红杉枝的Dh、Tt显著大于落羽杉和池杉(P<0.05,图4A和图4B),3种植物枝的管胞密度(Nt)均显著大于根(P<0.05,图4C)。北美红杉根的Tt、Nt显著大于落羽杉和池杉(P<0.05,图4B和图4C)。
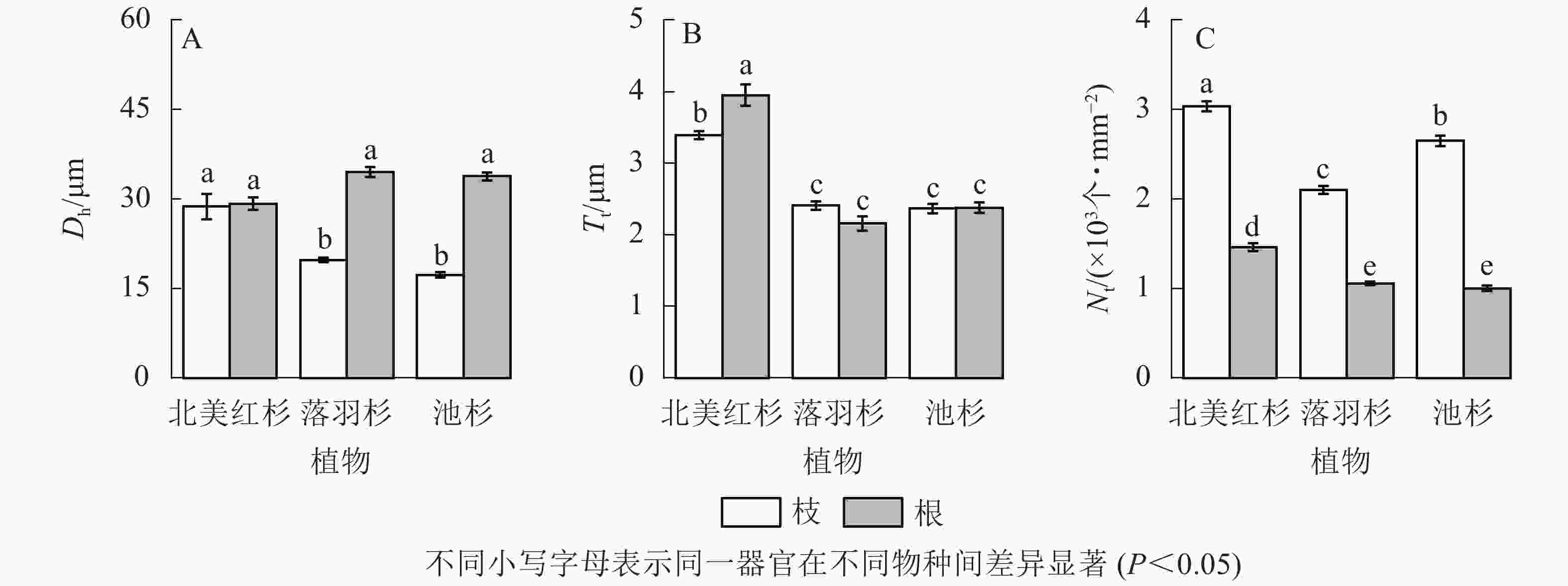
Figure 4. Hydraulic diameters (Dh), tracheid wall thickness (Tt) and tracheid density (Nt) of the branches and roots in the three species
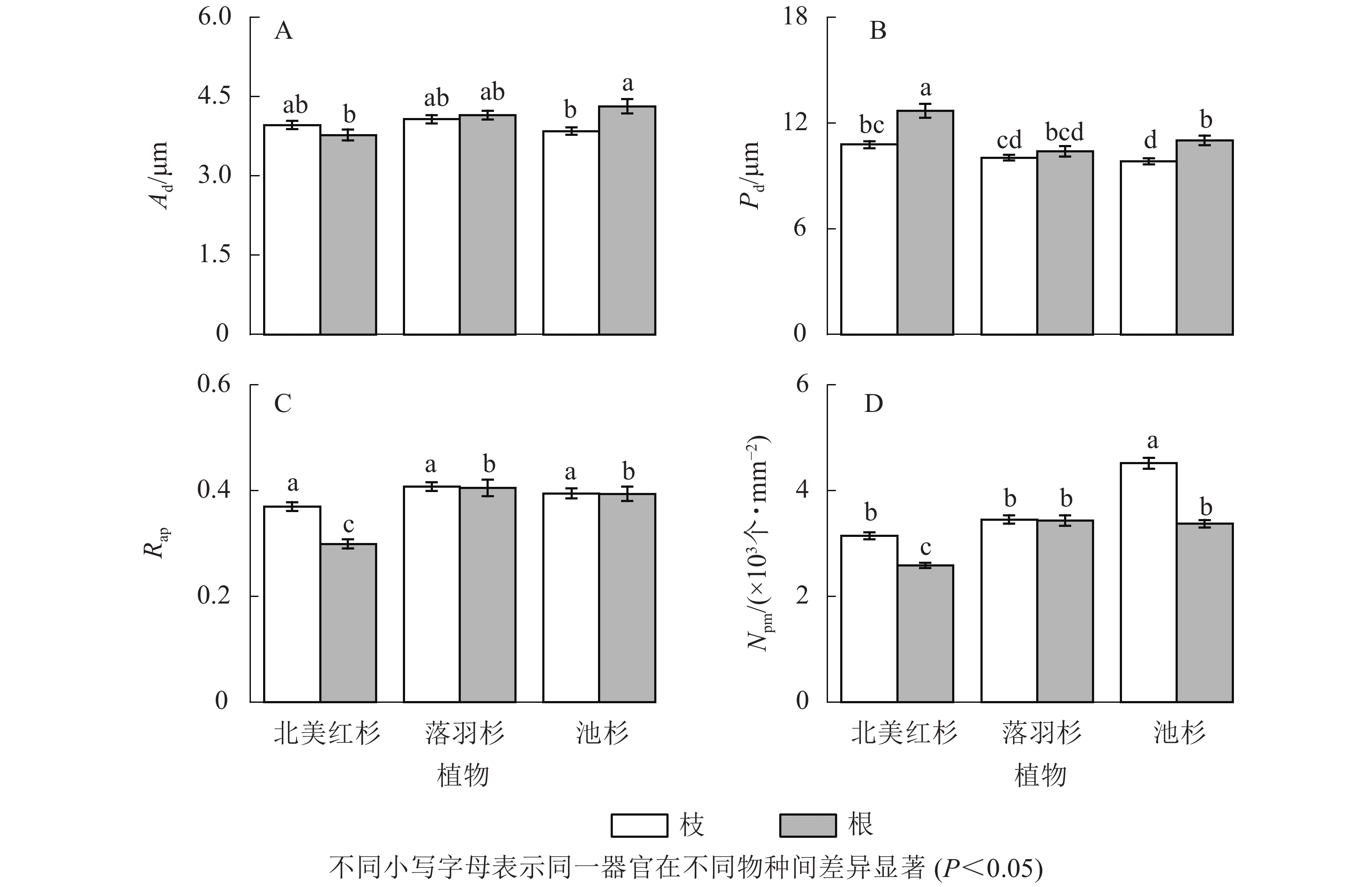
池杉根的纹孔口直径(Ad)显著大于枝(P<0.05,图5A),北美红杉和池杉根的纹孔膜直径(Pd)均显著大于枝(P<0.05,图5B),北美红杉根的Pd显著大于落羽杉和池杉(P<0.05,图5B),北美红杉枝的纹孔开口比(Rap)显著大于根(P<0.05,图5C)。池杉枝的纹孔密度(Npm)显著大于北美红杉和落羽杉,北美红杉和池杉枝的Npm显著大于根,落羽杉和池杉根的Npm显著大于北美红杉(P<0.05,图5D)。
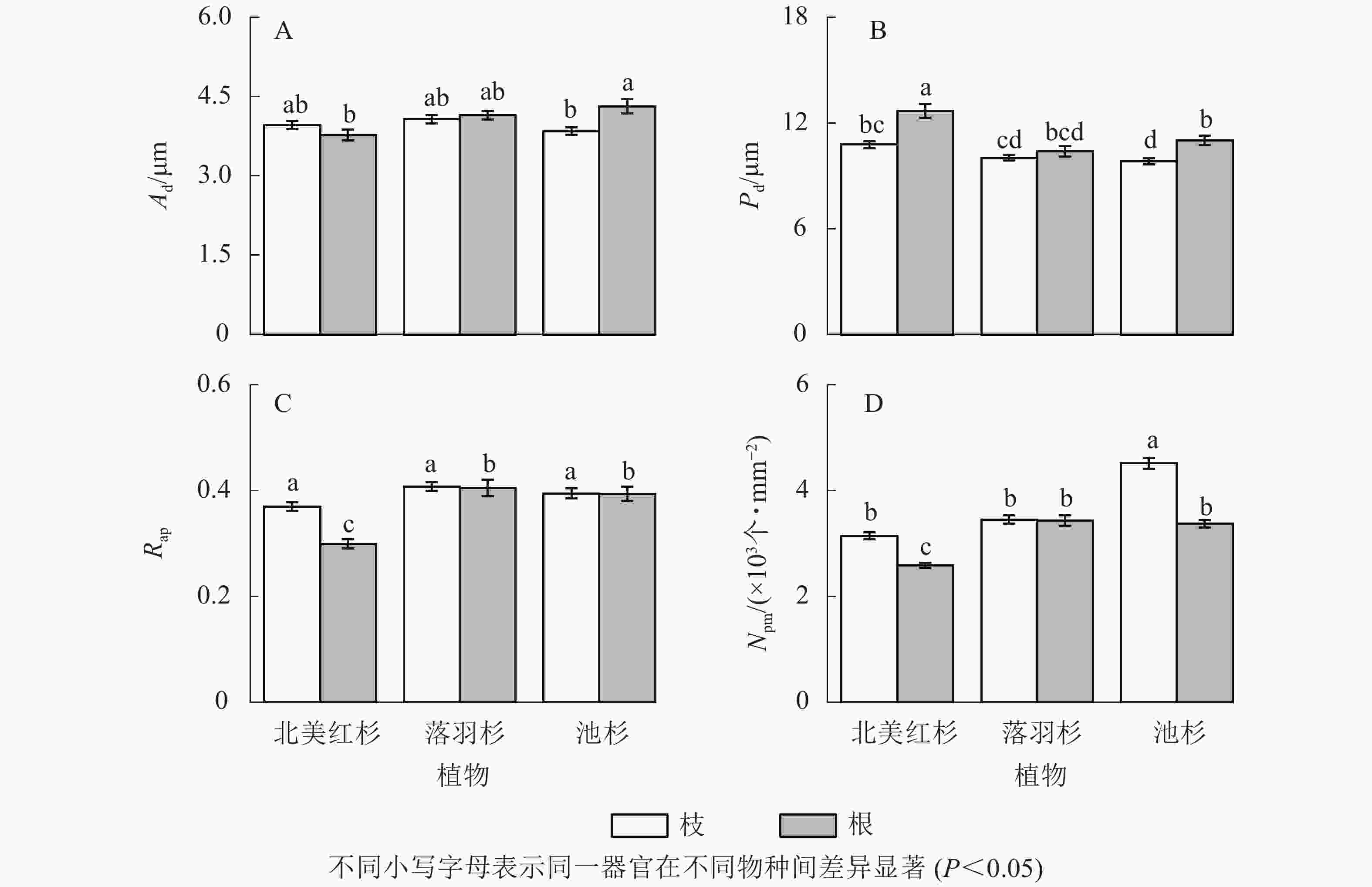
Figure 5. Pit aperture diameters (Ad), pit membrane diameters (Pd), aperture opening ratio (Rap), and pit density (Npm) of the branches and roots in three species
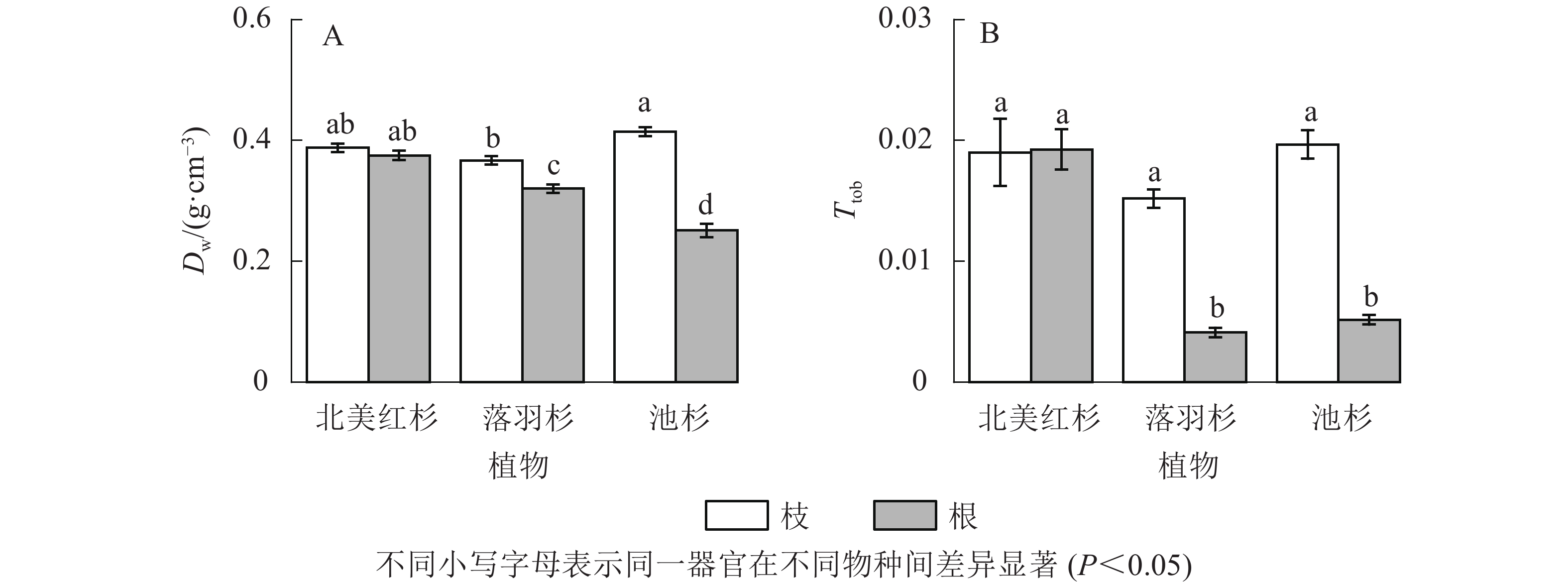
北美红杉枝与根的木质部密度(Dw)和厚度跨度比(Ttob)之间无显著差异(P>0.05),而落羽杉和池杉枝的Dw和Ttob均显著高于根(P<0.05,图6A和图6B)。在枝中,池杉枝的Dw最大,其次是北美红杉,落羽杉最低;3种植物枝的Ttob没有显著差异(P>0.05)。而在根中,北美红杉根的Dw最大,其次是落羽杉,池杉最低;北美红杉根的Ttob显著大于落羽杉和池杉(P<0.05),而落羽杉和池杉无显著差异(P>0.05)。
-
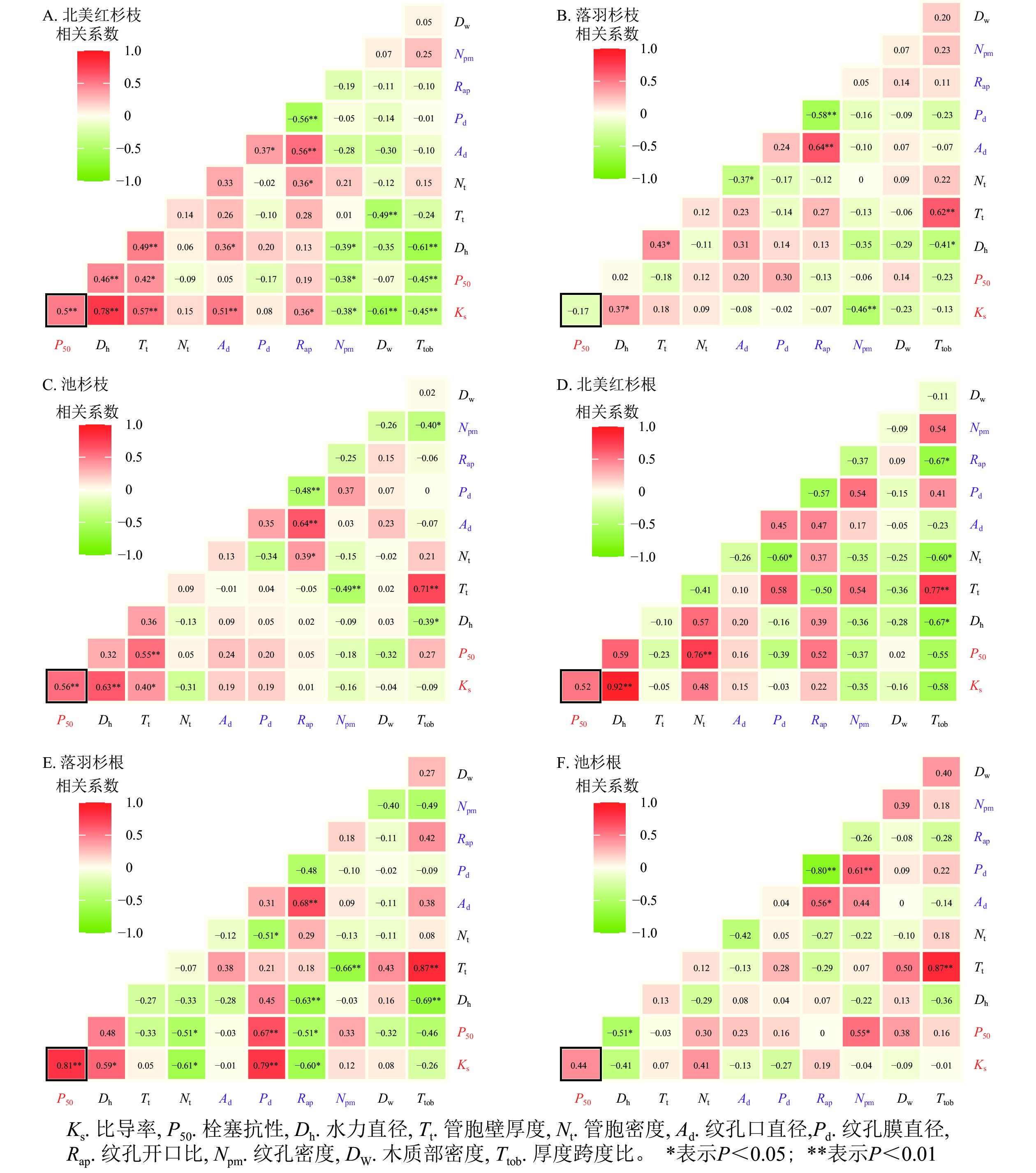
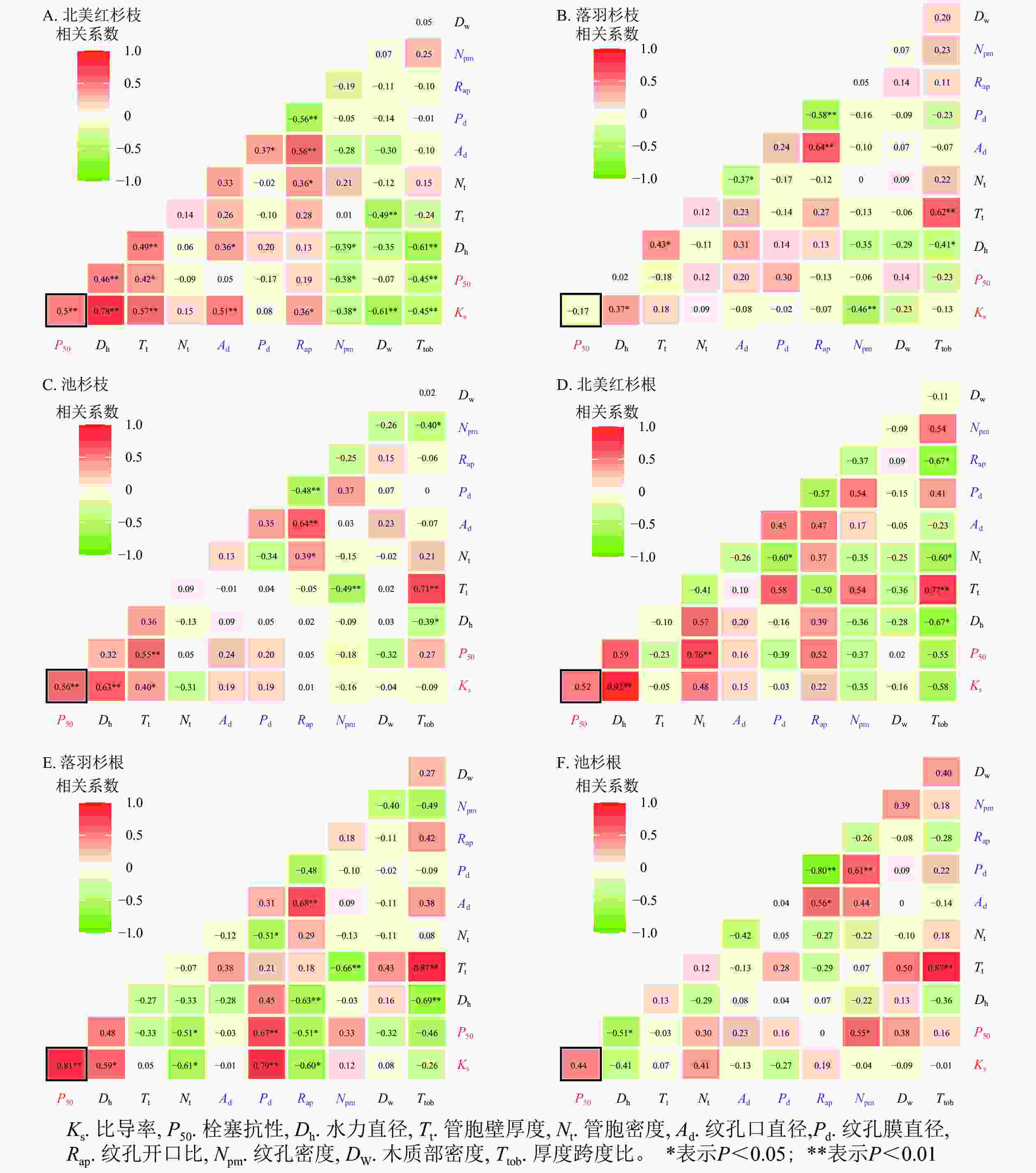
在种内水平上,北美红杉枝的Ks与Dh、Nt、Ad、Rap呈显著正相关(P<0.05),与Npm、Dw、Ttob呈显著负相关(P<0.05);枝的P50与Dh、Nt呈显著正相关(P<0.05),与Npm、Ttob呈显著负相关(P<0.05,图7A)。北美红杉根的Ks与Dh呈显著正相关(P<0.05),根的P50与Nt呈显著正相关(P<0.05,图7D)。落羽杉枝的Ks与Dh呈显著正相关,与Npm呈显著负相关(P<0.05,图7B)。落羽杉根的Ks与Dh、Pd呈显著正相关(P<0.05),与Nt、Rap呈显著负相关(P<0.05,图7E)。池杉枝的Ks与Dh、Tt呈显著正相关(P<0.05);P50与Tt呈显著正相关(P<0.05,图7C)。池杉根的P50与Npm呈显著正相关(P<0.05),与Dh呈显著负相关(P<0.05,图7F)。
-
本研究表明:3种植物根的导水率均高于枝条。根系吸收水分是土壤-植物-大气连续体的关键环节[38-39]。被子植物和裸子植物的根比枝更容易受到干旱的影响[13, 40],这与本研究结果一致。有研究表明:在干旱胁迫发生时,植物更倾向于增加地下生物量分配,发展根系,提高根冠比以增加抗旱性[41-42]。植物在严重干旱条件下保存根系的活力,提高了干旱胁迫过后的恢复能力[43]。生长在湿润地区的树木木质部栓塞抗性(尤其是根栓塞抗性)比干旱地区的树木弱,面对愈发严重、频繁的干旱,湿润地区的树木面临水力失效的风险更高。
-
本研究表明:3种植物枝/根均不存在效率-安全权衡,但是在跨物种间发现了权衡。GLEASON等[15]的研究发现了许多既低效又不安全的植物。与松科Pinaceae植物的Ks和P50相比[44],3种植物都属于低效率、低安全的物种。有研究表明:在管胞系统中构建冗余[45]或者新的木质部[15, 21],可以在栓塞积累的情况下维持叶片的水分供应。叶经济学光谱表明:一些物种具备“缓慢投资-收益”能力,即这些物种获取和利用资源的速度很慢,因此只需要低效率和低安全性[23-24, 46]。此外,大量研究表明:北美红杉和落羽杉属植物除了具有效率-安全权衡以外还有其他重要性状(如木质部的机械支持)的解剖学基础[15, 23],本研究结果也证实了这一观点。北美红杉枝的Ks与Dw呈显著负相关,根的Ks与厚度跨度比的相关系数为−0.55;落羽杉根的P50与厚度跨度比的相关系数为−0.46;池杉根的P50与木质部密度相关系数为0.38。这些结果表明:3种植物水力效率与机械强度之间存在着平衡,这与PRATT等[23]的研究结果一致。这是湿润区树种对水分充足生境适应的结果,因为水分充足,植物木质部输送水分安全性方面的威胁弱;反而高大的裸子植物其机械强度的需求在增加。此外,在跨物种水平上的幂函数曲线有助于解释本研究种内和其他研究中不存在效率-安全权衡的原因:幂函数的左端斜率为0,右端斜率为无穷大,因此,当所测量的数据处于幂函数两端时,效率-安全之间的权衡会因测量误差的微小变化而产生剧烈波动;只有当研究物种的Ks和P50处于幂函数曲线的中心部分,或所研究物种的Ks和P50变化范围很大时,这种权衡才会变得显著[47]。
一系列甚至单个解剖性状的权衡都有可能对效率-安全权衡进行解释。LENS等[18]研究了7种槭属Acer植物的解剖性状以及水力性状和解剖性状的相关关系网络发现:几乎所有测量到的解剖特征都是效率-安全权衡的因果基础。在本研究中,3种植物的枝和根都没有发现权衡关系。北美红杉枝的Ks和P50与Dh、Tt、Ad呈正相关,根的Ks和P50也随着Dh、Nt的增加呈升高的趋势。落羽杉根的Ks和P50与Dh、Pd有较强的正相关关系。池杉枝的Ks和P50也与Dh、Tt存在正相关关系。
由于导水率和栓塞抗性的结构需求不同,无法实现效率-安全权衡。这种对结构需求的独立性仅在本研究的3种植物中发现,然而,这种独立的物种特异性增加了对水力系统进化的理解[48]。
-
本研究表明:3种杉科植物不存在效率-安全权衡。效率-安全权衡不存在的原因是植物对高安全性和高效率的结构性需求不同,表明效率-安全权衡的结构基础并不存在。跨物种间输水效率与栓塞抗性存在的负幂函数关系或许存在于更多物种。效率、安全性和机械强度之间的权衡关系表明了木质部水力功能是由多种木质部共同决定的。为全面了解木质部导水系统,需要在整株水平(包括根、茎、枝、叶柄和叶)上进行更多的系统研究。
Relationships among water transport, mechanical strength and anatomical structure in branch and root xylem of Taxodiaceae species
doi: 10.11833/j.issn.2095-0756.20210248
- Received Date: 2021-03-28
- Rev Recd Date: 2021-09-10
- Available Online: 2022-03-25
- Publish Date: 2022-03-25
-
Key words:
- humid region /
- Sequoia sempervirens /
- Taxodium /
- hydraulic efficiency /
- cavitation resistance /
- efficiency-safety trade-off
Abstract:
| Citation: | CHEN Sen, LU Shitong, LI Yan, et al. Relationships among water transport, mechanical strength and anatomical structure in branch and root xylem of Taxodiaceae species[J]. Journal of Zhejiang A&F University, 2022, 39(2): 233-243. DOI: 10.11833/j.issn.2095-0756.20210248 |











 DownLoad:
DownLoad: