-
随着人们生活水平的提高,城市生活垃圾总量逐年增加,如何有效处理生活垃圾已成为人们关注的重点。目前生活垃圾的处理主要有卫生填埋、焚烧和堆肥等方式[1],其中垃圾焚烧可以极大地减少垃圾的原有体积,减少了对土地的占用。通过焚烧可以使垃圾的质量和体积分别减少70%和90%[2],同时也会产生原有垃圾质量20%~30%的炉渣[3]。目前炉渣可以与水泥混合制备混凝土[4],以及用来铺设路基[5]等。炉渣中成分复杂,属于强碱性物质,存在重金属含量超标等问题[6],虽然在碱性条件下比较稳定,重金属浸出含量较少[7],但具有潜在的浸出风险[8],直接处理有可能会对土壤环境造成潜在危害。同时炉渣在预处理过程中,如筛分、磁分选、水洗等,会产生一定量的炉渣泥[9]。炉渣泥中含有二氧化硅(SiO2)、氧化铝(Al2O3)、氧化铁(Fe2O3)、氧化钙(CaO)、氧化镁(MgO)、氧化钠(Na2O)等氧化物,这些物质恰好符合烧制陶粒的成分要求[10]。陶粒开始于1931年,后被成功烧制出页岩陶粒,并于1916年获得发明专利[11]。RILEY[12]认为:烧制陶粒的原料中,SiO2为48.0%~70.0%,Al2O3为8.0%~25.0%,助溶剂为4.5%~31.0%时,就可以制作出烧胀陶粒。随着固体废弃物产量的不断快速增长,人们开始使用固体废弃物替代部分黏土烧制陶粒。MI等[13]采用赤泥、粉煤灰和膨润土,经两级烧结工艺制备的高强度陶粒,具有抗压强度高,吸水率低,有害元素浸出含量少等性能。LI等[14]采用铁矿石尾矿、膨润土和铝土矿混合烧制出的陶粒,可用于制备轻质隔板。FAN等[15]使用铬(Cr)污染土壤烧制陶粒,铬元素在烧制过程中被固化,使其浸出含量远低于国家标准。因此,可以将炉渣泥与其他物质混合烧制陶粒,能够起到很好固定重金属的作用[16−17]。烧制陶粒除了原料成分配比外,烧制过程也是控制陶粒质量的关键因素[18],烧结温度过低过高都会对陶粒的孔隙率和强度造成影响,导致烧出的陶粒质量不理想[19−20]。只有合适的原料配比和烧制工艺同时作用,才可以烧出理想的陶粒。炉渣泥烧制成陶粒可以将固废资源化,能够在减少环境危害的同时创造一部分收益。本研究从垃圾焚烧炉渣中分出来颗粒较小的炉渣泥,通过与粉煤灰、石英粉和高岭土混合,在1 125~1 200 ℃下烧制5~20 min后得到陶粒,分析陶粒的基本性能以及陶粒的重金属浸出对环境的影响,以期为探索合理处置生活垃圾焚烧炉渣泥提供科学依据。
-
炉渣泥来自杭州市九峰垃圾焚烧厂,根据粒径大小从炉渣中分出颗粒更小的部分(100目以上)称为炉渣泥。炉渣泥与粉煤灰成分组成如表1所示。石英粉(分子式:SiO2,分析纯)、高岭土(分子式:Al2Si2O9H4 2H2O,化学纯)、粉煤灰(325目)购自龙泽环保科技有限公司,其他化学试剂药品均购自国药化学试剂有限公司。
材料 质量百分比/% SiO2 Al2O3 CaO Fe2O3 MgO Na2O 炉渣泥 13.70 6.46 53.61 7.39 2.73 1.57 粉煤灰 53.97 31.15 4.01 4.16 1.01 0.89 Table 1. Mass percentage of main components of slag sludge and fly ash
-
新鲜的炉渣泥pH为11.7,含水率为30.35%,有机质质量分数为14.00 g·kg−1,热灼减率为10.66%。对其进行自然风干,从中去除大颗粒的石子、碎玻璃、陶瓷碎片和未燃尽物质后,手工研磨,过100目方筛后,取适量放入烘箱中至质量不再变化。使用电感耦合等离子体发射光谱法和原子荧光法测定炉渣泥中多种重金属质量分数,具体操作按照HJ 781—2016、GB/T 22105.1—2008和GB/T 22105.2—2008标准执行。
-
陶粒的烧制主要分为原料混合、造粒、烘干、烧结、冷却等5个步骤[21]。首先将炉渣泥、石英粉、粉煤灰和高岭土,按质量比3∶3∶3∶1混合,手工搅拌至均匀。加入适量纯净水,将混合原料制作成直径为5~20 mm的球形颗粒[15]。在105 ℃下干燥6 h,将干燥后的球形颗粒放入马弗炉里烧结成陶粒。采用的烧制温度为1 125、1 150、1 175和1 200 ℃,烧制时间为5、10、15和20 min。为了防止陶粒受热不均匀而炸裂,在陶粒烧制前先进行预热处理,预热温度分别为300、400、500、600 ℃,预热时间分别为0、10、20、30 min。最后将烧制好的陶粒冷却至室温。试验设计如表2所示,在4个影响因素下共制备了16组陶粒样品。
样品编号 烧结温度/℃ 烧结时间/min 预热温度/℃ 预热时间/min 样品编号 烧结温度/℃ 烧结时间/min 预热温度/℃ 预热时间/min 1 1 125 15 400 20 9 1 200 15 300 20 2 1 150 15 400 20 10 1 200 15 400 20 3 1 175 15 400 20 11 1 200 15 500 20 4 1 200 15 400 20 12 1 200 15 600 20 5 1 200 5 400 20 13 1 200 15 400 0 6 1 200 10 400 20 14 1 200 15 400 10 7 1 200 15 400 20 15 1 200 15 400 20 8 1 200 20 400 20 16 1 200 15 400 30 Table 2. Ceramsite experimental design
-
根据GB/T 17431.2—2010《轻集料及其试验方法 第2部分:轻集料试验方法》对陶粒的堆积密度、表观密度和1 h吸水率进行测试。陶粒抗压强度测试采用万能力学试验机,以2 mm·min−1的位移速度均匀加荷,直到陶粒彻底压坏为止。
-
将烧制前的生陶粒和烧制后陶粒分别磨碎,置于扫描电子显微镜下观察拍照。
-
将烧制前后的陶粒分别磨碎,粉末放入X-射线衍射仪内测定炉渣泥的晶体成分。
-
由于炉渣泥中部分重金属超标,从环境保护方面考虑,对烧制好的陶粒进行重金属浸出测定,按照HJ/T 299—2007《固体废物 浸出毒性浸出方法 醋酸缓冲溶液法》标准进行,首先制备浸提剂(17.5 mL冰乙酸加纯水稀释到1 L,pH 2.64±0.05),液固质量比按照20∶1混合浸提。将烧杯放入温度为23 ℃的往返式振荡器中振荡18 h,振荡器转速为110 r·min−1。然后以4 000 r·min−1的转速离心5 min,取上清液过0.45 μm滤膜,放入4 ℃冰箱冷藏保存,最后使用电感耦合等离子体质谱仪(ICP-MS)进行测试。
-
以GB 4284—2018《农用污泥污染物控制标准》为判定依据,发现炉渣泥中共有2种重金属质量分数超标,分别为镉(Cd)和锌(Zn)。其中Cd质量分数超标严重,实际值为限定值的2.9倍,Zn质量分数略微超标,实际值为限定值的1.1倍(表3)。主要原因是生活垃圾中物种品类成分复杂,经过高温焚烧之后,垃圾体积减少,重金属大量富集到炉渣泥中,导致其质量分数增加。因此需要从源头控制重金属,可以通过适当增加生活垃圾在焚烧之前的分拣次数,优化分拣工艺流程,提高资源的回收利用,减少重金属在炉渣泥中的残留。
判定依据 质量分数/(mg·kg-1) Cr Cu Cd Pb As Hg Zn Ni 本研究 24.4 864 43.7 406 32.1 0.088 3360 72.5 GB 4284—2018 1000.0 1500 15.0 1000 75.0 15.000 3000 200.0 判定 达标 达标 超标 达标 达标 达标 超标 达标 Table 3. Chang of heavy metal content of slag sludge
-
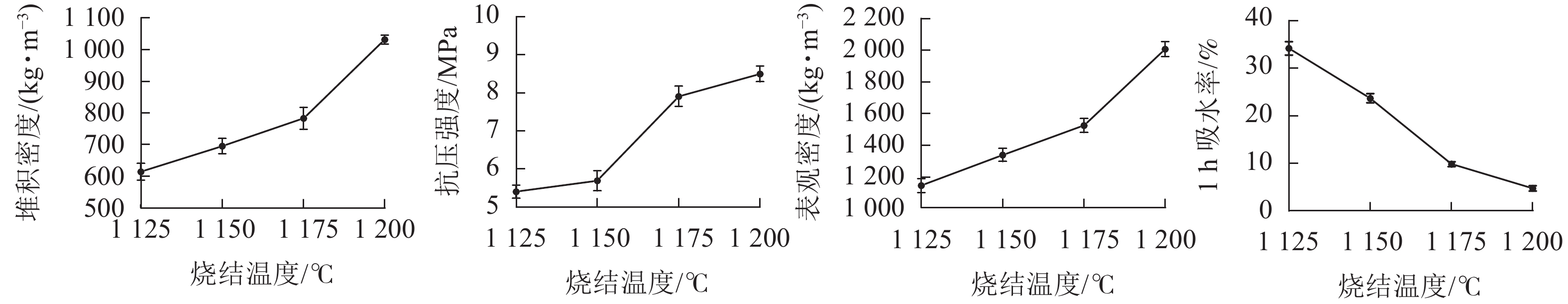
在预热温度为400 ℃,预热时间为20 min时,分别在1 125、1 150、1 175和1 200 ℃温度下烧结15 min制备了4种不同烧结温度的陶粒,测定陶粒的基本性质。从图1可见:随着烧结温度的升高,陶粒的抗压强度逐渐上升,在1 150 ℃之前抗压强度较小,且增长缓慢。当烧结温度从1 150 ℃上升到1 175 ℃时,陶粒抗压强度有较大的提高,而当烧结温度进一步由1 175 ℃提高1 200 ℃时,陶粒抗压强度变化较小,趋于稳定。说明陶粒在未达到最佳烧结温度之前,适当提高温度可以促进烧结[22]。陶粒的堆积密度、表观密度和1 h吸水率的重要转折点均在1 175 ℃。说明这个温度前后陶粒内部发生大量物理化学反应,较多釉质层在这时产生,填充了陶粒内部空隙,陶粒孔隙率有所下降,同时在液相的作用下,陶粒体积逐渐收缩,导致陶粒的堆积密度和抗压强度增加,吸水率下降[23]。所以最佳烧结温度为1 175 ℃。
-
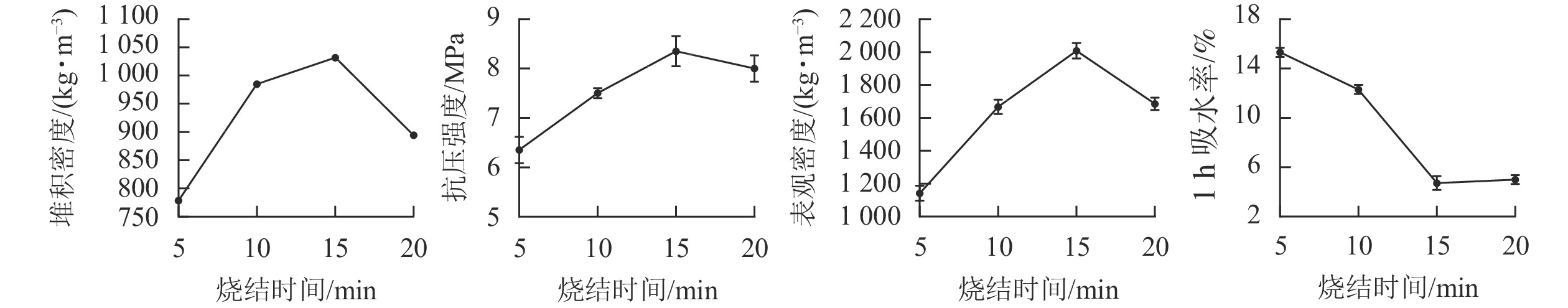
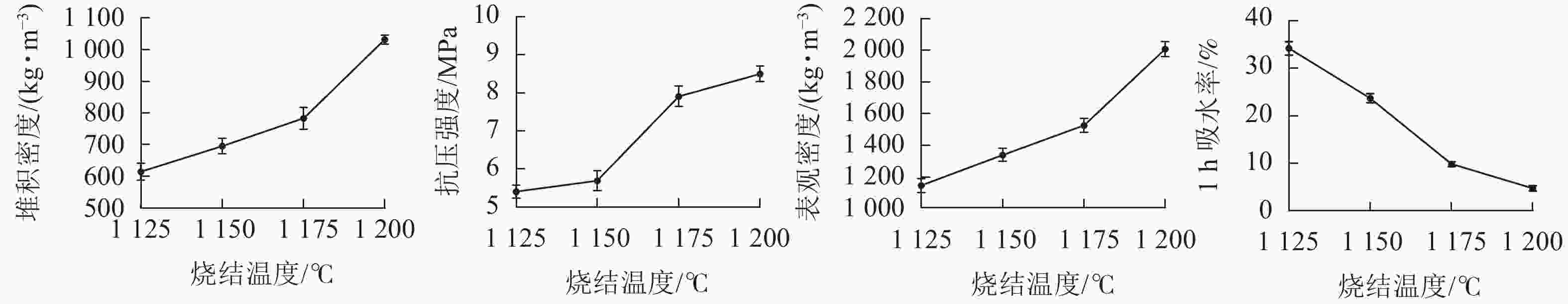
在预热温度为400 ℃,预热时间为20 min,烧结温度为1 200 ℃时,分别烧制5、10、15和20 min,制备4种不同烧结时间的陶粒。从图2可见:烧制5、10、15和20 min的陶粒抗压强度依次为6.35、7.50、8.35和8.00 MPa;堆积密度依次为778.5、985.0、1 031.7和894.4 kg·m−3;表观密度依次为1 141.4、1 666.7、2 007.2和1 685.0 kg·m−3。陶粒1 h的吸水率先降低后升高,其中在10~15 min的变化幅度最大,从12.3%降低至4.7%,烧结时间在15 min时,陶粒1 h吸水率达到了最低点符合国家标准中对建筑轻集料陶粒要求,所以最佳烧结时间为15 min。
-
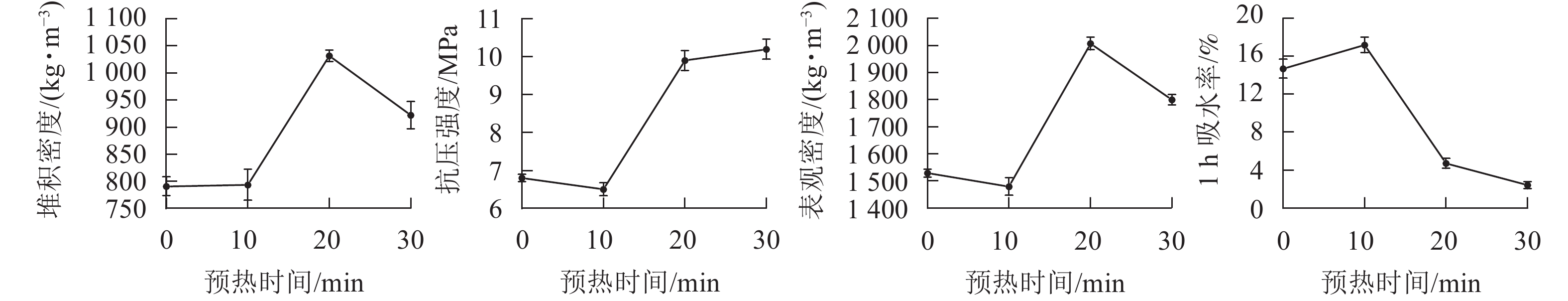
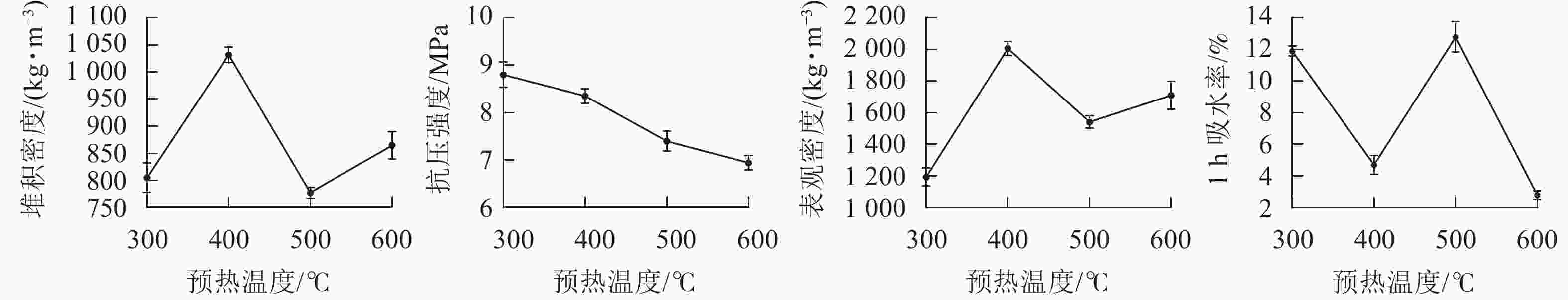
预热的主要作用是去除陶粒中的水分,防止陶粒在温度剧烈变化时开裂,同时控制陶粒中有机质的分解和气体释放[24]。在预热时间为20 min,烧结温度为1 200 ℃,烧结时间为15 min时,设置预热温度分别为300、400、500和600 ℃,制备4种不同预热温度的陶粒。从图3可见:陶粒的抗压强度随着预热温度的升高而逐渐降低,主要原因是随着预热温度的升高,陶粒中有机质分解不断加剧,温度在没有到达陶粒产生液相之前分解了过度的有机质。而前期过多的有机质分解,会造成后期陶粒内部无法产生足够的气体去支撑起表面液相物质,烧成的陶粒效果不理想。预热温度在400 ℃时,陶粒抗压强度较大,1 h吸水率也比较小,尽管陶粒在预热温度为300和500 ℃时的堆积密度和表观密度都比较小,但他们的吸水率偏高,不适合陶粒在轻集料建材中使用。综合考虑,400 ℃预热温度为制备陶粒的适宜温度。
-
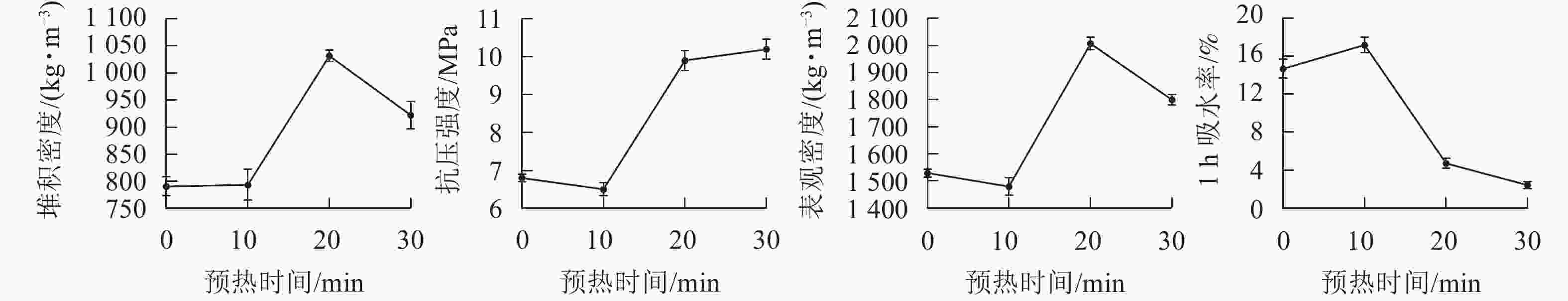
在预热温度为400 ℃,烧结温度为1 200 ℃,烧结时间为15 min条件下,设置预热时间分别为0、10、20和30 min,制备4种不同预热时间的陶粒。从图4可见:当预热时间小于10 min时,陶粒的各个性能指标几乎没有变化。说明预热时间太短,不足以引起陶粒内部产生反应或者反应不足,起不到预热的基本需求。预热在10~20 min时,抗压强度急速增加,同时吸水率也明显降低,而预热时间在20~30 min时,抗压强度和1 h吸水率趋于稳定。同时预热时间不宜过长,预热时间过长会导致陶粒内部产气物质过度分解,容易造成后期陶粒膨胀效果减弱[25]。从节约能源的角度来看,预热时间在20 min时最合适。
-
通过以上筛选,确定了最终的陶粒烧制条件,即烧制温度为1 175 ℃,烧结时间为15 min,预热温度为400 ℃,预热时间为20 min。测试的各项指标均符合国家标准(表4),其中炉渣泥陶粒堆积密度略小于市场陶粒,抗压强度和1 h吸水率均高于市场陶粒。
研究方法 堆积密度/
(kg·m−3)抗压强
度/MPa1 h吸
水率/%表观密度/
(kg·m−3)本研究陶粒 783 7.9 9.8 1 524.8 市场陶粒 417 5.3 4.2 − GB/T 17431.1—2010 700~800 ≥4.0 ≤10.0 − 说明:−表示没有相关数据或标准。 Table 4. Various indexes of ceramsite under the best firing conditions
-
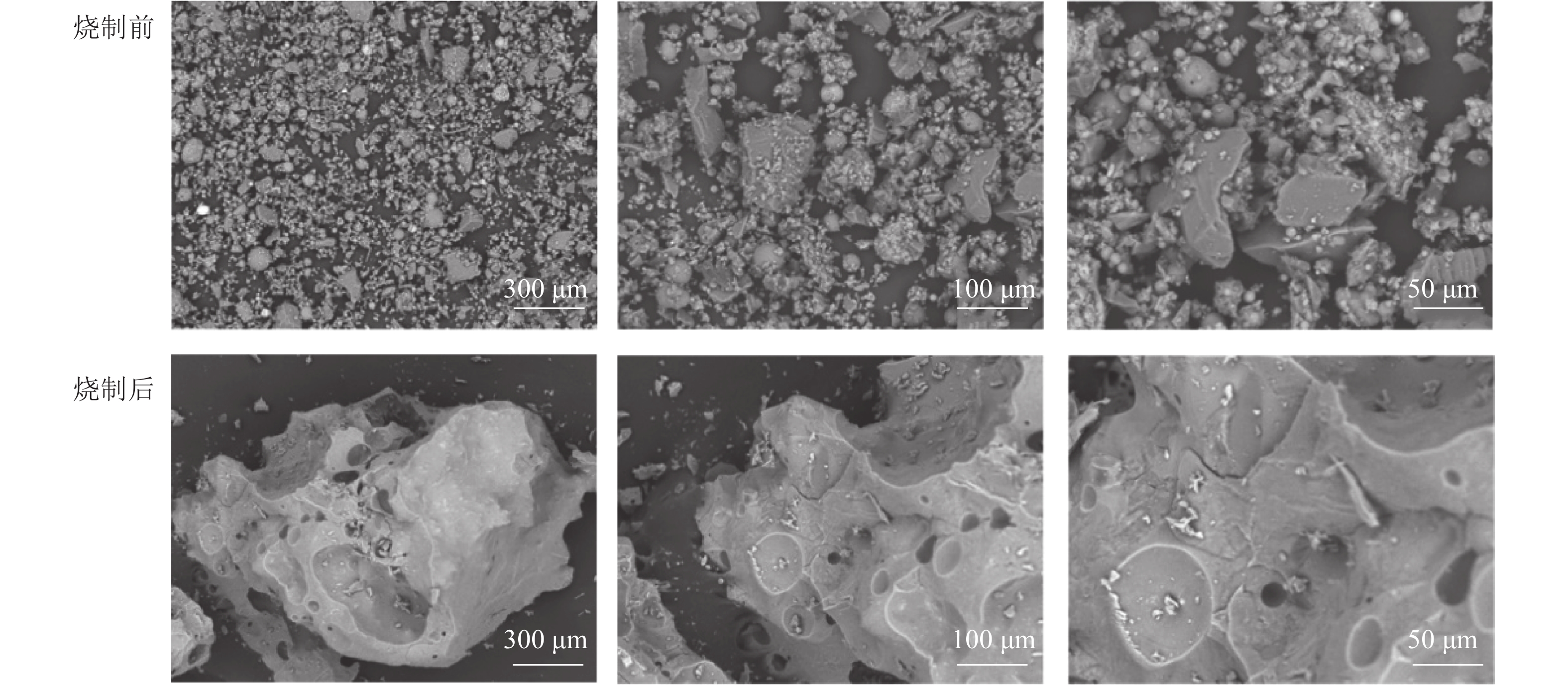
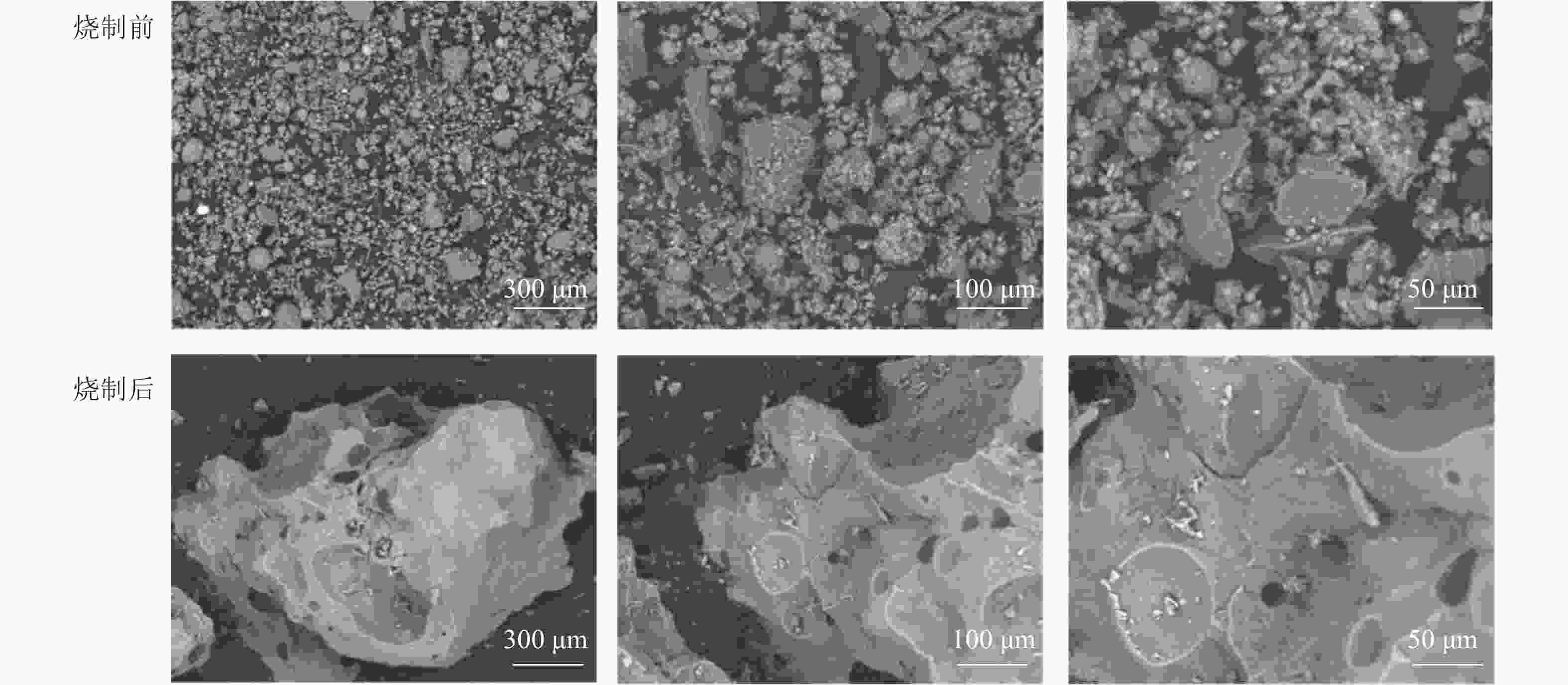
将最佳烧制条件下烧制前后的陶粒研磨,分别在扫描电镜下观察。如图5所示:烧制前的陶粒结构分散,颗粒大小不一且形态各异,结合性能差。烧制后的陶粒有大量晶体覆盖,光滑且多孔,有效降低了陶粒的吸水率。说明在此温度下陶粒表面和内部产生液相,高温产生的气体被封在陶粒内部,无法溢出,导致陶粒内部产生大量孔隙[26]。陶粒体积发生显著膨胀,有效降低了陶粒密度。
-
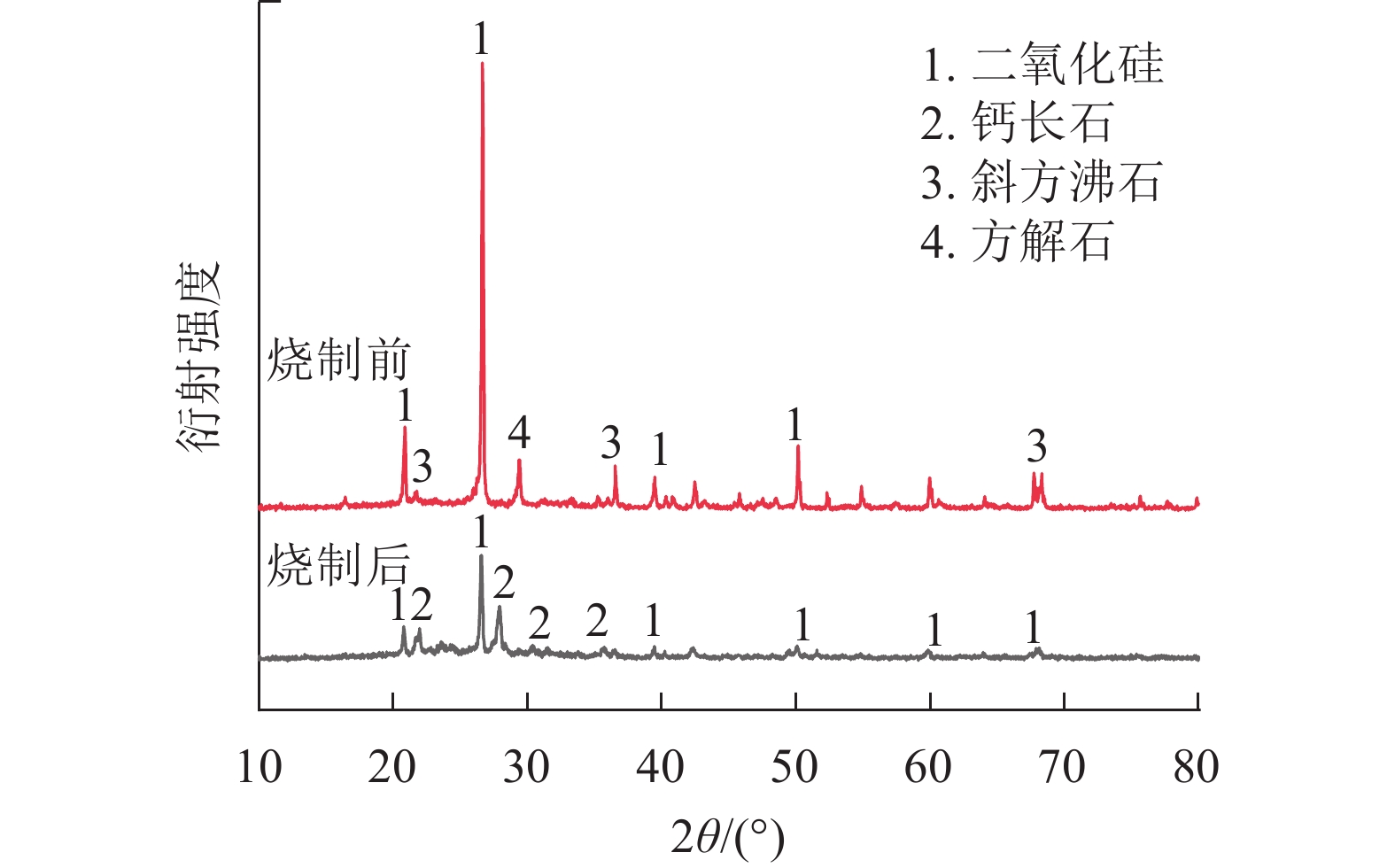
由图6可知:在最佳烧制条件下,生陶粒主要含有石英、方解石和斜方沸石,烧制后陶粒主要为石英和钙长石。陶粒主要结晶相为SiO2,这与前期研究结果一致[27]。烧制后的SiO2衍射峰显著降低,说明烧结过程中陶粒表面有大量熔融物质产生,形成一定体量的釉质层,有效提高了陶粒抗压强度。斜方沸石衍射峰减弱,钙长石衍射峰增强,说明陶粒烧制过程中一部分的斜方沸石转化成为了钙长石。
-
如表5所示:在最佳工艺条件下烧制的陶粒,重金属浸出比炉渣泥本身明显下降,其中Cd和Zn的浸出质量浓度都大大降低,且使用炉渣泥烧制的陶粒重金属Zn浸出质量浓度低于市场陶粒。可见,将炉渣泥烧制成陶粒,其烧结过程中内部产生的液相,将重金属包裹起来,对重金属起到了很好的固化作用[28]。
名称 质量浓度/(mg·L−1) Cd Zn 炉渣泥 0.730 0 32.300 0 陶粒 0.006 5 0.511 7 市场陶粒 0.002 3 1.321 5 GB/T 5083.3—2007 1.000 0 100.000 0 Table 5. Leaching content of heavy metals in ceramsite
-
3种添加剂粉煤灰、石英粉、高岭土的单价分别为80.0、130.0、110.0元·t−1,且每消耗1 t炉渣泥可以节约159.0元·t−1的填埋处理费。根据陶粒原料配比和堆积密度可知:每生产1 m3的陶粒需要消耗炉渣泥0.235 t,粉煤灰0.235 t,石英粉0.235 t,高岭土0.078 t。综上所述,生产陶粒的原料成本为20.5元·m−3。实验室采用的马弗炉容量为400 mm×250 mm×160 mm,一次可以烧结0.016 m3的陶粒。最佳烧结工艺(1 175 ℃和15 min)一次耗电为0.2 kW·h,因此,烧制1 m3陶粒需要消耗电量12.5 kW·h。每千瓦时电费价格按0.6元计算,制作陶粒的电力成本则为7.5元·m−3,因此,在实验室中制备陶粒的总成本为28.0元·m−3。目前国内陶粒市场价格约130.0元·m−3,故通过炉渣泥生产陶粒的利润可以达102.0元·m−3。
-
城市生活垃圾成分复杂,焚烧后所得炉渣泥存在重金属超标等问题[6],本研究采用九峰垃圾焚烧厂的炉渣泥,其中Cd和Zn超标。SHAO等[23]研究表明:城市固体垃圾焚烧后的飞灰和底灰可以作为生产陶粒的原料,而且陶粒的制作需要同时满足多种物质成分,比如SiO2、Al2O3、Fe2O3和多种助溶剂。本研究采用炉渣泥和粉煤灰、石英粉、高岭土混合,按照质量比为3∶3∶3∶1来制备陶粒。通过添加石英粉和高岭土以提高陶粒的强度和可塑性[24]。在烧结温度为1 175 ℃,烧结时间为15 min,预热温度为400 ℃,预热时间为20 min时,烧制出的陶粒具有抗压强度高和吸水率低的特点,这与MI等[13]研究结果一致。最佳烧制工艺烧出来的陶粒质量符合GB/T 17431.1标准,其中堆积密度为783 kg·m−3,抗压强度为7.9 MPa,1 h吸水率为9.8%。FAN等[15]使用铬污染土壤烧制陶粒,铬元素在烧制过程中被固化,浸出含量降低,本研究炉渣泥烧制的陶粒,Cd和Zn浸出毒性远远低于GB/T 5083.3—2007标准,有效减少了对环境的潜在危害性。从经济性方面看,炉渣泥烧制陶粒,每生产1 m3陶粒可以创造102.0元经济效益。对于烧制膨胀效果更好的陶粒还需深入研究,因为引入不同的添加剂或着改进烧制工艺都会对陶粒的膨胀效果产生影响。综上所述,通过将生活垃圾焚烧炉渣泥烧制成陶粒,可以有效减少炉渣泥对环境的潜在危害,实现炉渣泥的资源化利用,创造可观的经济价值。
Preparation and properties of ceramsite from domestic waste incineration slag sludge
doi: 10.11833/j.issn.2095-0756.20220518
- Received Date: 2022-08-09
- Accepted Date: 2023-04-03
- Rev Recd Date: 2023-03-01
- Available Online: 2023-07-13
- Publish Date: 2023-08-20
-
Key words:
- domestic waste incineration slag /
- ceramsite /
- slag sludge /
- heavy metal /
- leaching toxicity
Abstract:
| Citation: | MA Peng, ZHANG Cheng, SONG Chengfang, et al. Preparation and properties of ceramsite from domestic waste incineration slag sludge[J]. Journal of Zhejiang A&F University, 2023, 40(4): 867-874. DOI: 10.11833/j.issn.2095-0756.20220518 |











 DownLoad:
DownLoad: