-
红花石蒜 Lycoris radiata是石蒜科Amaryllidaceae石蒜属 Lycoris的多年生球根花卉,分布于亚热带地区,喜温暖湿润气候,耐旱,不喜强光[1]。一般在10月出叶,翌年5月叶片枯萎凋落,地上部进入休眠期,至翌年9月上旬无叶期开花[2]。其花形奇特、花色红艳,极具观赏价值。鳞茎富含多种生物碱和淀粉,药用和工业价值较高[3]。因鳞茎生长缓慢、种球自然繁殖率低[4],石蒜的产业化开发受限。
碳水化合物在植物器官发育及种子萌发过程中起重要作用[2]。糖和淀粉是维持鳞茎碳水化合物平衡的主要物质[5],在植物休眠期间还充当信号物质[6],也是鳞茎打破休眠开始萌发的能量与物质基础[7]。其中,非结构性碳水化合物(NSC)是重要的能源物质,对鳞茎的“源—库”平衡至关重要,其动态变化反映了植物体内的碳收支情况以及对外界环境的适应[8]。稳定同位素标记法是研究光合碳固定、分配及转移的重要工具,具有安全、稳定、易操作等优点,在生物及化学研究中被广泛应用。目前,已在小麦Triticum asetivum [9]、玉米Zea mays [10]、水稻Oryza sativa [11]等农作物及山毛榉Fagus longipetiolata[12]、马尾松Pinus massoniana[13]和冷杉 Picea abies [14−15]等树木中采用同位素标记法开展同化物的分配研究,且大多采用脉冲标记法。通过测定非结构性碳水化合物中单一化合物的同位素比率(δ13C),有助于理解光合作用后续代谢及生理生化过程中的碳同位素分馏效应[16]。
目前,有关石蒜体内非结构性碳水化合物的研究多集中在石蒜不同生长发育阶段[7, 17−18]、不同器官或部位内的非结构性碳水化合物含量变化[19]等方面,较少涉及非结构性碳水化合物的合成、转运及代谢过程。为此,本研究以红花石蒜为研究材料,采用13C同位素脉冲标记法追踪光合碳在不同器官和非结构性碳水化合物各组分中的分配与累积量,旨在探析石蒜体内光合碳的运输和分配规律,为进一步阐明石蒜体内糖类物质的积累、转化及代谢过程,揭示库—源转化的生理机制提供依据。
-
研究地点位于江西农业大学园林与艺术学院花卉盆景实训基地(28°45′41′′N,115°50′28′′E),海拔50 m,属亚热带湿润季风气候,四季分明,雨水充沛。研究于2021年11月中旬(红花石蒜生长旺盛期)进行,供试材料为3年生的盆栽红花石蒜,共9株(1株1盆),其中6株被标记13C,其余3盆未标记作为δ13C参考。供试的盆栽石蒜种球大小基本一致(种球直径为2.8~3.0 cm),健康无病虫;种植盆的规格为130 mm (口径)×95 mm (底径)×200 mm (高)。种植基质为蛭石∶泥炭土=1∶1(质量比)。
-
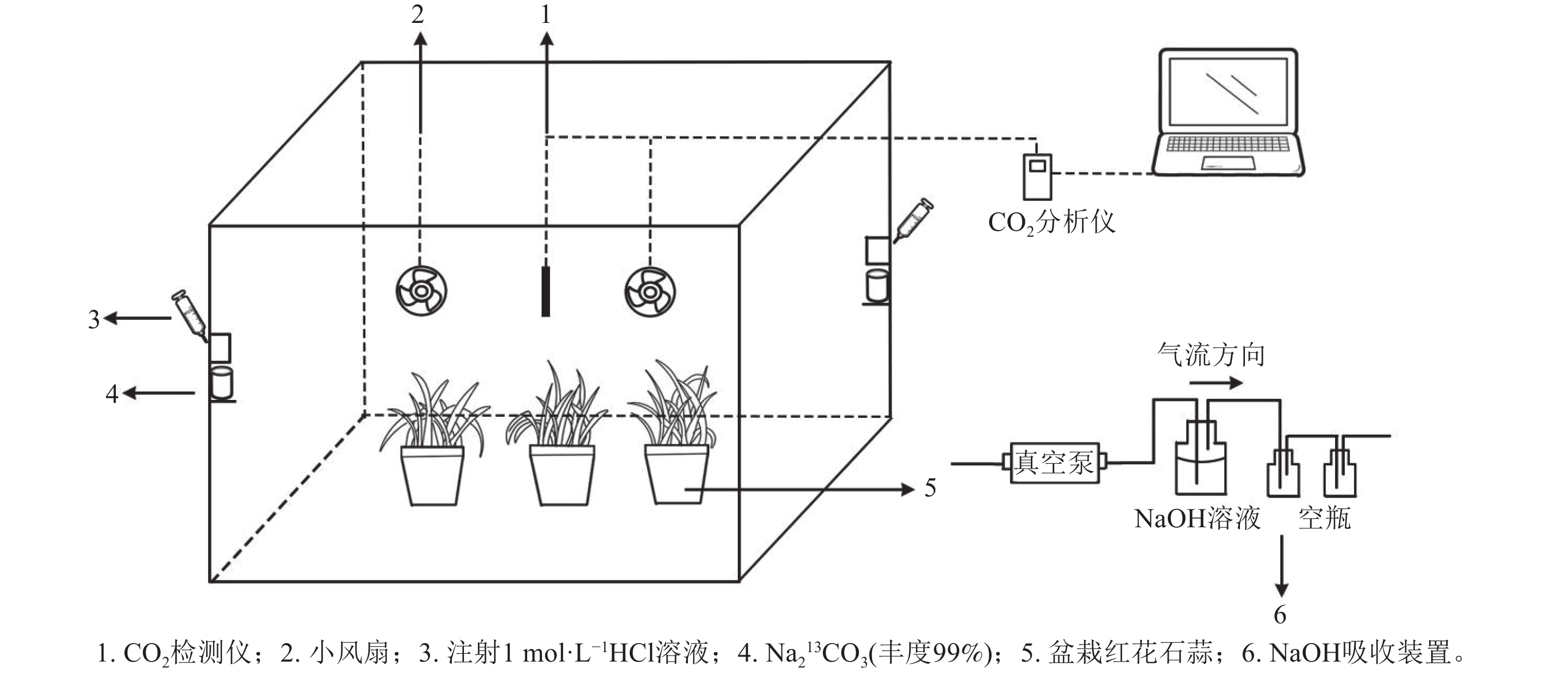
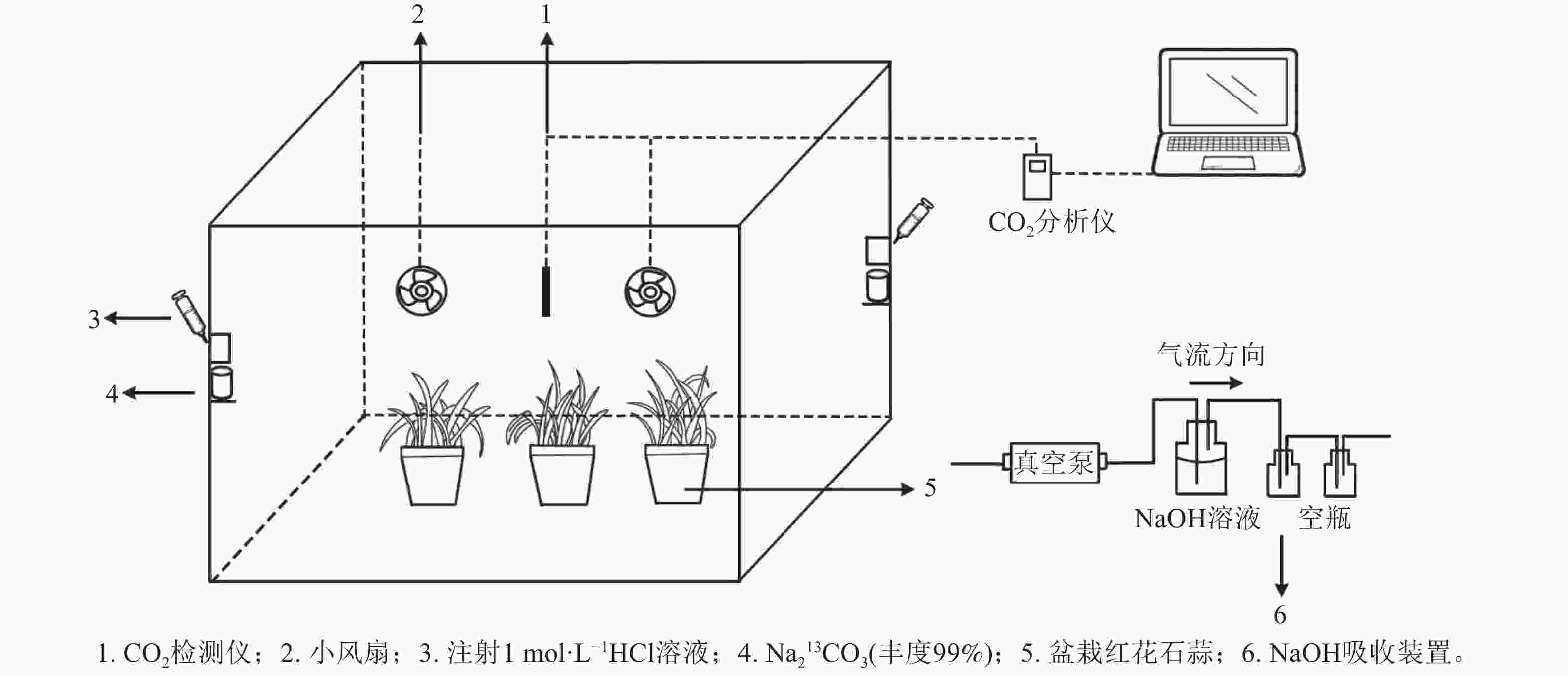
采用的脉冲标记法参照HU等[20]执行,采用亚克力板搭建标记室(长×宽×高为0.7 m×0.6 m×0.7 m),置于光照充足的户外,植物13C标记在标室中进行(图1)。标室内配有小风扇(用以均匀分配产生的13CO2)、冰块(降温)和烧杯等,并采用气球法检查标室气密性。
标记前,先利用装有1 mol·L−1 氢氧化钠(NaOH)的吸收装置吸除标记室内的12CO2,再将6盆待标记植物移入标室内,同时将其余3盆置于距离标记室10 m以外的地方,作为空白对照(ck)。标记开始时,先用注射器将适量1 mol·L−1盐酸(HCl)注入装有Na213CO3的烧杯,反应产生13CO2;当标室内CO2被植物吸收浓度降低到一定值后,再在另一个装有Na213CO3的烧杯中注入等量盐酸。标记从9:00开始,至17:00结束。期间,使用CO2监测仪(Li-850)实时监测标记室内的CO2浓度。待全部反应完毕后,再将适量盐酸注入装有Na212CO3的烧杯中,使其产生12CO2,以提高箱内CO2浓度,促进残留的13CO2同化。待植物不再吸收CO2进行光合作用时,关闭吸收装置。
-
在标记结束后,同时将被标记和对照处理的红花石蒜从盆中取出,冲洗干净并吸干表面水分后,分别采集石蒜植株的根、叶和鳞茎,再切取鳞茎的内层鳞片(第2~4片)、中层鳞片(第5~8片)、外层鳞片(第9~12片)样品。将样品先置于105 ℃ 烘箱中杀青处理30 min,再放于65 ℃干燥箱中干燥24 h至恒量,称取各样品干质量,并计算其生物量。随后,再将烘干的样品研磨成细粉,分成2份。其中,一份用于测定不同非结构性碳水化合物组分的有机碳和δ13C值;另一份则用于测定样品中的蔗糖、葡萄糖、果糖及淀粉质量分数。
-
①可溶性总糖的提取。取0.05~0.10 g干样细粉置于具盖的5 mL塑料离心管中,加入2 mL的纯净水,在80 ℃水浴下浸提10 min;再以3 000 r·min−1离心5 min,取上清液装入10 mL的具塞刻度试管中沉淀,再用蒸馏水提取2次,每次2 mL。收集并合并离心后的上清液,提取液定容至10 mL,用于可溶性糖(葡萄糖、果糖和蔗糖)的13C测定,残渣则用于淀粉水解分析。② 淀粉水解。取①中可溶性糖测定时离心管内残渣,加入2 mL水,在沸水浴中加热糊化10 min;经冷却后再加入1 mL 9.2 mol·L−1高氯酸,置冷水浴中提取15 min后,以3 500 r·min−1离心5 min,取上清液。在残渣中,再加入2 mL 4.6 mol·L−1高氯酸,继续在冷水浴中浸提10 min,后离心取上清液。再将残渣用2 mL水洗涤,离心,取上清液,合并以上3步上清液,将其定容至10 mL,即为淀粉测定样液。将样液置于冷冻干燥机内浓缩(至少2 d)成浓缩液或干粉,用于淀粉的13C测定。③稳定同位素分析。采用质谱仪与EA-HT元素分析仪、LC-isolink联用法测定同位素比率。首先,将样品在元素分析仪中高温燃烧后生成CO2,再用质谱仪检测通过的CO2中的13C与12C比率,经与国际标准物(PDB)进行比对后,再计算得出样品的δ13C值。其中,含碳量是通过比较待测样品和3个工作标样的出峰面积计算而得(精度:δ13C 为±<0.1‰;含碳量为±<0.5%)。
-
淀粉、蔗糖、葡萄糖、果糖使用分光光度法测定,具体操作参照苏州科铭生物有限公司的试剂盒说明书执行。
-
根据文献[21]将同位素质谱仪得出的δ13C值换算成13C原子百分比[AT13C (%)]。参照邓秀秀等[13]的计算方法计算各器官(或部位)中含碳总量(mg)、库活力、各器官中非结构性碳水化合物(NSC)积累量(mg)以及不同NSC组分在各器官的13C分配比例(%)。各器官及不同NSC组分的13C分配率和13C富集率的计算公式如下:
其中:i为不同器官,Si为不同NSC组分;13Ci各器官固定的13C,13C(Si)各器官中不同NSC组分固定的13C;Ci为各器官或部位的含碳总量;C(Si)为NSC各组分中的含碳总量;Cup为标记溶液所产生的13C量。
-
利用Excel 2021整理试验数据,利用SPSS 25.0进行差异显著分析(Duncan法),利用OriginPro 2019b、Photoshop 2019制作图表。
-
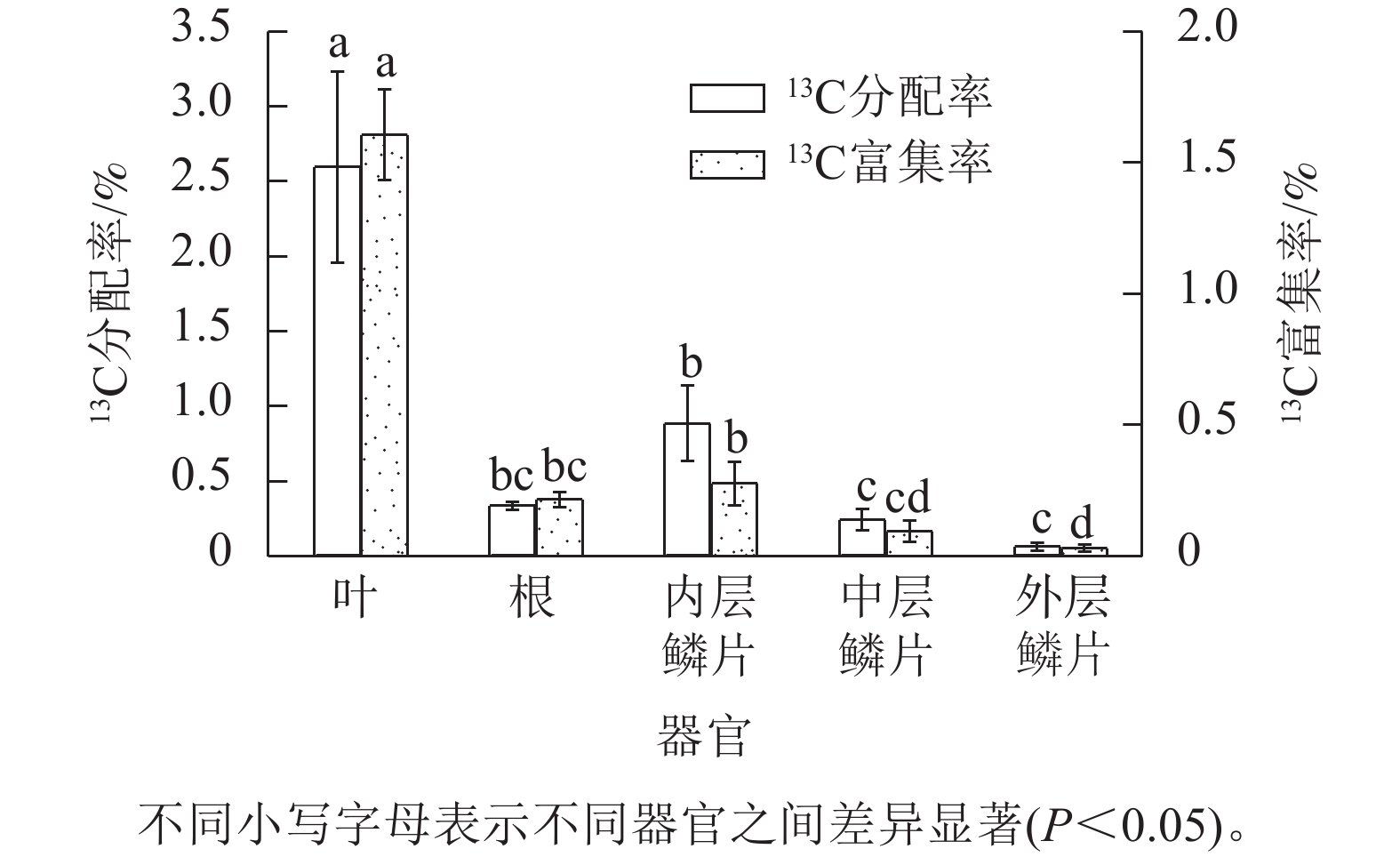
由图2可知:红花石蒜不同器官之间的13C 分配率差异显著(P<0.05),且总体表现为新同化的13C光合产物分配率呈现从上(叶)到下(根)逐渐减少趋势,即新同化的碳分配率从高到低依次为叶(2.59%)、鳞茎(0.39%)、根(0.33%)。此外,13C分配率在鳞茎横向分配中表现出由内到外逐渐递减趋势,即从高到低依次为内层鳞片(0.88%)、 中层鳞片(0.23%)、外层鳞片(0.05%)。经统计分析鳞茎3个不同部位的数据可知,鳞茎内层鳞片13C分配率显著高于中层鳞片和外层鳞片13C分配率,但中层鳞片与外层鳞片之间并无显著差异。另外,根中的13C分配率虽然高于中层鳞片和外层鳞片中的13C分配率,但差异并不显著。说明新同化的碳在石蒜体内的“纵向”运输效率要优先于“横向”,可能与绿叶期红花石蒜的叶和根部均处于旺盛的生长状态,而同期的中层鳞茎和外层鳞片膨大相对较弱有关。从新同化的碳对相应碳库的相对贡献视角发现,以红花石蒜叶中13C富集率最高(即其在相应碳库中新同化的13C占比为1.60%),说明叶中新同化的13C对相应碳库的相对贡献最大;其次为根(0.21%),而以鳞茎中(3个部位的平均值)的13C 富集率最低(仅为0.13%)。在鳞茎中,不同部位鳞片中的13C 富集率从高到低依次为内层鳞片(0.27%)、中层鳞片(0.09%)、外层鳞片(0.02%),这与13C的分配规律趋同。
-
由13C标记的非结构性碳水化合物各组分在红花石蒜不同器官中的分配比例差异显著(P<0.05)。其中,13C标记的蔗糖在不同部位中的分配比例从高到低依次为内层鳞片、叶、中层鳞片、根、外层鳞片,且内层鳞片中的分配比例显著高于其他4个器官(P<0.05)(图3 A)。13C标记的葡萄糖却在叶片中的分配比例显著高于其他4个器官(图3 B),其他4个器官之间无显著差异。13C标记的果糖则在叶片和内层鳞片中的分配比例显著大于其他3个器官(P<0.05),根中的分配比例大于鳞茎的中层鳞片和外层鳞片,但三者之间差异并不显著(图3 C)。13C标记的淀粉也以叶中的分配比例最大;在鳞茎中,以内层鳞片中的占比最大,其次为中层鳞片,外层鳞片最低;根中13C标记淀粉的分配比例大于外层鳞片中的分配比例(图3 D)。这些结果说明:鳞茎是光合产物的运输和储藏中心,在叶绿期鳞茎内的新淀粉合成以内层鳞片为主,其次为中层鳞片,外层鳞片的淀粉代谢活动最弱。另外,根中的糖代谢活动也较为活跃(如蔗糖的水解),因为此时的根系正处于旺盛生长期。
-
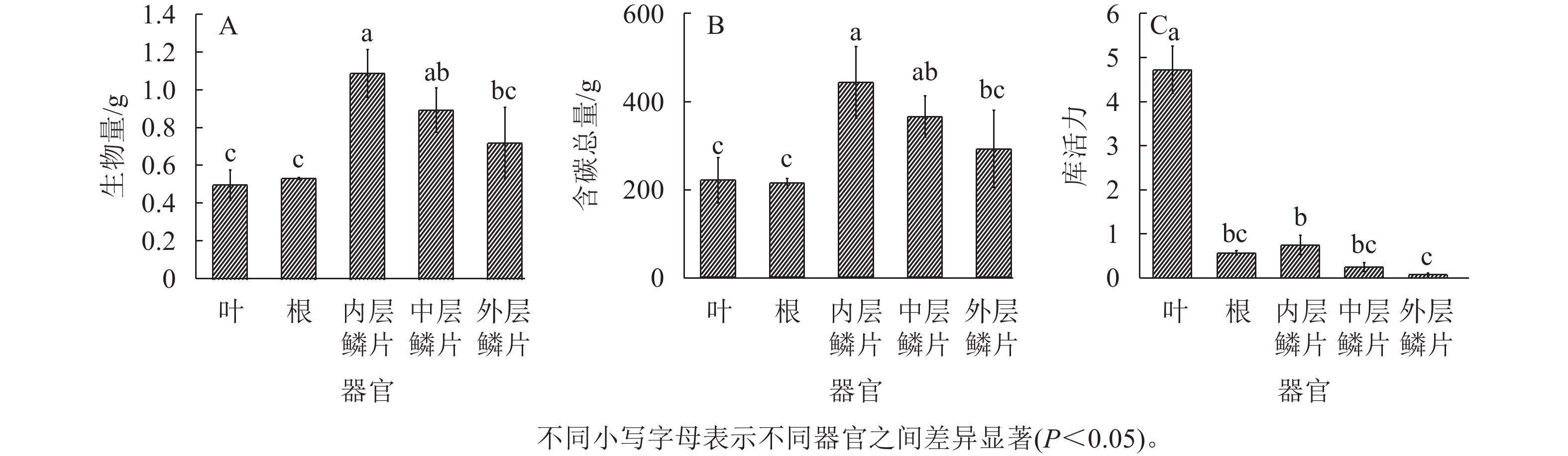
从图4A~B得知:在红花石蒜的不同器官中,生物量、含碳总量均以鳞茎(3个部位的平均值)最大。在鳞茎的3个不同部位之间,均以内层鳞片的生物量、含碳总量最高,且显著大于外层鳞片的生物量、含碳总量(P<0.05)。另外,不同器官的库活力从高到低依次为叶、内层鳞片、根、中层鳞片、外层鳞片(图4C),其中,叶片的库活力分别为内层鳞片、根部、中层鳞片和外层鳞片的6.3、8.3、19.1和65.7倍。在鳞茎中,库活力依然为从内到外逐渐降低。根中的库活力大于中层鳞片和外层鳞片,表明红花石蒜根的单位生物量吸收同化产物的速率高于中层鳞片和外层鳞片。
-
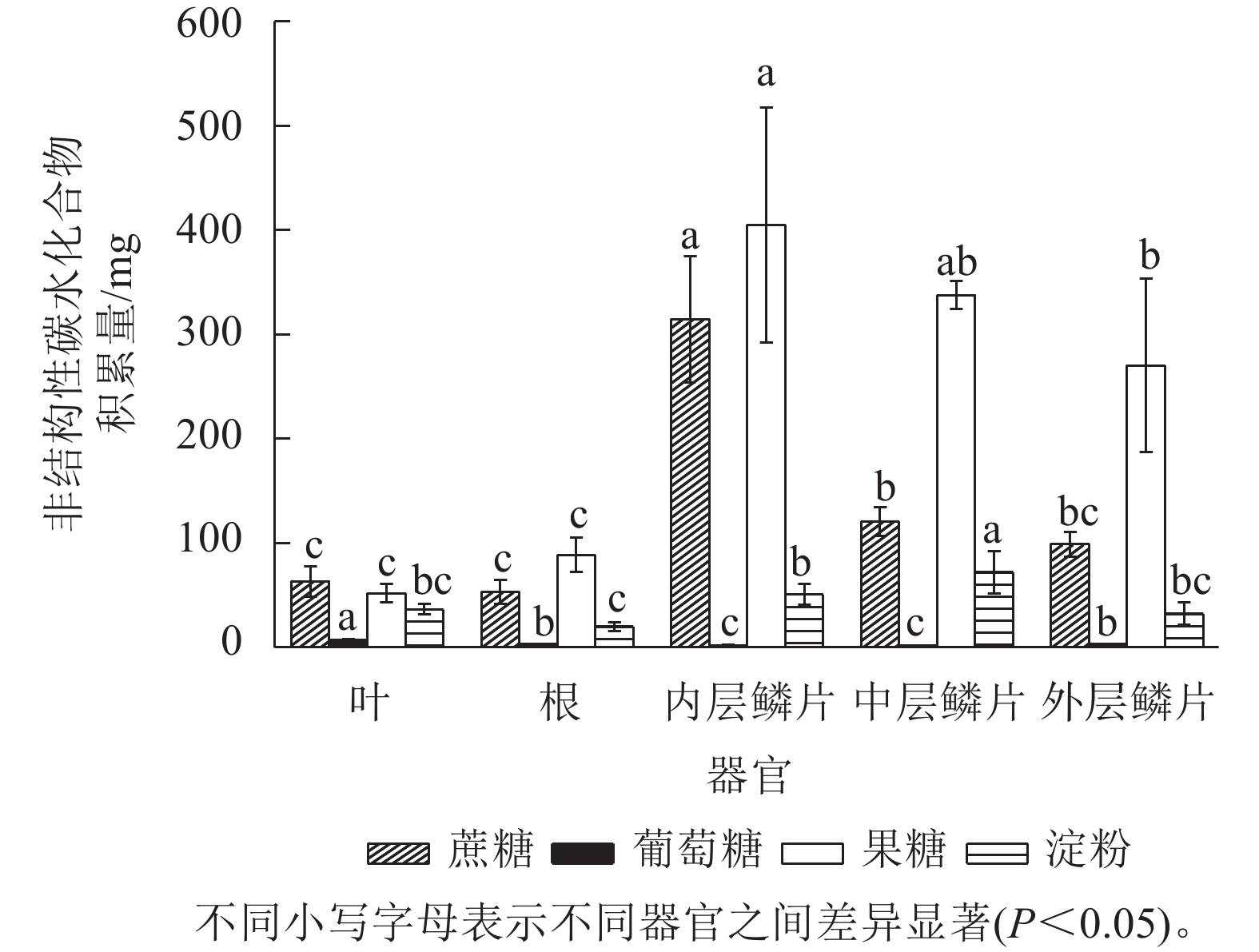
由图5可知:非结构性碳水化合物(主要包括蔗糖、葡萄糖、果糖和淀粉等物质)在红花石蒜各部位中的积累量存在显著差异(P<0.05),总体上从高到低依次为内层鳞片、中层鳞片、外层鳞片、根、叶片。其中,各器官中可溶性糖积累量(蔗糖、葡萄糖、果糖之和)均远高于淀粉,而在可溶性糖中又主要以果糖积累量最高,其次为蔗糖,葡萄糖较少。另外,在5个不同部位中,以内层鳞片中可溶性糖积累量最高。说明内层鳞茎是石蒜体内碳水化合物(尤其是可溶性糖)的转运中心和果糖的积累库。
-
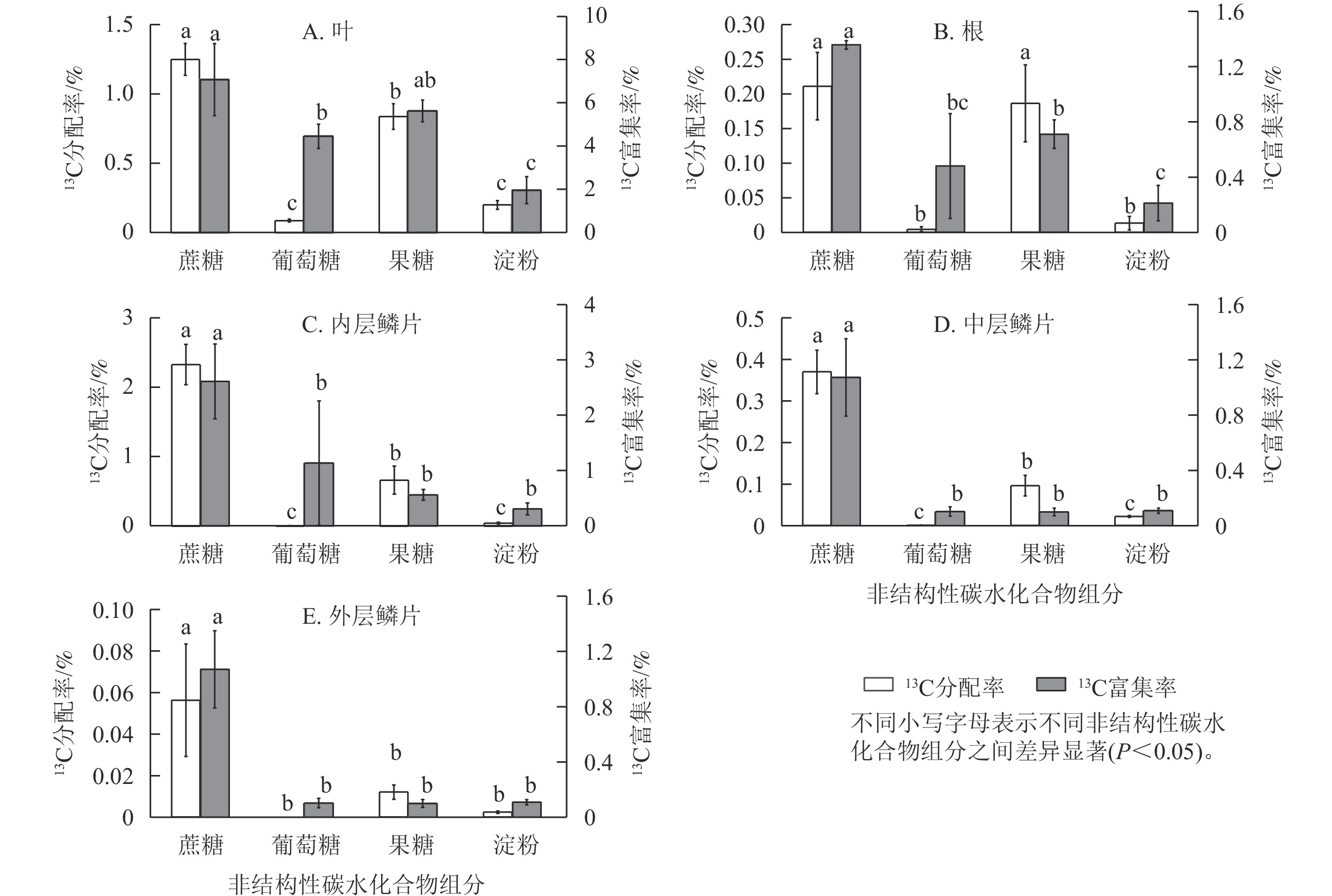
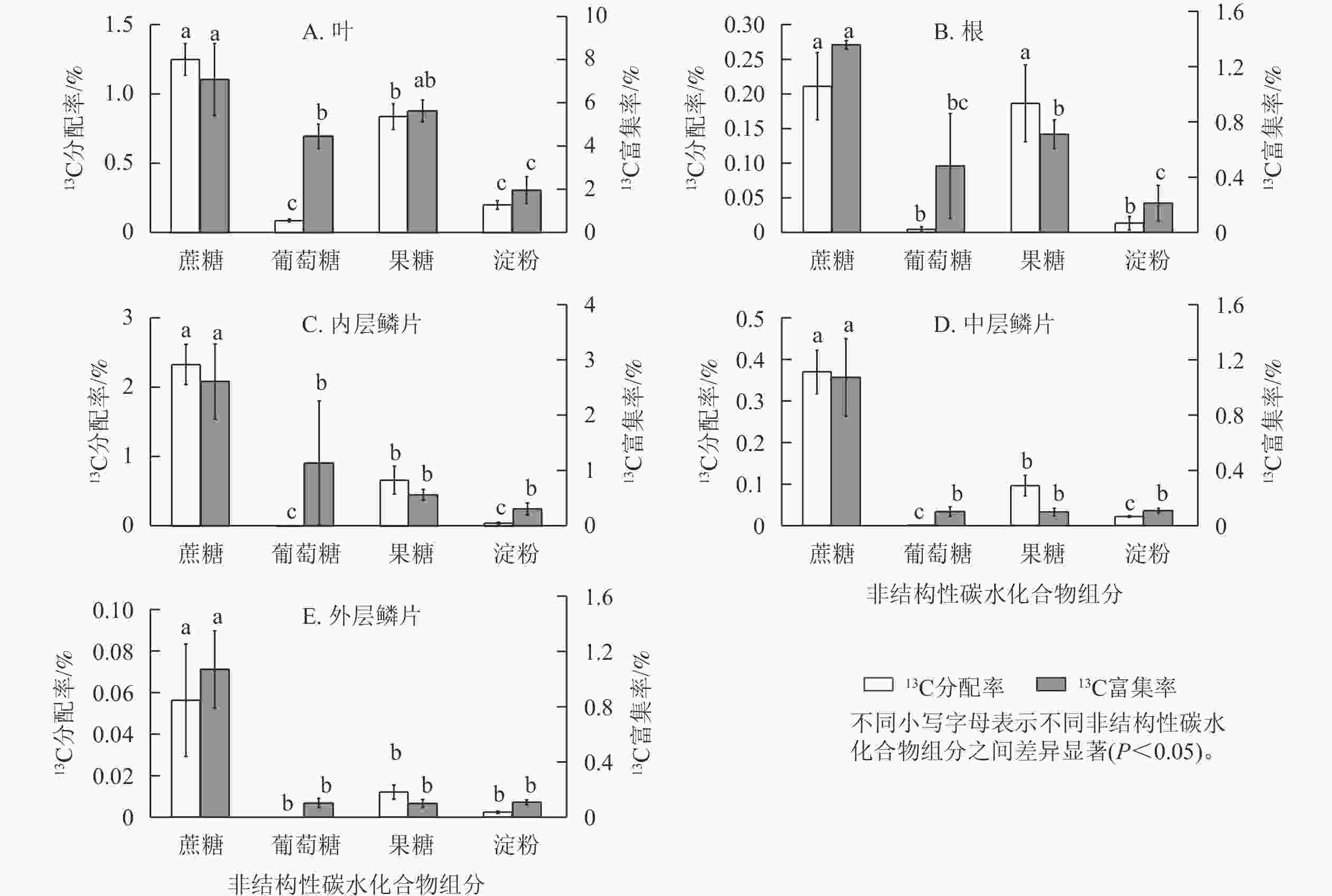
由图6可知:红花石蒜经过光合作用在各部位中新合成的4种糖类(蔗糖、葡萄糖、果糖和淀粉)的13C分配率之间存在显著差异(P<0.05)。其中,在红花石蒜各器官内非结构性碳水化合物的13C分配率均以蔗糖最多,显著高于其他3种组分(除根中13C标记的蔗糖与果糖无显著差异外);其次为13C标记的果糖,而各部位中13C标记的葡萄糖甚微。说明蔗糖不仅是石蒜体内主要的光合产物,也是非结构性碳水化合物运输的主要形式。另外,在石蒜根、内层鳞茎和中层鳞片中均有少量13C标记的淀粉,而在外层鳞片中几乎检测不到含13C标记的淀粉(图6 B~E);在石蒜叶、根和内层鳞片中,果糖的13C分配率均显著高于葡萄糖的13C分配率(P<0.05),依次为13C标记的葡萄糖的9.6、41.5和118.1倍;在中层鳞片和外层鳞片中,几乎没有检测到带13C标记的葡萄糖。此外,在石蒜的各个部位中,除外层鳞片外(外层鳞片中13C标记的淀粉分配率与果糖分配率无显著差异),其余部位淀粉13C分配率均显著低于蔗糖和果糖13C分配率(P<0.05),但与葡萄糖13C分配率却无显著差异。
石蒜体内的蔗糖不仅13C分配率最高,而且13C 富集率也最大。在叶、根、内层鳞片、中层鳞片、外层鳞片中,蔗糖中新同化的13C分别占蔗糖碳库的7.0%、1.3%、2.6%、1.1%、0.2%。蔗糖13C富集率较高表明:这类化合物合成及转化的速率较快,化学反应较活跃。相反,淀粉的13C 富集率较低,说明淀粉的合成及转化速率较慢。尽管在石蒜的叶、根和内层鳞片中13C标记的葡萄糖13C分配率均低于淀粉,但葡萄糖的13C富集率却高于淀粉(图6 A~C)。这说明新合成的葡萄糖虽然比淀粉少,但却对叶、根及内层鳞片中葡萄糖碳库产生了极大贡献。另外,在叶和根中不仅13C分配率较高,13C 富集率也较高,且均大于葡萄糖13C 富集率,与分配模式相同;由果糖同化的13C分别占果糖碳库的5.6%、0.7%。而在内层鳞片中,果糖13C 富集率却低于葡萄糖13C 富集率,与分配模式相反。
-
光合碳同化是指利用光反应形成的同化力将CO2还原形成糖类物质的过程,主要在叶绿体基质中发生,是由多种酶参与的一系列化学反应。新合成的糖类再通过韧皮部运输到植物的其他部位,从而完成由源到库的长距离运输[22]。其中,新合成碳的分配不但受光合作用同化物供应的控制,同时也取决于不同器官利用供应物的能力[23]。
由于叶片为光合产物的主要吸收源,新同化的碳被优先大量分配在叶中[24]。本研究发现:13C在红花石蒜中的分配率由高到低依次为叶、鳞茎(茎)、根,这与山杨 Populus davidiana 和栎树 Quercus [25]、玉米[26]的研究结果相似。本研究还发现:红花石蒜鳞茎中13C分配在横向分布上呈现一定的规律性,即新同化的碳分配表现为由内层(0.88%)向外层(0.05%)逐渐降低的趋势,且新合成的13C标记的淀粉、蔗糖、果糖、葡萄糖分配也表现出类似现象。说明在石蒜鳞茎中,代谢活动从内到外呈逐渐减弱态势。此外,尽管鳞茎内层鳞片中的13C分配率大于根,但中层鳞片与外层鳞片中的13C分配率却小于根;且根中也有更多的蔗糖被分解。这可能与供试石蒜鳞茎的特殊结构、石蒜所处的特定的生长时期及植物体内有机物的纵向运输要优于横向运输有关。因为植物的器官特异性(鳞茎是由鳞叶与鳞茎盘构成)及韧皮部的路径和长度也可能对碳吸收能力和转移速率有一定的影响[27]。可见,掌握石蒜体内的碳分配规律可以实现对鳞茎库强的调控。
13CO2在红花石蒜体内的运输路径大致可分为2个步骤:首先,叶片(成熟叶片系内层鳞芽转变而来)在吸收CO2进行光合作用后,将新合成的光合产物从叶片运输至地下部的鳞茎盘、内层鳞片,继而沿纵向运输至根部以供其生长;其次,再将多余的有机物经鳞茎盘(转运平台)进行横向运输(即从鳞茎的内层鳞片依次运往中层鳞片和外层鳞片),最终转化成临时储藏或长时储存的物质与能量。
-
植物具有固定碳的特殊能力,并以碳水化合物的形式储存运输碳和能量,因而糖代谢在植物的生长发育中一直发挥着核心作用[28]。本研究表明:在石蒜的5种不同器官(或组织)中,13C在非结构性碳水化合物各组分中的分配均以蔗糖中最多,葡萄糖中最少。这与罗琦[7]认为石蒜属植物鳞茎中的还原性糖质量分数占比较小,而蔗糖才是转运的主要形态的研究结果相似。另外,13C标记的蔗糖在不同器官中的分配比例以内层鳞片最高,且内层鳞片的含碳总量与库活力大小也均大于中层与外层鳞片,可能是由于内层鳞片不仅是石蒜体内碳水化合物的转运中心,还是为花芽原基生长发育提供营养物质的首要场所。
各部位中蔗糖的高13C富集率表明了这类化合物的合成及转化速率较快,相反,淀粉的13C 富集率均为最低,说明淀粉的合成及转化速率较慢。本研究表明:在石蒜的叶、根和内层鳞片中13C标记的葡萄糖分配率虽低于淀粉,但葡萄糖的13C富集率却高于淀粉。这可能是由于跟淀粉相比,葡萄糖具有更大的可溶性且易被利用,新合成的葡萄糖虽然比淀粉少,但却对叶、根及内层鳞片中碳库产生了极大贡献。这与张琳梅等[29]发现红壤中添加葡萄糖外源有机碳对可溶性有机碳库的贡献大于淀粉的结果相似。
此外,带13C标记的淀粉在叶中的分配比例显著高于其他器官(P<0.05)。这是因为在叶绿体内合成的是瞬时同化淀粉,这种淀粉不同于其他异养器官中新生成的储藏型淀粉,在夜晚会分解为麦芽糖和葡萄糖,后转运出叶绿体,用于合成蔗糖,维持叶片的呼吸和植物代谢。中层鳞片中的带13C标记的淀粉的分配比例要大于根,这从侧面说明了此时的鳞茎中层主要发挥“库”的功能。
-
当蔗糖被运输到各库组织中,可以被转化酶水解生成葡萄糖和果糖,或被蔗糖合成酶裂解生成果糖和尿苷二磷酸葡萄糖。这2种蔗糖裂解酶均能产生游离果糖,之后果糖再被果糖激酶或己糖激酶磷酸化。其中,果糖激酶对果糖的亲和力远高于己糖激酶,是果糖代谢的中心,能够与蔗糖合成酶协同调节蔗糖的代谢,从而增加特定库组织的活性,增加库强度[30]。
占主导地位的蔗糖裂解途径是激活鳞茎再生潜力的关键[31]。在休眠期,红花石蒜鳞茎内的果糖质量分数显著高于葡萄糖,并以游离状态存在于鳞片内的浆液中[19]。本研究结果表明:在红花石蒜的各个部位中,非结构性碳水化合物组分中果糖的13C分配率远远高于葡萄糖,意味着新合成的果糖远多于葡萄糖。其中,在叶、根和内层鳞片,果糖的13C分配率依次分别是葡萄糖13C分配率的9.6、41.5、118.1倍。这表明新合成的果糖不是单纯由蔗糖通过转化酶分解而来,而是主要或优先通过蔗糖合成酶分解蔗糖途径而来(该途径不直接产生葡萄糖)。这与XU等[32]认为蔗糖和尿苷二磷酸葡萄糖焦磷酸化酶途径是小鳞茎形成过程中主要的蔗糖代谢途径的研究结果基本一致。导致该结果的原因可能与通过转化酶的蔗糖分解代谢途径比通过蔗糖合成酶途径需要更多的能量[33]或鳞茎内的蔗糖合成酶的活性要显著高于转化酶[34]有关。
鳞茎内高质量分数的果糖或果聚糖对石蒜的生长发育具重要意义。有研究表明:在培养基中添加质量浓度为30 g·L−1的果糖会导致5种新的生物碱的积累(包括异胺),而在添加其他碳水化合物的培养基中却未发现[35]。果聚糖还可充当渗透调节剂,保持膜的完整性和防止蛋白质变性,以防止植物受到严寒或干旱等非生物胁迫的伤害[36]。因此,巧妙利用好果聚糖合成酶也可以改变植物库器官的强度,实现从源到库组织的更高分配,有效提高目标产量。
-
本研究利用同位素技术,分析了红花石蒜光合作用固定的13C分配到各个器官及其非结构性碳水化合物各组分中的情况,发现了蔗糖与果糖的主要代谢方式,初步解析了光合碳在石蒜体内的空间分配特征,阐明了果糖对于石蒜生长发育的重要意义,为进一步探析石蒜属植物的鳞茎发育提供了理论依据。然而,植物中利用的碳来源及转化过程较复杂,某种程度上限制了对碳分配途径的明确追踪。在今后的研究中,可以从标记后的不同时间采样,或者对红花石蒜体内更细致的代谢物进行特异性标记,以便更高效地研究石蒜体内新光合产物在时间上的动态分配特征或更具体的潜在代谢途径。
Distribution and transport patterns of NSC in Lycoris radiata based on 13C tracing
doi: 10.11833/j.issn.2095-0756.20230196
- Received Date: 2023-03-06
- Accepted Date: 2023-12-11
- Rev Recd Date: 2023-12-06
- Publish Date: 2024-04-01
-
Key words:
- carbon allocation /
- metabolite tracing /
- non-structural carbohydrates /
- photosynthetic products /
- glucose metabolism
Abstract:
| Citation: | YANG Yu, ZHANG Yun, WEI Xuying, et al. Distribution and transport patterns of NSC in Lycoris radiata based on 13C tracing[J]. Journal of Zhejiang A&F University, 2024, 41(2): 252-261. DOI: 10.11833/j.issn.2095-0756.20230196 |













 DownLoad:
DownLoad: