-
水体富营养化容易导致藻类暴发,不仅破坏水域景观生态,还会造成严重的生态失衡[1−2]。据《2021中国生态环境状况公报》显示:209个国控重点湖泊(水库)中,轻度富营养状态占23.0%,中度富营养状态占4.3%[3]。以太湖为例,2021年3—10月,卫星遥感监测到蓝藻水华现象96次,平均发生面积为117 km2 [4]。水华治理的方法主要有物理、化学和生物法,其中水生生物控藻技术因成本较低且环境友好的特点已成为当前研究的重点和热点[5−6]。水生植物化感作用利用植物所释放的次生代谢产物(化感物质)影响周围藻类生长代谢,从而达到抑藻的目的[7]。沉水植物整株浸没于水中,其根、茎、叶不仅能够吸收水中氮、磷等营养物质,而且可直接向水体中分泌化感物质抑制藻类等浮游生物的生长,在藻类水华治理中具有明显的优势[8−9]。但大部分沉水植物生长季节性较强,如伊乐藻Elodea nuttallii、菹草Potamogeton crispus不耐高温,穗花狐尾藻Myriophyllum spicatum、金鱼藻Ceratophyllum demersum和苦草Vallisneria natans不能越冬,因而不能稳定有效地发挥抑藻作用[10−12]。
圆叶节节菜Rotala rotundifolia为一种可挺水、浮水和沉水生长的水生植物,它的沉水态为多年生草本植物,温度适应范围广,为4~30 ℃;圆叶节节菜对氮、磷去除效果大于80%[13],在水生态系统恢复方面具有较强的应用潜力,但其抑藻性能和作用机制尚不明了。鉴于此,本研究采用超声辅助溶剂浸提圆叶节节菜化感活性物质,研究其对铜绿微囊藻Microcystis aeruginosa的抑制性能和量效关系,对比圆叶节节菜与6种常见沉水植物对铜绿微囊藻生长、光化学效率和抗氧化系统的化感作用,分析圆叶节节菜的抑藻效能和作用机制,以期为圆叶节节菜在藻类水华治理工程应用方面提供理论数据。
-
圆叶节节菜取自温州市仙岩风景区内池塘,穗花狐尾藻、苦草、水盾草Cabomba caroliniana、伊乐藻、金鱼藻和轮叶黑藻Hydrilla verticillata购自江苏宁高水生植物基地。铜绿微囊藻(编号FACHB-905)来源于中国科学院水生生物研究所,采用灭菌后的BG-11培养基于QHX-250B人工气候培养箱中扩大培养,光照强度为3 000 lx,光照时间为12 h (光)∶12 h (暗)[14]。
-
新鲜的植株体用自来水洗干净后,自然晾干除去表面水分,然后通过真空冷冻干燥进一步去除组织内水分,得到的冻干组织磨碎后,于−20 ℃下保存备用。使用时,称取2 g冻干粉末加入一定溶剂中,采用超声提取沉水植物有效成分,主要参数为m(料)∶V(液)=1∶50,时间为40 min,温度为25 ℃,重复2次。粗提液经双层粗纱布过滤后合并,过0.45 μm有机滤膜,滤液经旋转蒸干除去原溶剂,加入无水乙醇洗脱并定容至10 mL。用植物质量变化表示化感物质的相当量(g·L−1),得到质量浓度为200 g·L−1的植物提取物母液,于4 ℃储存备用。
-
选择水、乙醇(体积分数75%)和石油醚(沸点60~90 ℃)等3种不同极性的溶剂,按1.2.1提取方法制备圆叶节节菜提取物。将对数生长期的藻细胞转接至灭菌后的BG-11培养基中,初始细胞密度约5.0×109个·L−1。100 mL锥形瓶中加入50 mL藻液,设置提取物添加量(质量分数)为0.25%、0.50%、0.75%、1.00%,即每组分别加入125、250、375、500 μL的母液,以不加提取物的处理为对照。每个处理设3个平行。分别于第1天、第3天、第5天取样2 mL,测定铜绿微囊藻的叶绿素a (Chl a)质量浓度。
-
设置石油醚提取物的添加量 (质量分数)为0.05%、0.10%、0.20%、0.40%、0.80%,即分别添加25、50、100、200、400 μL母液至50 mL藻液中,以不加提取物的处理为对照,每组处理重复3次,分别于第1天、第3天、第5天取样2 mL,测定铜绿微囊藻的Chl a质量浓度。
-
100 mL锥形瓶中加入50 mL初始细胞密度为5.0×109个·L−1的藻液,按0.5%的质量分数分别加入250 μL圆叶节节菜、穗花狐尾藻、苦草、水盾草、伊乐藻、金鱼藻和轮叶黑藻的石油醚提取物,以不加提取物的处理为对照,每组处理重复3次,分别于第1天、第3天、第5天取样2 mL,测定铜绿微囊藻的Chl a质量浓度。分析处理24 h的最大光化学效率(Fv/Fm)和超氧化物歧化酶(SOD)活性。
-
取2 mL藻液,暗适应15 min,采用浮游植物分类荧光仪(Phyto-PAM)分析Chl a和Fv/Fm[15]。
-
取2 mL藻液,8 000 r·min−1离心5 min收集藻细胞,加入预冷的生理盐水(质量分数为0.9%) 100 μL,使用高通量冷冻组织研磨仪破碎细胞,4 ℃下6 m·s−1速率震荡30 s,间隔60 s,循环6次,即得到酶液。采用南京建成生物有限公司试剂盒的羟胺法测定SOD活性。
-
各处理组对铜绿微囊藻生长抑制率(IR)计算公式为:IR=(N0−Ns)/N0×100%。其中,N0和Ns分别为对照组和实验组的Chl a质量浓度(μg·L−1)。
-
采用GraphPad Prism 8.0.2进行逻辑回归(Logistic regression)分析,根据提取物质量(取对数)和抑制率之间的关系曲线,得出半最大效应质量浓度(EC50)。
-
采用SPSS 26.0进行单因素方差分析,显著性水平为0.05,数据为平均值±标准差。采用GraphPad Prism 8.0.2软件作图。
-
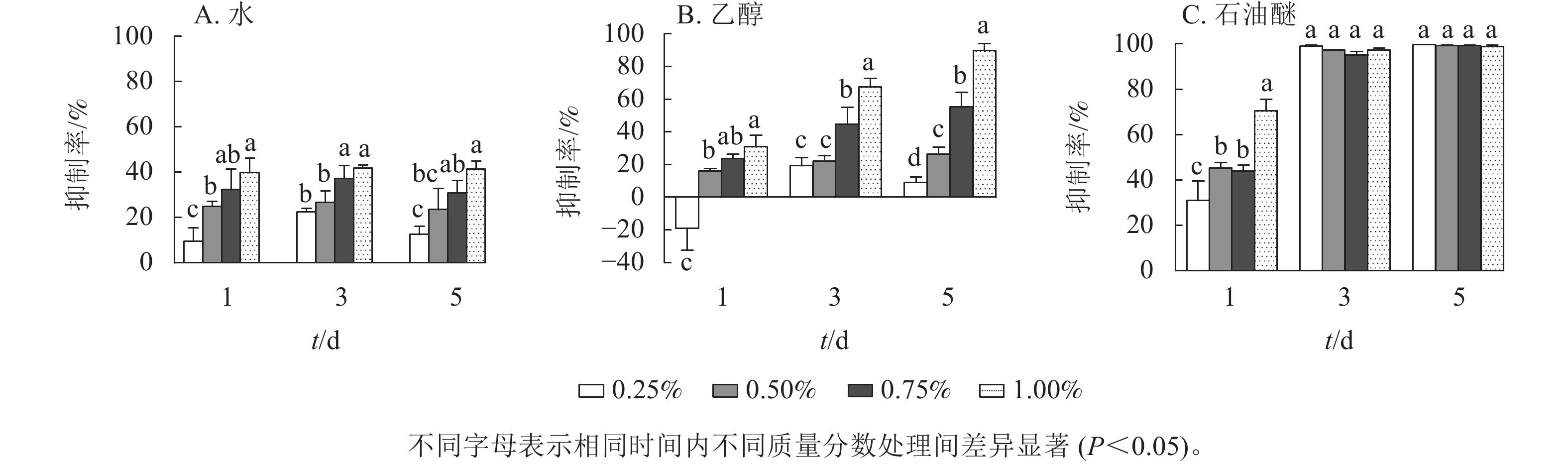
由图1可见:3种提取物处理下,藻细胞的生长受到了不同程度的抑制,并表现出剂量效应。圆叶节节菜的水提取物对铜绿微囊藻有一定的抑制效果(图1A),各处理下抑制率均小于50%,且抑制率受处理时间的影响较小,抑制率的阈值为9.7%~41.8%。由图1B所示:第1天,乙醇提取物的抑制率均低于30%,且提取物低质量分数(0.25%)处理下抑制率为负;第3天提取物高质量分数(1.00%)处理对铜绿微囊藻的抑制效果显著(P<0.05),抑制率达67.5%,其他处理下抑制率均小于50%;第5天提取物质量分数为0.75%和1.00%的处理有显著抑制效果(P<0.05),抑制率分别为55.2%和89.6%。如图1C所示:石油醚提取物对铜绿微囊藻具有显著的抑制作用(P<0.05),第1天提取物1.00%质量分数处理组抑制率达70.5%,第3天各处理组均有完全抑藻效果,且差异不显著,第5天各处理组抑制率基本不变。由此可见,圆叶节节菜水、乙醇和石油醚3种提取物的化感抑藻效应强度不同,抑藻能力由强到弱依次为石油醚、乙醇、水。
-
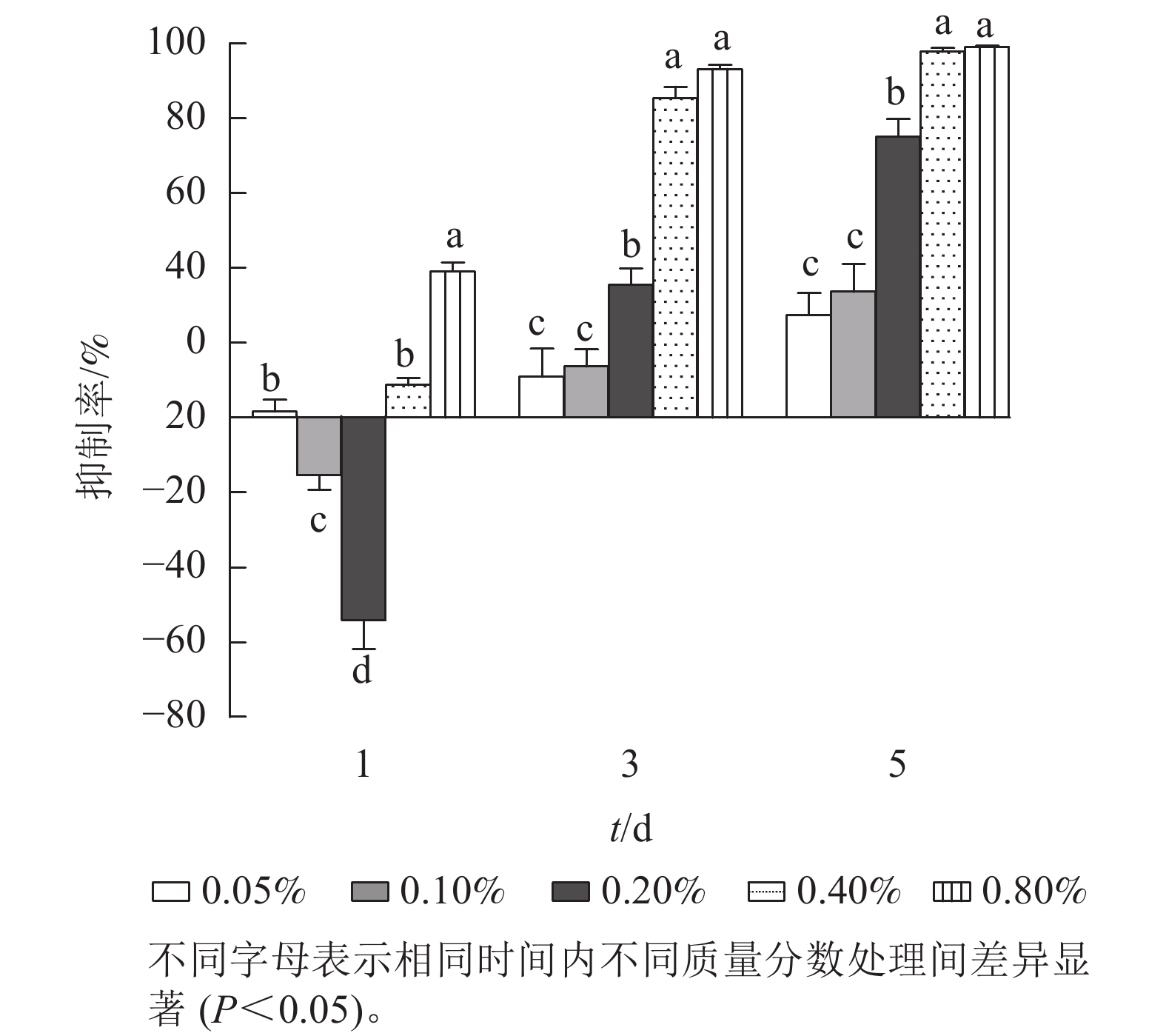
进一步研究低质量分数处理下石油醚提取物对铜绿微囊藻抑制率的剂量-效应关系(图2)。第1天,各质量分数处理下,铜绿微囊藻的生长响应差异显著(P<0.05),质量分数为0.10%和 0.20%的处理组,抑制率为负值。质量分数为0.80%的处理组抑制率较高(38.0%,P<0.05)。第3天,各处理组对铜绿微囊藻增殖均有一定的抑制效果,且处理组质量分数越高,抑制率越高,出现明显剂量依赖关系;第5天,各处理组的抑制率显著上升(P<0.05),质量分数为0.40%和0.80%的高剂量组,抑制率高达97.8%和99.2%。将石油醚提取物质量分数(0.05%、0.10%、0.20%、0.40%和0.80%)转化为提取物质量浓度(5、10、20、40、80 g·L−1),分别以各采样时间的提取物质量为横坐标,以抑制率为纵坐标,进行Logistic回归分析(表1),结果表明:圆叶节节菜石油醚提取物对铜绿微囊藻的半最大效应质量浓度随暴露时长的增加而降低,第3天和第5 天均具有显著的剂量-效应关系,发挥效应所需的剂量质量浓度分别为23.15和11.56 g·L−1。
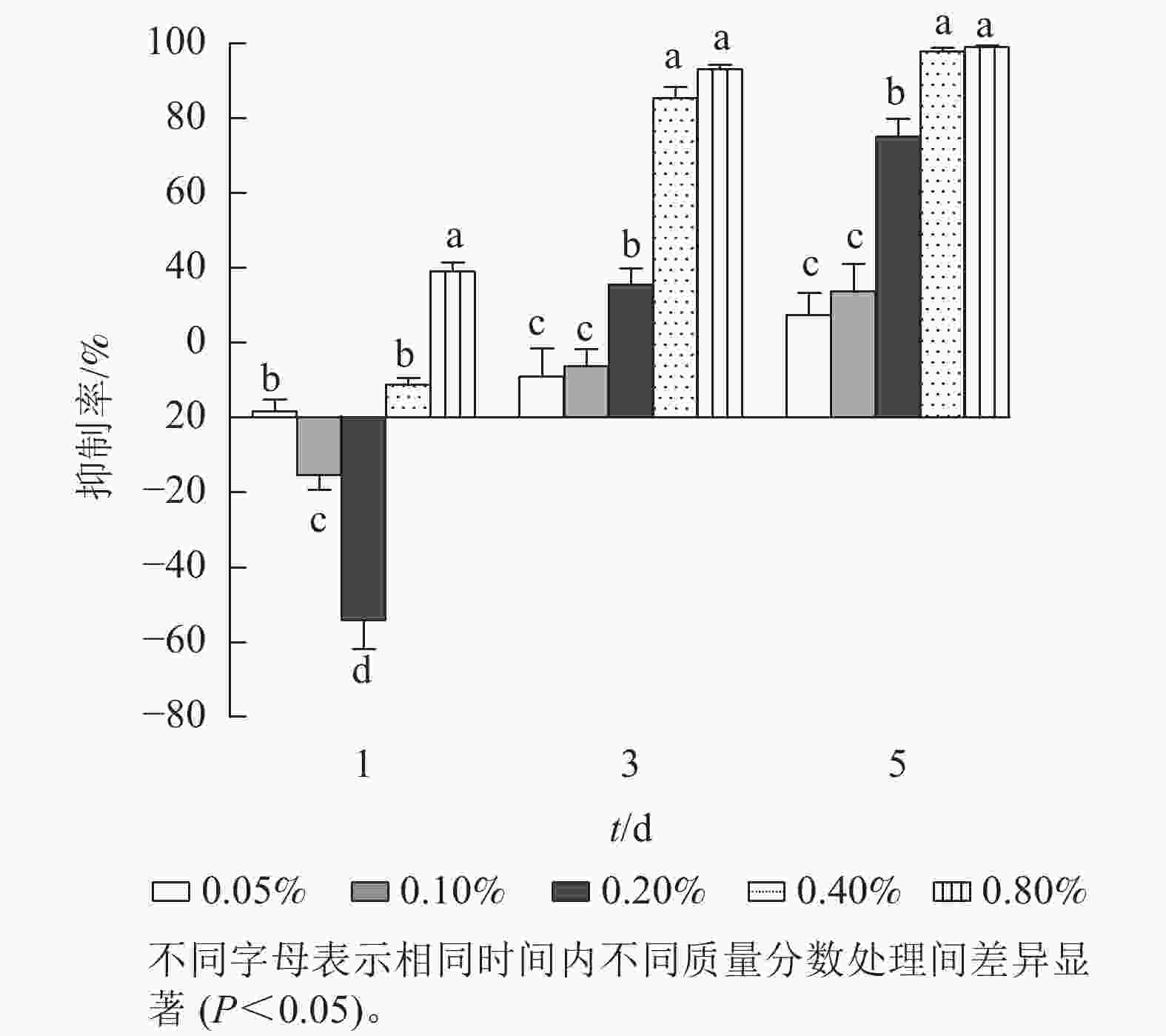
Figure 2. Effect of petroleum ether extract concentration on inhibition rate of M. aeruginosa under different treatment times
t/d 拟合方程 决定系数(R2) EC50 /(g·L-1) 1 y=0.328 8x−0.467 9 0.208 4 87.54 3 y=0.784 5x−0.543 2 0.906 9 23.15* 5 y=0.689 7x−0.231 0 0.911 9 11.56* 说明:x表示提取物质量(取对数);y表示抑制率。*表示差异显著(P<0.05)。 Table 1. Half maximal effective concentration of petroleum ether extract for algae inhibition
-
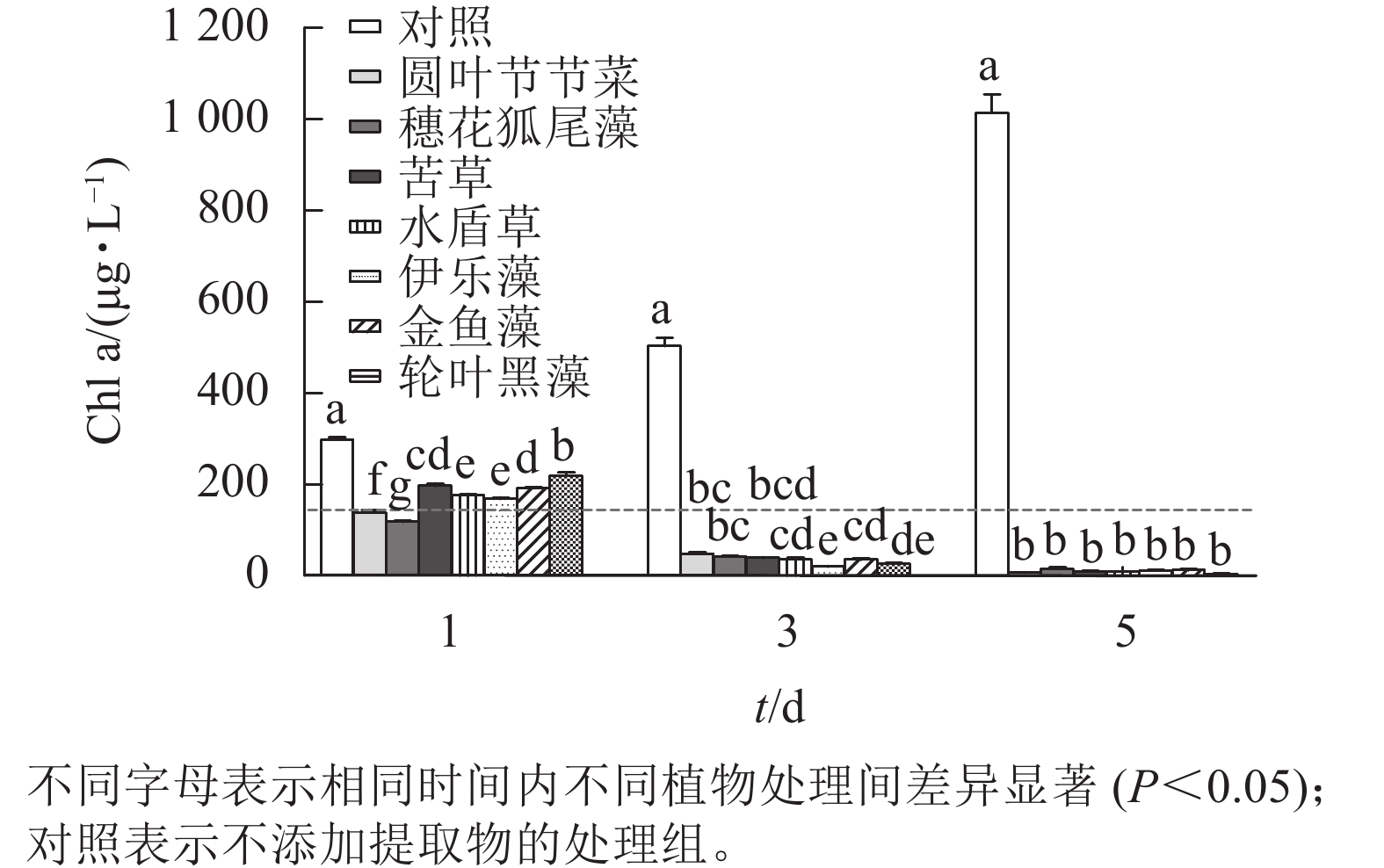
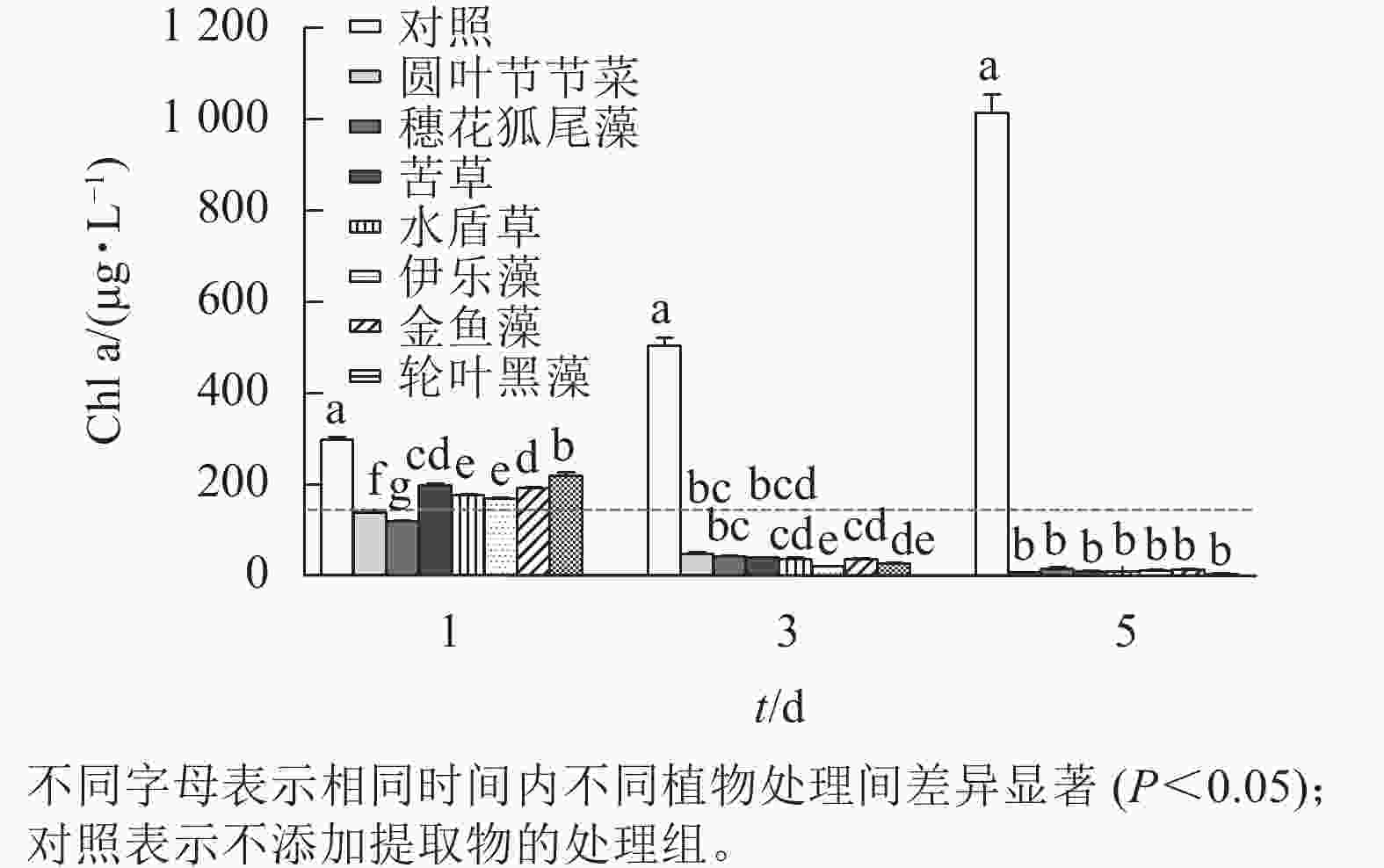
本研究所采用的沉水植物提取物对铜绿微囊藻均有一定的抑制效果(图3)。第1天,相较于对照组,所有植物提取物处理对铜绿微囊藻的生长均有显著抑制作用(P<0.05),且圆叶节节草和穗花狐尾藻处理铜绿微囊藻Chl a显著低于其他处理(P<0.05),说明这2种沉水植物抑藻响应时效较快。随时间的增加,植物处理组Chl a均呈下降趋势,第5天,Chl a差异不显著,此时Chl a低于20 μg·L−1。而对照组不受胁迫,Chl a随时间增加而升高,第5天Chl a 高达1 013.89 μg·L−1。
-
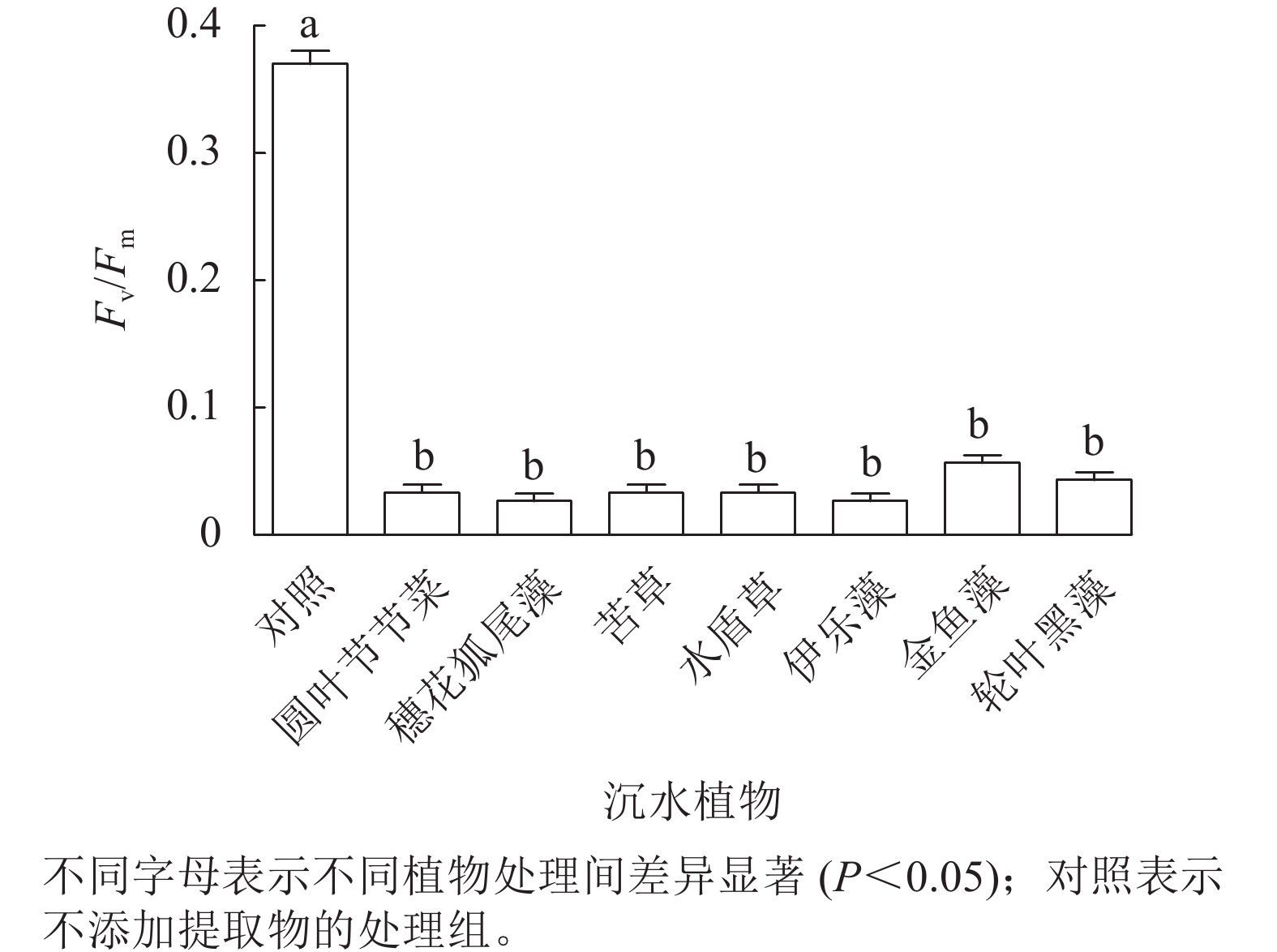
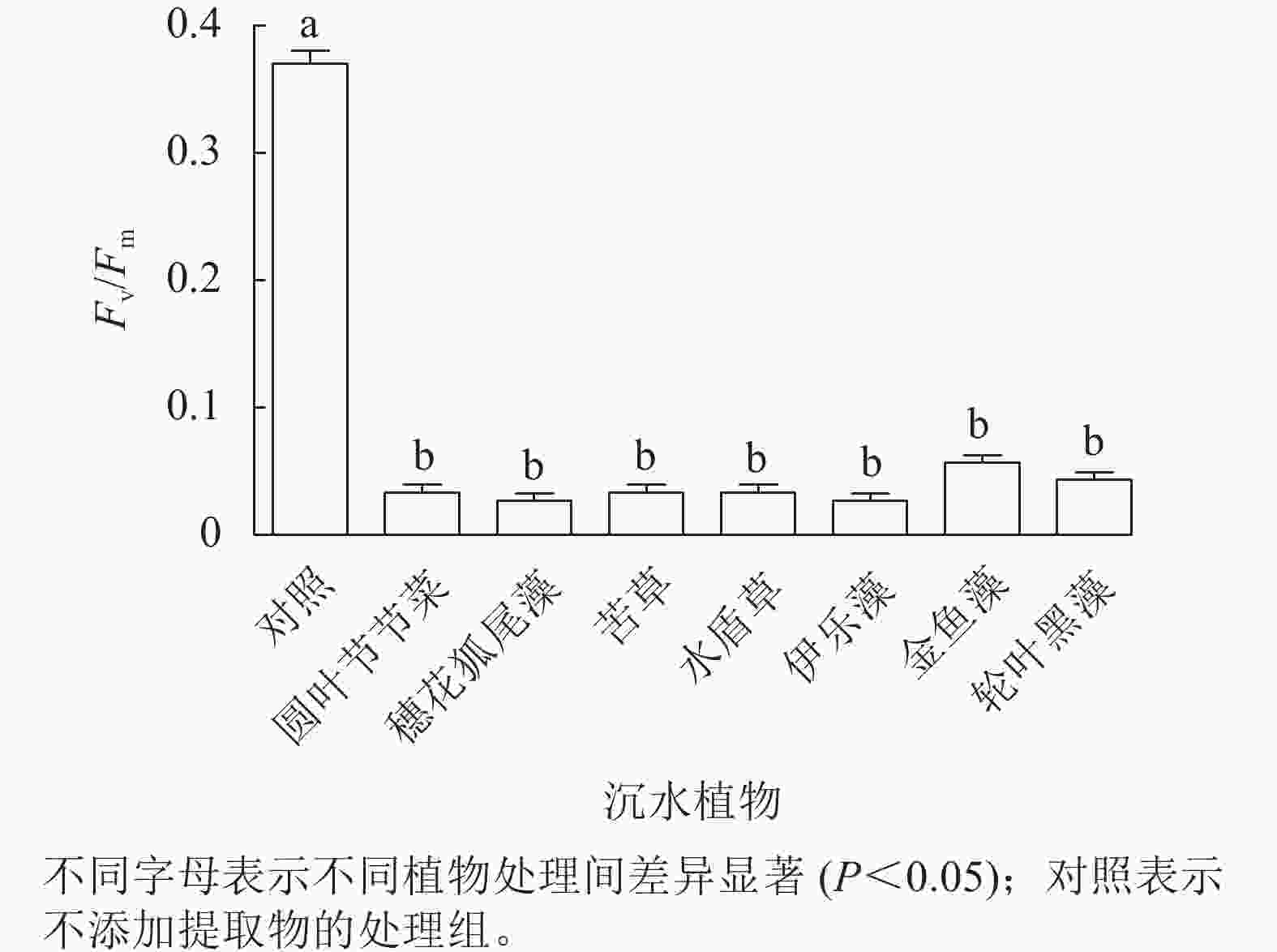
由图4可见:处理24 h,对照组Fv/Fm稳定在较高水平,植物处理组的Fv/Fm为0.02~0.06,为对照组的8.3%~13.9%,均显著低于对照组(P<0.05),但是处理组之间藻细胞的Fv/Fm差异不显著。由此可见,铜绿微囊藻的光系统PS Ⅱ对这几种沉水植物提取物的胁迫均较为敏感。
-
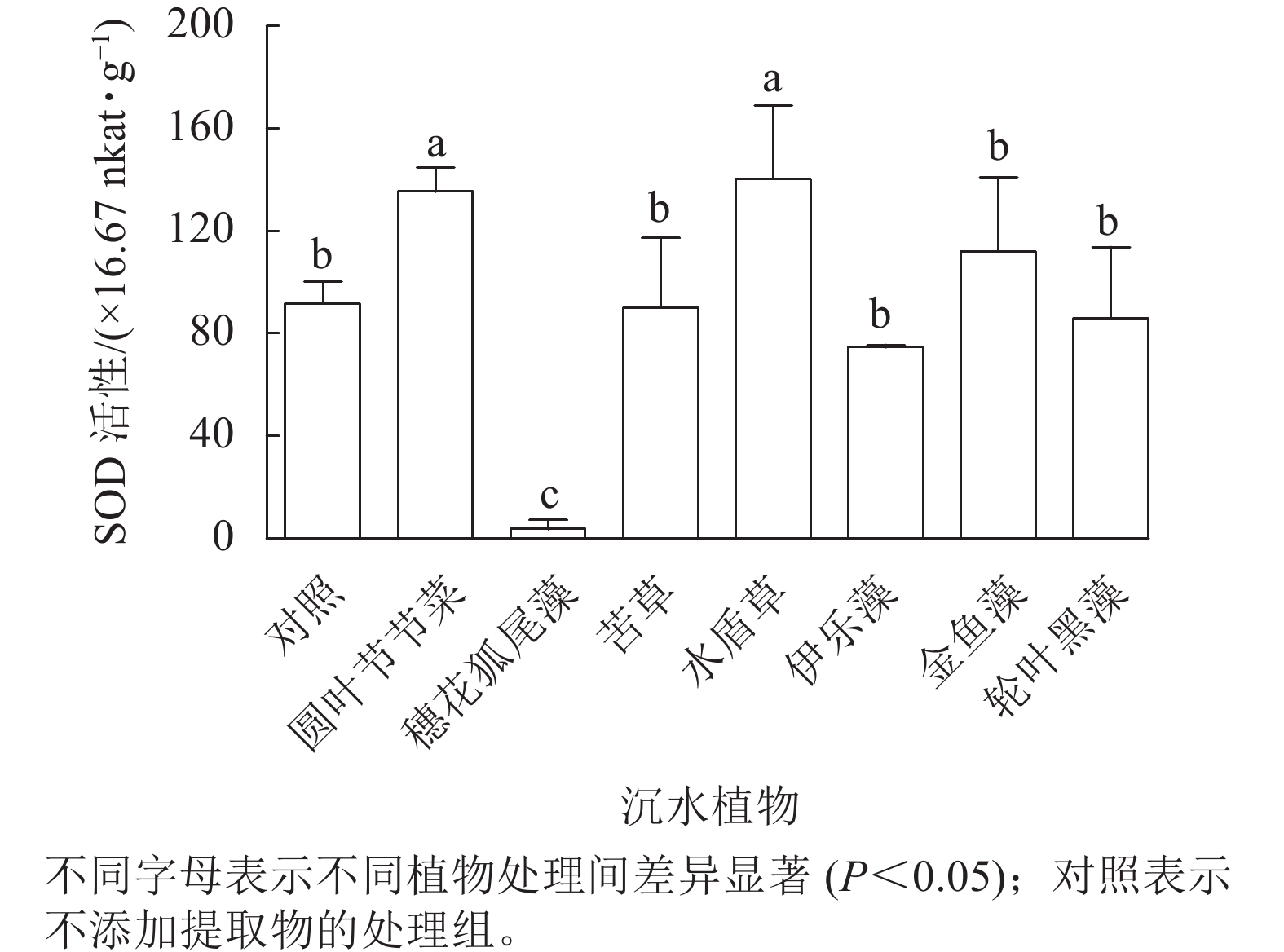
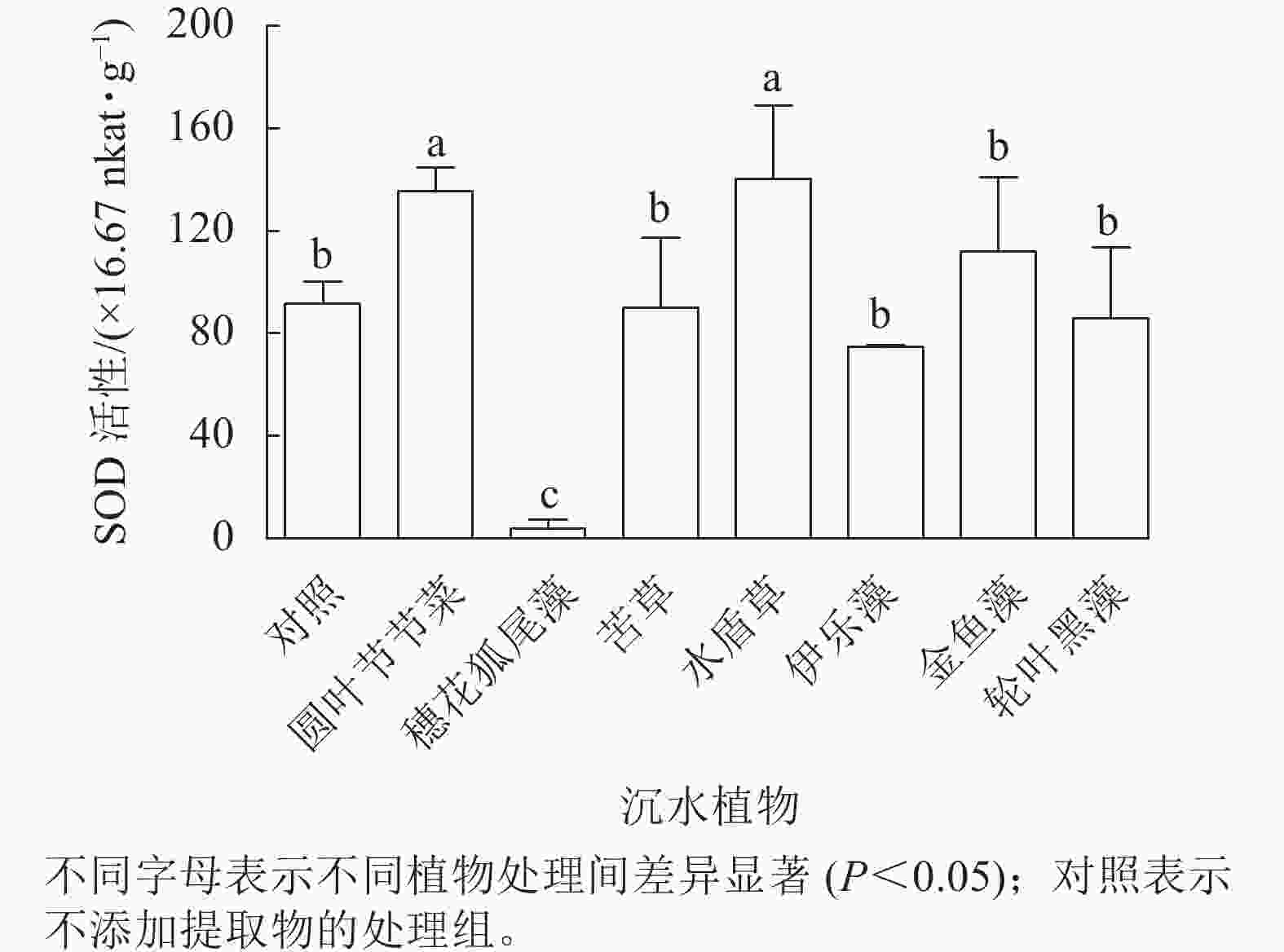
从图5可见:圆叶节节菜和水盾草提取物处理组的胞内SOD活性分别是对照组的1.48和1.53倍,显著高于对照组和其他植物处理组(P<0.05)。穗花狐尾藻处理组的SOD活性显著低于对照组(P<0.05),而苦草、金鱼藻、伊乐藻和轮叶黑藻提取物处理后藻细胞SOD活性与对照组无显著差异。
-
植物抑制藻类生长主要归因于不同种类化感物质的胁迫,根据生物合成途径,已报道的植物化感物质可分为酚类、萜类、脂肪酸类和生物碱类等4种主要类型[16]。本研究中,圆叶节节菜水、乙醇和石油醚3种溶剂提取物对铜绿微囊藻的抑制效果差异显著,抑藻率从大到小依次为石油醚、乙醇、水。由此可见,非极性的石油醚提取物对铜绿微囊藻有较好的抑制效果。张胜娟[17]在研究槐叶萍Salvinia natans提取物对铜绿微囊藻抑制效应时,也得到了类似的结果,发现抑制作用由强到弱依次为乙酸乙酯、丙酮、乙醇、水,其中乙酸乙酯粗提物检测到不饱和脂肪酸类、酯类和芳香类化合物质。但是高云霓等[18]采用正己烷、二氯甲烷、乙酸乙酯、丙酮和甲醇这5种极性跨度较大的溶剂洗脱苦草分泌物的化感物质后,发现甲醇洗脱组分抑藻活性最强,最佳活性抑藻组分为咖啡酸、原儿茶酸、香草酸等9种酚酸类化感物质。说明不同种类植物的化感物质可能有较大的差异。溶剂的极性大小决定了植物组织内各种成分在不同溶剂中的溶解性,极性溶剂获得较多亲水性化感物质,非极性溶剂获得较多亲脂性化感物质[19]。本研究中,3种提取液颜色差异明显,水、乙醇和石油醚提取液分别为棕黄色、深绿色和明黄色,后续研究将对这3种提取物的主要成分进行分析比较,研究其抑藻效能。不同极性溶剂提取物抑藻效应的作用方式不同,导致藻细胞受到胁迫时敏感性不一,从而影响起效时间和有效时长[20]。目前研究较多的亲水性化感物质组分为酚酸类,如阿魏酸、丙二酸可增加细胞膜通透性,导致细胞内溶物外流和诱导脂质过氧化,从而造成藻细胞生长抑制或细胞坏死[21];香草酸和肉桂酸可使Chl a和藻胆蛋白下降,破坏铜绿微囊藻细胞的捕光天线[22]。亲脂性化感物质的作用方式则是直接接触破坏脂溶性生物细胞膜进入细胞,在胞内溶解、吸收、分布和转运,从而影响一系列生理生化活动[23]。例如2-甲基乙酸乙酯可能引起藻细胞质膜完整性改变,造成K+、Ca2+泄漏[24];N-苯基-2-萘胺可能通过破坏细胞膜、增加超氧阴离子自由基$ {\mathrm{O}}_{2}^{\cdot -} $含量、降低Chl a等方式抑制铜绿微囊藻细胞的生长[25]。
本研究发现:第3天和第5天均具有显著的剂量-效应关系。而研究初期(第1天),2个低质量分数(0.10%和0.20%)处理组抑制率出现负值,说明圆叶节节菜化感物质对藻细胞生长也符合低促高抑的Hormesis效应。这一效应也出现在了圆叶节节菜乙醇提取物对铜绿微囊藻作用的第1天,即低质量分数(0.25%)处理下抑制率为负。这与董颖娜等[26]的研究结果一致,分析原因认为可能低质量分数的化感物质改变了藻细胞的通透性,减小了物质传输阻力,促进了藻细胞对营养盐的吸收和利用。
光合作用是藻类生长过程中最重要的生理过程之一。圆叶节节菜和其他沉水植物提取物处理后,铜绿微囊藻Chl a质量浓度均显著下降。穗花狐尾藻和圆叶节节菜响应最快,原因是不同植物源的化感物质活性不同,抑藻效果也不尽相同。随着处理时间的延长,各沉水植物提取物的抑藻效果无显著差异。张之浩等[27]的研究也得到了类似的结果,即苦草、轮叶黑藻、狐尾藻Myriophyllum verticillatum等常见沉水植物衍生的化感物质能显著降低富营养化水体中的Chl a质量浓度,对水体中浮游藻类的抑制效应较明显且稳定。Fv/Fm是衡量藻类PSⅡ光合性能的主要参数,是表征藻类光合作用效率的重要指标[28]。PSⅡ是化学物质作用于藻类细胞的重要位点之一,孙东等[29]研究了天然植物成分原儿茶酸抑制米氏凯轮藻Karenia mikimotoi的生长机制,发现其阻碍了米氏凯轮藻的PS Ⅱ电子传递和电子吸收途径。熊珍珍等[30]在调查水杨酸对刚毛藻Cladophora的抑制机制时,发现其Fv/Fm迅速下降,PSⅡ光合活性显著降低。本研究不同沉水植物化感物质处理24 h后,铜绿微囊藻细胞Fv/Fm均大幅降低,可见藻细胞PSⅡ会受到各种沉水植物提取物的胁迫损伤,降低藻光合作用效率,使同化产物减少,从而抑制其生长。
藻细胞在正常代谢下,由于氧化磷酸化过程,细胞器会产生适量的活性氧(ROS)。受到外界环境胁迫时,胞内ROS水平升高造成细胞损伤,但藻细胞的抗氧化系统也有自身的调节机制[31]。SOD是植物抗氧化系统的首要防线,在清除ROS方面发挥着重要作用,其活性可直接反映生物体抵御不良环境的能力[32−33]。本研究中,圆叶节节菜与水盾草提取物处理24 h显著提高了铜绿微囊藻细胞的SOD活性,说明铜绿微囊藻抗氧化系统通过升高SOD活性以平衡ROS来减轻机体伤害。但穗花狐尾藻处理组的SOD活性显著低于对照组。赵文婧[34]采用穗花狐尾藻提取物处理铜绿微囊藻也观察到了这个现象,当提取物质量浓度为5、10和20 g·L−1时,铜绿微囊藻的SOD活性均先升高后降至显著低于对照。可能当ROS过高时,SOD合成结构遭到破坏,活性氧不能被抗氧化酶及时清除,藻细胞大量死亡,抗氧化酶活性下降[35]。
综上所述,沉水态圆叶节节菜水、乙醇、石油醚提取物对铜绿微囊藻均有一定的抑制效应,其中以石油醚提取物效果最佳,并具有显著的剂量-效应关系。圆叶节节菜提取物和穗花狐尾藻提取物的抑藻能力相较其他沉水植物具有一定的快速响应优势。圆叶节节菜化感物质胁迫铜绿微囊藻细胞生长具有多方面的生理作用,可降低藻细胞Chl a质量浓度和Fv/Fm,升高抗氧化酶SOD活性。
Allelopathic and algal inhibition effects of submerged aquatic plant Rotala rotundifolia
doi: 10.11833/j.issn.2095-0756.20220568
- Received Date: 2022-09-09
- Accepted Date: 2023-04-18
- Rev Recd Date: 2023-03-23
- Available Online: 2023-07-13
- Publish Date: 2023-08-20
-
Key words:
- Rotala rotundifolia /
- submerged plant /
- Microcystis aeruginosa /
- plant extracts /
- algal inhibition
Abstract:
| Citation: | CHEN Jingying, ZHOU Weijie, LIN Guo, et al. Allelopathic and algal inhibition effects of submerged aquatic plant Rotala rotundifolia[J]. Journal of Zhejiang A&F University, 2023, 40(4): 765-772. DOI: 10.11833/j.issn.2095-0756.20220568 |









 DownLoad:
DownLoad: