-
在全球范围内,土壤含碳大约是大气的3.0倍,是生物碳库的4.5倍[1],其中约1 500 Gt的碳以有机碳的形式存在于土壤中,使得土壤碳库成为陆地生态系统最大的碳库,因此土壤碳库微小的变化必将会对全球碳平衡和气候变化产生重大影响[2−3]。土壤有机碳(SOC)矿化是指土壤有机碳分解并向大气释放二氧化碳(CO2)的过程,该过程是土壤有机碳损失的重要途径,有助于促进土壤营养元素的循环[4−5]。土壤有机碳矿化及其影响因素的研究已成为全球气候变化的热点之一[6]。草地生态系统是陆地生态系统的重要碳库之一。据统计,全球草地生态系统碳储量为761 Gt,占整个陆地生态系统碳储量的34%,而中国草地面积约4 亿hm2,草地生态系统碳储量为44 Gt,约占世界草地生态系统碳储量的8%[7]。青藏高原生态系统碳库容量巨大,具有重要的生态安全屏障作用,在全球碳循环调控中发挥着重要作用[8−9],因此,研究高寒草地土壤有机碳矿化对于预测温室气体排放及管理土壤碳平衡具有重要意义。
土壤有机碳矿化是由生物和非生物过程共同决定的[10]。土壤有机碳分解通常被认为是由生物过程驱动的,然而,热降解、光降解、活性氧氧化、细胞外氧化代谢和无机化学反应等非生物过程也可以产生CO2,但非生物过程的贡献往往被忽略[11−13]。研究发现:尽管灭菌后的土壤中缺乏活细胞,但土壤CO2产生的活跃生化途径仍在继续[14]。非生物土壤有机碳矿化是无处不在的,平均贡献了土壤CO2排放量的24%,其对土壤CO2排放的贡献度随着可溶性有机碳(DOC)的增加而增大[15]。此外,非生物过程的贡献程度还与土壤含水量关系密切,当土壤含水量为10%时,非生物过程对土壤有机碳矿化过程的贡献为18.6%~49.2%,且土壤含水量越低,非生物过程的影响越大[16]。然而,在人们普遍认为是生物活动占主导的陆地生态系统中,非生物过程对土壤CO2排放有何贡献仍缺乏足够的数据支撑[13]。
牛尿排泄物是草地生态系统中的一个重要养分来源,但牛尿返还对土壤CO2排放的影响却不尽一致[17]。一般认为,放牧牲畜的尿液向土壤中输入了碳源和氮源,可促进土壤有机碳矿化[18],往往会增加土壤CO2的排放[19]。研究表明:尿液添加可提高草甸土壤的累积碳矿化率[20],由于尿液诱导的激发效应,尿液添加后增强了土壤有机碳的分解[21−22];再者,尿液还能刺激土壤微生物水解尿素,释放CO2[23−24]。但也有研究发现:添加氮源虽可促进土壤活性有机碳的分解,但更能促进难分解性有机碳的积累,减少总有机碳的损失,进而有利于土壤有机碳稳定性的维持或增加[25−26]。土壤CO2排放量可随尿液添加量的增加而增大[27],但高氮输入会导致饱和响应,土壤CO2排放不会随氮素输入量的增加而无限制增加,因为氮供应充足情况下资源限制可能会从氮素转向其他因素,比如水分、磷素和光照等[28−29]。由此可知,不同尿液返还量对CO2排放的促进效应不一定会随着尿液返还量的增加呈持续增大趋势。
在以往研究中,土壤灭菌通常被认为可使得微生物活性得到最大程度的降低,最终抑制土壤有机碳的矿化。也有研究认为:土壤有机碳矿化与微生物活性或土壤微生物生物量的大小无关[30]。虽然目前对非生物过程有初步研究,但关于土壤有机碳非生物矿化过程的量化研究还鲜有报道[13]。此外,土壤有机碳矿化对尿液添加如何响应?生物和非生物过程对尿液添加下的土壤有机碳矿化贡献多大?仍需进一步明确。鉴于此,本研究通过对高寒草甸土壤进行灭菌与不灭菌处理,设置0、250、500、750、1 000 kg·hm−2·a−1的尿液返还量,研究不同灭菌方式下草地土壤CO2排放对尿液添加的响应,以期为应对放牧草地生态系统中温室气体排放增加提供理论依据。
-
土壤采自于四川省阿坝藏族羌族自治州红原县境内的中国科学院若尔盖湿地生态系统定位站(32°58′08″N,102°37′08″E,海拔为3 450 m)[31]。研究区年均气温为1.9 ℃,夏季月均最高气温为20.0 ℃,年均降水量为747.0 mm,其中77.0%集中在生长期(5—9月)[32]。草地类型为高寒草甸,植被优势种是小嵩草Kobresia pygmaea、垂穗披碱草Elymus nutans和矮嵩草Kobresia humilis,土壤为高山草甸土。
研究区于2014年5月起开始实施禁牧,2020年7月通过五点取样法用土钻取0~20 cm土层的土壤,充分混匀剔除根系、石块等杂物。将新鲜土样经过风干研磨后,过孔径为2 mm的不锈钢筛,密封备用。取部分风干土用于采样地基础理化性质测定,测得土壤pH 5.71,土壤有机碳为63.84 g·kg−1,全氮为6.20 g·kg−1。取部分鲜土测得可溶性有机碳为67.78 mg·kg−1,溶解性总氮为64.67 mg·kg−1,铵态氮(NH4 +-N)为36.33 mg·kg−1,硝态氮(NO3 −-N)为10.68 mg·kg−1。
-
称取40 g风干土壤(基于干土质量)于100 mL的培养瓶中,用去离子水将土壤含水量调整为30%土壤持水量(WHC),在黑暗中20 ℃下预培养7 d。预培养期间每隔1 d补充培养瓶内水分以保证含水量稳定。实验设置5个牛尿返还量(施氮0、250、500、750、1 000 kg·hm−2·a−1,相当于土壤中增加的氮质量分数为0、96、192、288、384 mg·kg−1)和3种灭菌方法(不灭菌、高压蒸汽灭菌、氯仿熏蒸灭菌),共15种处理,每个处理4个重复。对于尿液添加处理,考虑到牛尿收集不易且易发生变质,依据牛尿配方:尿素17.7 g·L−1、马尿酸7.4 g·L−1、肌酐酸0.2 g·L−1、尿囊素0.4 g·L−1、尿酸0.1 g·L−1、氯化铵0.9 g·L−1、碳酸氢钾14.2 g·L−1、氯化钾10.5 g·L−1的方法配置合成牛尿(9.0 g·L−1)[33]。预培养结束后,将需要灭菌处理的土壤样品分别进行高温高压蒸汽灭菌和氯仿熏蒸灭菌。高温高压蒸汽灭菌时将样品在121 ℃和0.3 MPa条件下灭菌30 min。氯仿熏蒸灭菌需将土壤样品放入干燥器,在2个单独的烧杯中分别加入30 mL氯仿(加入防沸材料)、30 mL稀氢氧化钠,打开阀门抽真空后关闭阀门,土壤样品在室温下暴露于氯仿中24 h[34]。将灭菌组样品和非灭菌组的土壤添加相应返还量的人工尿液,并同时添加无菌水(去离子水高温高压蒸汽灭菌法灭菌)将土壤含水量调整至60%土壤持水量,然后立即盖上已消毒的瓶盖,使用抽真空装置置换瓶内的气体为无菌空气(0.22 μm滤膜过滤后装入气袋),瓶内起始CO2的体积分数即为无菌空气中CO2的体积分数,将培养瓶置于20 ℃ (基于夏季月平均最高气温来设置)培养箱内避光进行培养,培养6 h (施加尿液后土壤排放的CO2主要集中在此期间,且土壤灭菌后的效果在此期间能得到较好地维持)后测定瓶内的CO2体积分数。
-
土壤CO2体积分数测定:抽取20 mL通入培养瓶内的无菌空气作为起始气体,培养6 h后,立即用注射器在每个待测样瓶中抽取20 mL气体样品,用气相色谱仪(Agilent 7890B)测定气体样品中的CO2体积分数。根据培养前后所抽取气体样品中的CO2体积分数差值计算土壤CO2排放速率,其计算公式为:
其中:ER为土壤CO2排放速率(mg·kg−1·h−1);22.4为标准状态下(273.2 K,1013.0 kPa)气体的摩尔体积(L·mol−1);P为取气样时培养瓶内的气压(kPa);P0和T0分别为标准状态下空气的绝对气压(kPa)和绝对温度(K);T为取气样时培养瓶内的绝对温度(K);ΔC为CO2体积分数(μL·L−1)在密闭时间内的增加值;Δt为密闭时间(h);V为培养瓶内的空气体积(mL);M为培养土样的干质量(g)。土壤CO2累积排放量为土壤CO2排放速率与培养瓶密闭时间的乘积。贡献比为生物过程或非生物过程对有机碳矿化的贡献百分比。
于培养结束后进行破坏性采样,取5 g培养结束后的土壤样品(包括灭菌组和不灭菌组)加入2 mol·L−1氯化钾溶液,按m(液)∶m(土)=5∶1浸提1 h,浸提后在170 r·min−1、25 ℃下充分震荡30 min后过滤,滤液采用靛酚蓝比色法测定铵态氮质量分数,双波长差法分析硝态氮质量分数。另取5 g培养后的鲜土土样用去离子水浸提,m(水)∶m(土)=5∶1,170 r·min−1振荡30 min后,在20 ℃下以3 500 r·min−1离心20 min,离心后取浸提液过0.45 μm微孔滤膜,使用TOC分析仪(Multi C/N 3100, 德国)测定土壤可溶性有机碳和溶解性总氮。土壤pH用煮沸的去离子水浸提,m(水)∶m(土)=2.5∶1.0,置于170 r·min−1摇床上振荡10 min后静置30 min,上清液用pH计测定。风干土壤过100目筛后,用凯氏定氮法测定土壤全氮质量分数,SOC质量分数采用高温外热重铬酸钾氧化-容量法测定。
-
利用Excel 2019进行数据预处理,在检验了数据的正态性和方差齐性后,采用SPSS 26的单变量方差分析及Duncan法检验不同尿液添加量和不同灭菌方法下CO2排放量之间的差异显著性(显著性水平为0.05)。对于不符合正态分布和方差齐性的数据,采用非参数检验的独立样本检验分析不同处理的结果之间是否存在显著性差异(显著性水平为0.05)。利用线性回归分析方法评估不同处理间CO2排放量与土壤可溶性有机碳之间的关系,采用Pearson系数对CO2排放量与土壤理化性质进行相关分析,用Origin 2022绘图。文中CO2数据皆为4个重复的平均值。
-
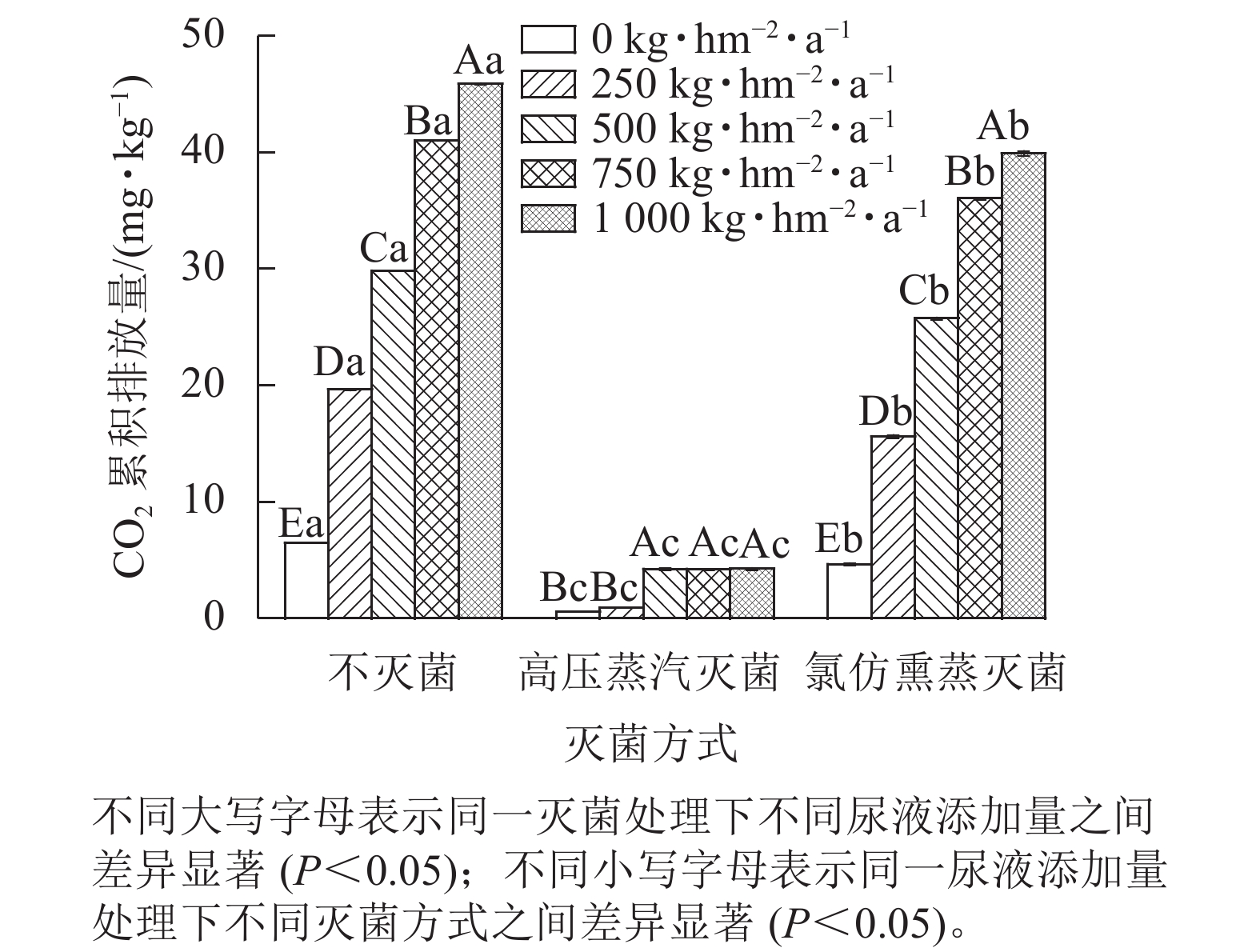
同一灭菌条件下,土壤CO2排放量随着尿液添加量的增加总体呈上升趋势(图1)。不灭菌与氯仿熏蒸灭菌处理下土壤CO2排放量随着尿液添加量的增加而增大,而高压蒸汽灭菌处理的土壤CO2排放量在尿液添加量达500 kg·hm−2·a−1后则不再变化。同一尿液添加量下,不灭菌方式下土壤CO2排放量显著高于灭菌处理(P<0.05),其中不灭菌方式的土壤CO2排放量比高压蒸汽灭菌处理高6~20倍,比氯仿熏蒸灭菌处理高14%~39%。再者,氯仿熏蒸灭菌处理下土壤CO2排放量也显著高于高压蒸汽灭菌处理,前者高出后者5~16倍(P<0.05)。
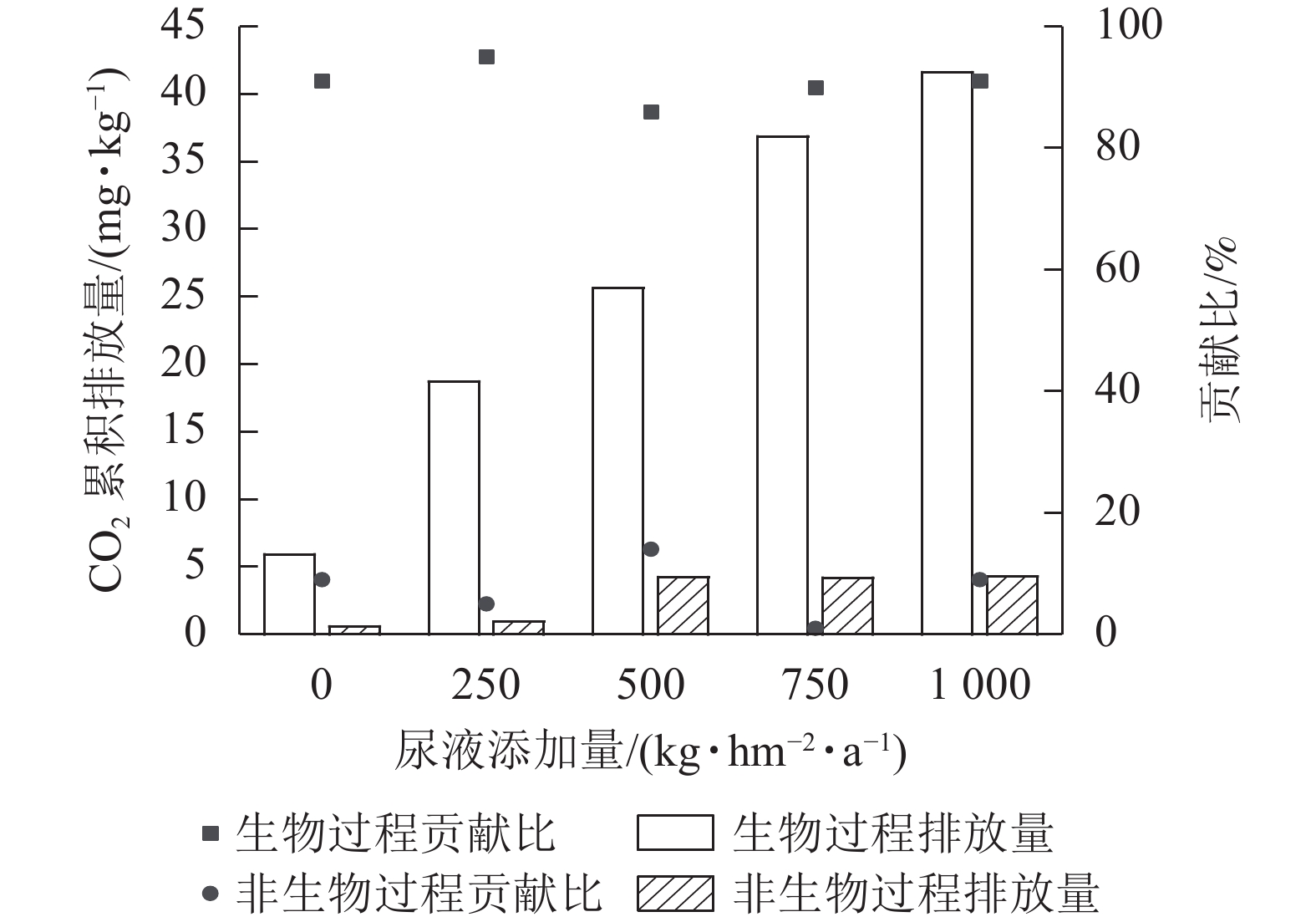
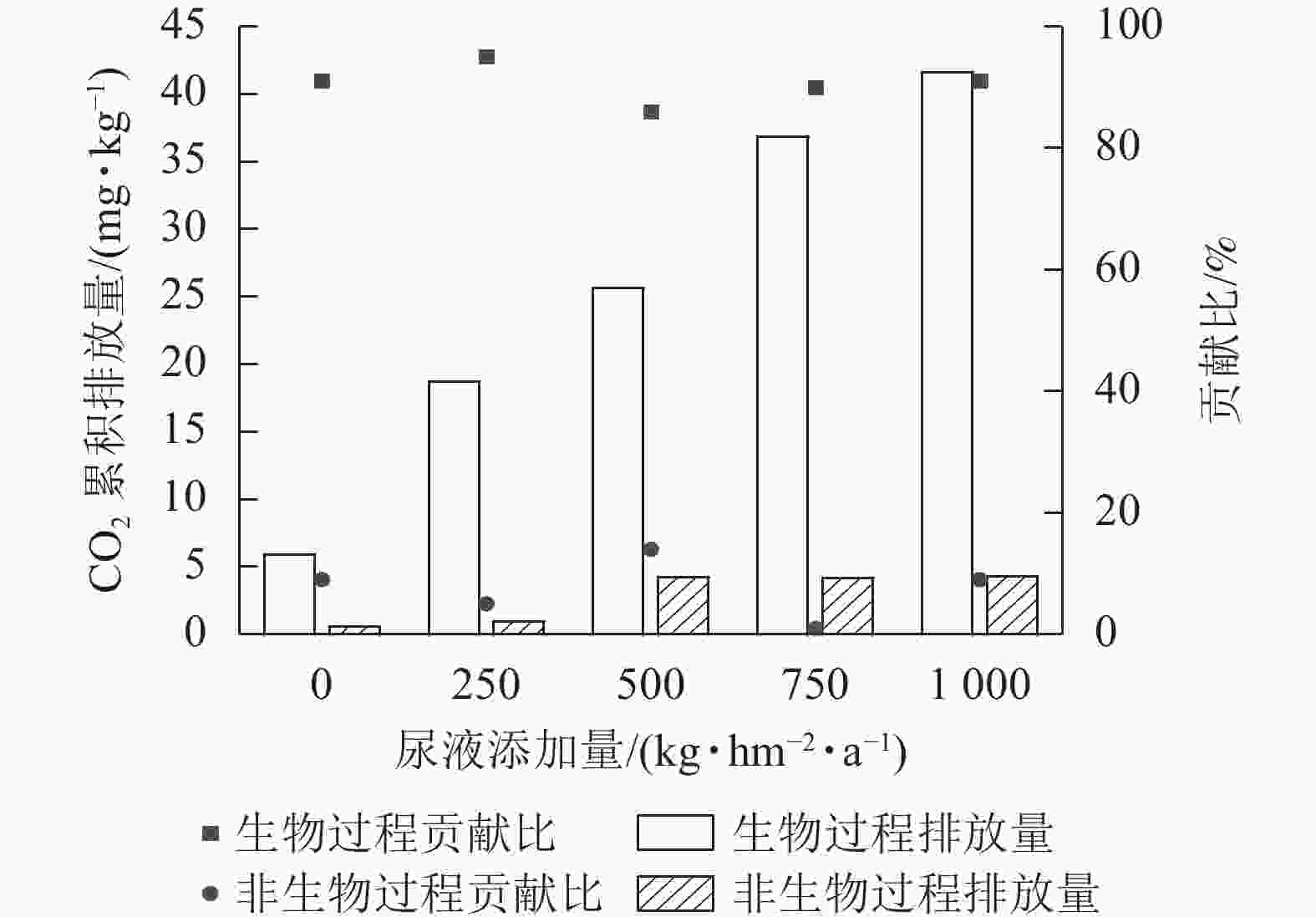
综上可知,灭菌处理可减少土壤CO2排放,但氯仿熏蒸灭菌的抑制效果较差,所以本研究基于不灭菌和高压蒸汽灭菌处理来计算生物和非生物过程对土壤CO2排放的贡献。生物和非生物过程对CO2排放的贡献为86%~95%和5%~14% (图2)。在尿液添加量为500 kg·hm−2·a−1时,非生物过程对CO2排放的贡献最大,达14%;在尿液添加量为250 kg·hm−2·a−1时,生物过程对CO2排放的贡献最大,达95%。
-
不灭菌方式下,土壤有机碳、全氮质量分数随尿液添加量的增加总体呈递减趋势,土壤pH、可溶性有机碳、溶解性总氮、铵态氮、硝态氮则呈现递增趋势,其中pH、可溶性有机碳、硝态氮在不同尿液添加量之间差异显著(P<0.05)。高压蒸汽灭菌条件下,土壤溶解性总氮、铵态氮、硝态氮随尿液添加量的增加呈递增的趋势,不同尿液添加量之间土壤有机碳、全氮、pH、可溶性有机碳差异不显著。氯仿熏蒸灭菌方式下,土壤pH、溶解性总氮、铵态氮、硝态氮呈现递增的趋势且各处理之间差异显著(P<0.05)。对于相同的尿液添加量,高压蒸汽灭菌处理的土壤可溶性有机碳和溶解性总氮高于不灭菌和氯仿熏蒸灭菌处理;而不灭菌处理的土壤pH和铵态氮总体上高于灭菌处理(表1)。
灭菌方式 尿液添加量/
(kg·hm−2·a−1)有机碳/
(g·kg−1)全氮/
(g·kg−1)pH 可溶性有机碳/
(mg·kg−1)溶解性总氮/
(mg·kg−1)铵态氮/
(mg·kg−1)硝态氮/
(mg·kg−1)不灭菌 0 63.95 a 6.20 a 6.08 e 265.32 c 45.76 b 92.95 b 20.77 d 250 59.58 b 5.57 bc 6.24 d 277.86 c 66.63 ab 165.81 ab 21.48 cd 500 56.97 b 5.55 c 6.36 c 320.48 b 96.41 ab 254.75 ab 25.07 c 750 59.30 b 5.80 b 6.48 b 337.21 b 132.36 a 348.65 a 48.74 b 1 000 57.90 b 5.73 bc 6.61 a 396.17 a 150.13 a 330.67 a 53.27 a 高压蒸汽灭菌 0 55.35 a 5.40 a 5.50 a 1 439.26 a 101.44 b 117.43 b 34.35 b 250 55.15 a 5.43 a 5.50 a 1 480.46 a 132.23 b 129.29 a 35.55 b 500 55.40 a 5.53 a 5.50 a 1 536.71 a 159.28 ab 124.44 ab 49.98 a 750 55.13 a 5.45 a 5.48 a 1 579.73 a 171.40 ab 118.96 ab 43.34 ab 1 000 55.43 a 5.35 a 5.48 a 1 548.92 a 189.43 a 120.87 ab 41.60 ab 氯仿灭菌 0 53.60 b 5.15 b 5.64 d 250.07 a 59.05 d 106.59 b 35.19 b 250 56.90 ab 5.53 ab 5.85 c 242.11 ab 66.95 c 140.95 ab 36.39 b 500 57.40 ab 5.60 ab 6.03 b 222.34 b 76.45 b 195.46 ab 50.82 a 750 60.43 a 5.80 a 6.15 a 243.17 ab 92.43 a 245.57 ab 44.18 ab 1 000 55.30 ab 5.10 b 6.23 a 237.76 ab 97.45 a 292.04 a 41.98 ab 说明:不同小写字母表示同一灭菌方式处理下不同尿液添加量之间差异显著(P<0.05)。 Table 1. Effects of sterilization and urine addition on soil chemical characteristics
-
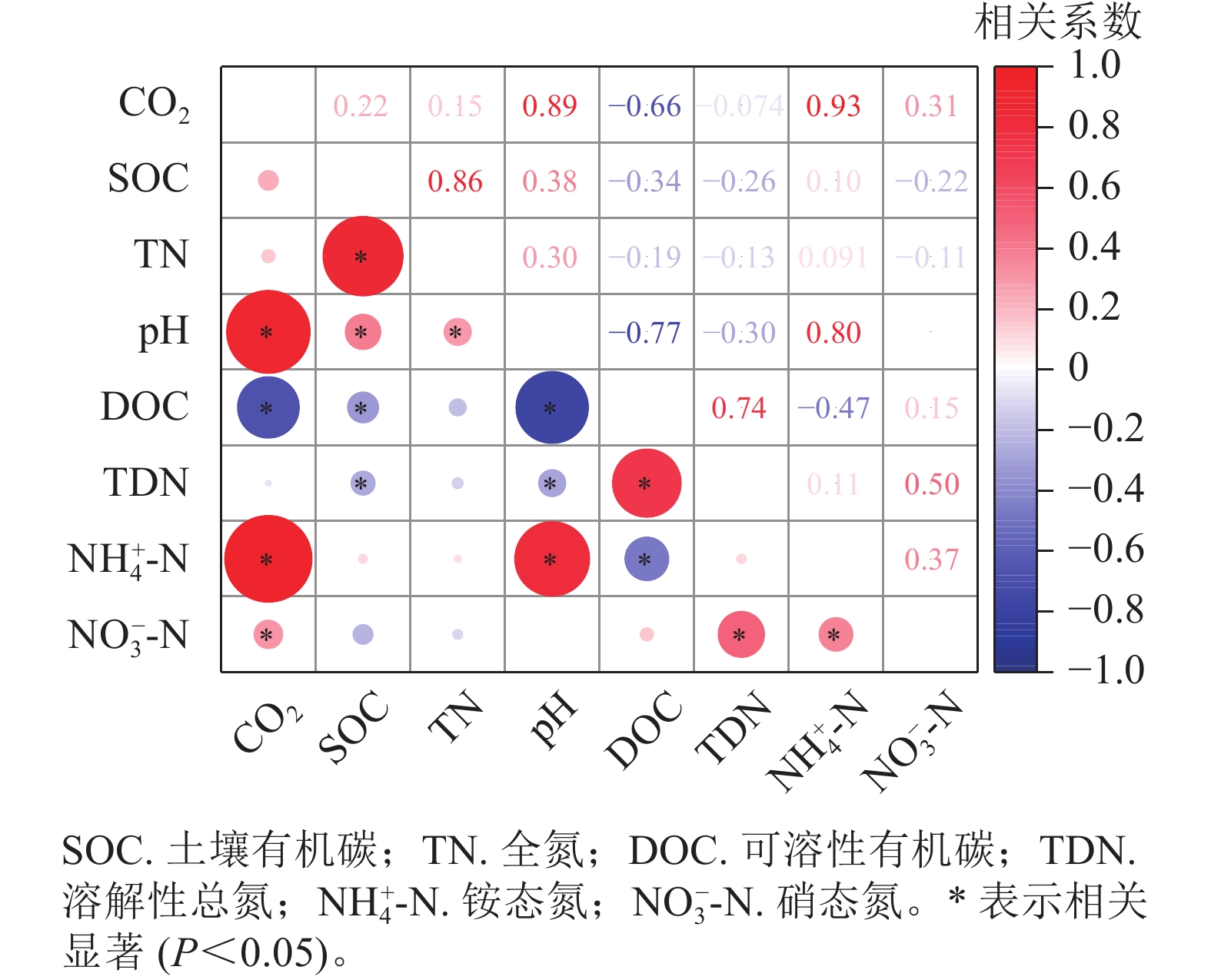
相关分析(图3)发现:土壤CO2排放量与土壤pH、铵态氮、硝态氮呈显著正相关(P<0.05),与有机碳、全氮、溶解性总氮不存在相关性。
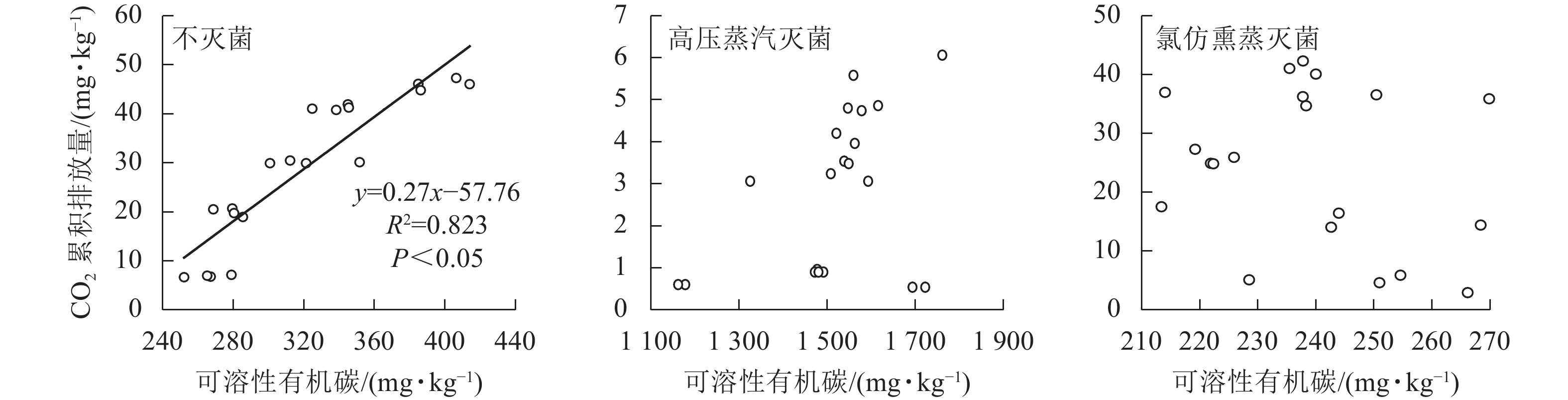
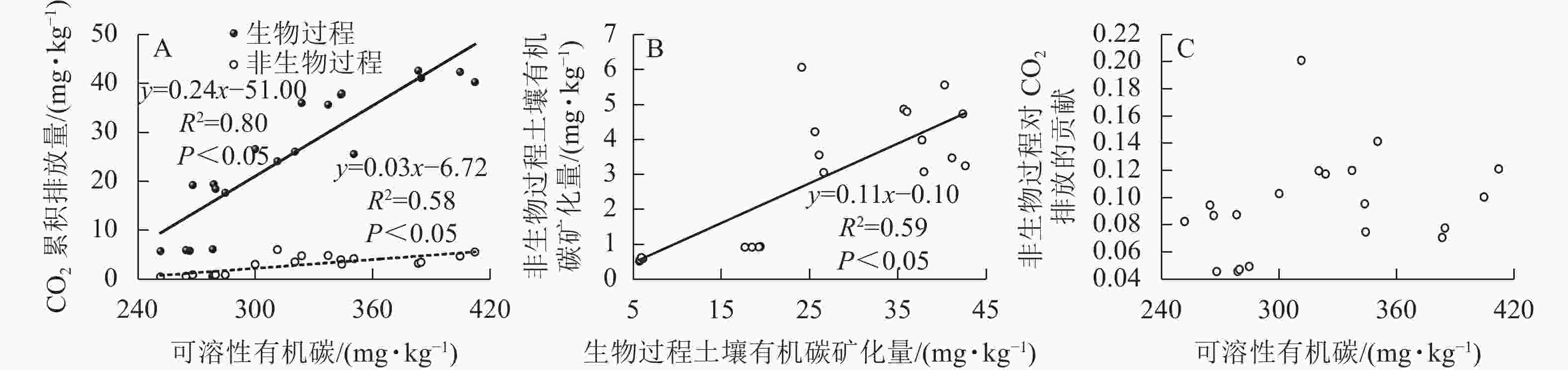
由于CO2的排放量与土壤可溶性有机碳质量分数(培养结束后测定值)总体呈现明显负相关,与以往结论相反,故对不同处理分别展开相关性分析。不灭菌方式处理下的土壤CO2排放量与土壤可溶性有机碳质量分数呈现正相关,经过高压蒸汽灭菌和氯仿熏蒸处理后的CO2排放量与土壤可溶性有机碳质量分数不存在线性相关(图4)。基于灭菌处理引起的土壤可溶性有机碳质量分数改变,导致了土壤可溶性有机碳与CO2排放量相关性的丧失,使得整体呈现负相关。

Figure 4. Relationship between soil dissolved organic carbon content and cumulative CO2 emission from the soils affected by sterilization
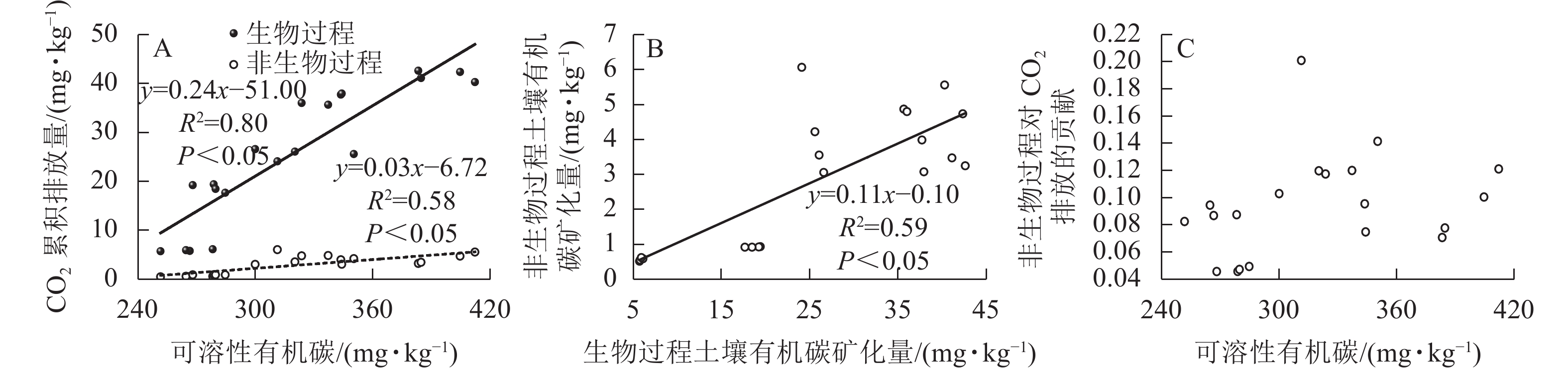
生物和非生物过程排放的CO2均与土壤可溶性有机碳质量分数呈正相关,但生物与非生物过程对土壤可溶性有机碳的敏感度不同,前者的敏感度更高(图5A)。生物和非生物有机碳矿化是正相关的(图5B),非生物过程对土壤CO2排放的贡献与土壤可溶性有机碳不存在相关性(图5C)。
-
不论是否进行灭菌处理,土壤CO2排放量均会随着尿液添加量的增加而增大,不过在高压蒸汽灭菌处理下土壤CO2排放量仅在部分尿液添加量处理间呈现显著差异。本研究结果与蒋梦蝶等[35]的研究一致。尿液添加量的增加为微生物提供了氮源,从而增强了微生物活性,促进有机碳矿化以及尿素分解后产生CO2,两者共同作用,导致CO2排放量增加。也有研究认为:添加尿液可造成土壤碳的分解以及土壤碳的溶解和浸出[36],从而促进土壤CO2排放,这可能是影响灭菌处理组CO2排放的主要机制。但在高温高压灭菌处理中,由于有机碳矿化以非生物过程为主,因此尿液添加量的增加对土壤CO2排放的促进效果并不十分明显,所以仅在部分尿液添加量处理间呈现显著差异。
相同尿液添加量下,灭菌处理显著降低了土壤CO2排放量,且氯仿熏蒸处理的土壤CO2排放量显著高于高压蒸汽灭菌处理。氯仿熏蒸可有效破坏土壤微生物的细胞膜结构,导致细胞裂解并将细胞内容物碳、氮等释放到土壤中[14]。然而,有研究发现:熏蒸处理后的土壤微生物整体表观活性并未有效降低[37];即使90%以上的土壤微生物被氯仿熏蒸破坏,在去除熏蒸剂和熏蒸剂杀死的微生物量碳的矿化后,有机碳矿化速率与未熏蒸土壤基本相同[30, 38]。由此可见,氯仿熏蒸灭菌效果并不完全。本研究发现:高压蒸汽灭菌法对于土壤CO2排放有明显抑制作用但不完全阻碍土壤CO2的排放,这可能与高压灭菌破坏细胞内外生化过程,但却同时保持非生物过程完整有关[14]。土壤经高压蒸汽灭菌处理后,微生物大量死亡,导致土壤CO2排放作用减弱,此时有机碳矿化以非生物过程为主。本研究发现:土壤CO2排放量与土壤可溶性有机碳总体呈负相关,这与以往的结论相悖。通过将数据按照不同灭菌处理拆分分析发现:高压蒸汽灭菌和氯仿熏蒸灭菌处理下的CO2排放量与土壤可溶性有机碳均无显著相关性。据此推断,土壤CO2排放量与土壤生化指标的正相关性很大程度上与生物过程的参与有关。廖添怀等[39]研究指出:有生物过程参与的土壤CO2排放与土壤可溶性有机碳呈正相关。然而,经过高压蒸汽灭菌处理后,土壤CO2排放量远低于不灭菌和氯仿熏蒸灭菌处理,但同时其土壤可溶性有机碳质量分数却远高于其他处理组,这是由于高温高压处理中蒸汽杀死土壤中大量微生物[40],死亡的生物细胞快速释放出有机质进入土壤,使得土壤中的可溶性有机碳质量分数显著增加[41]。由此推测,高压蒸汽灭菌处理使得土壤环境发生剧烈改变,从而出现土壤可溶性有机碳质量分数骤增而CO2排放量大幅度下降,将高压蒸汽灭菌处理的数据并入分析是导致负相关趋势的关键原因。不过,高压高温环境导致的土壤可溶性有机碳质量分数骤增如何影响非生物CO2产生过程还有待进一步研究和探讨。
生物和非生物过程产生的CO2均与土壤可溶性有机碳显著正相关,其中生物过程对土壤CO2排放的贡献比非生物过程更大,这一结论与BENOIT等[15]的研究结果相近。再者,BENOIT等[15]还发现:非生物过程对土壤CO2排放量的贡献随着可溶性有机碳质量分数的增加而增大,而本研究中非生物过程对土壤CO2排放的贡献与土壤可溶性有机碳不存在相关性。这可能是灭菌方法不同而导致的。本研究中高压蒸汽灭菌法使得土壤可溶性有机碳质量分数明显提升,因此两者之间不存在相关性。在以往研究中发现:当生物过程较强时,非生物过程对土壤CO2排放的影响可以忽略不计;然而,如果生物过程较弱,非生物过程可能主导土壤CO2排放[10]。虽然高压灭菌法并不是区分非生物和生物过程CO2排放的准确方法,但其是各种土壤灭菌方法中的最佳方法,所得结果可视为接近真实情况的参考值[42]。
-
不论是否进行灭菌处理,尿液返还对土壤CO2排放的促进效应会随着尿液返还量的增加而呈增大趋势,但在尿液添加量超过一定梯度时,土壤CO2排放量不再受尿液添加量的影响。在本研究中,生物和非生物有机碳矿化过程对不同尿液返还量的响应趋于相同,但生物过程对土壤可溶性有机碳的敏感度更高,且生物过程对土壤CO2排放的贡献是非生物过程的6~19倍。非生物过程在某些条件下对土壤CO2排放具有重要贡献,因此,非生物过程CO2排放对不同人类活动或全球气候变化的响应机制还需深入研究。
Response of grassland soil CO2 emissions to urine addition under different sterilization methods
doi: 10.11833/j.issn.2095-0756.20230149
- Received Date: 2023-02-16
- Accepted Date: 2023-06-14
- Rev Recd Date: 2023-05-16
- Available Online: 2023-11-23
- Publish Date: 2023-11-23
-
Key words:
- soil sterilization /
- bovine urine addition /
- soil CO2 emission /
- organic carbon mineralization /
- biotic and abiotic processes
Abstract:
| Citation: | JIANG Shuzhen, LIAN Yichen, HUA Runxin, et al. Response of grassland soil CO2 emissions to urine addition under different sterilization methods[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1241-1249. DOI: 10.11833/j.issn.2095-0756.20230149 |










 DownLoad:
DownLoad: