-
香榧Torreya grandis‘Merrillii’为红豆杉科Taxaceae榧属Torreya植物,是榧树Torreya grandis中的变异类型经无性繁殖形成的品种[1−2]。香榧为常绿针叶乔木,主要生长在中国南方较为湿润的地区,包括浙江、江苏、安徽、福建等省份。香榧种仁是珍贵的干果,油脂含量高,尤其富含金松酸等多种功能性多不饱和脂肪酸,对预防心脑血管疾病、防治中风和老年痴呆方面具有显著疗效[3−4]。关于香榧油脂的研究主要包括油脂组成、提取方法和功能特性等方面[5−7]。近年来,香榧油脂的分子调控机制研究表明:油脂合成相关基因和一些关键转录因子对香榧油脂合成产生重要作用[8−9]。基于此,本研究对香榧种仁营养成分中的油脂及其合成调控机制进行了综述,以便为香榧油脂的深度开发和利用提供参考。
-
香榧干果口感香脆、经济价值高,这主要得益于其高含油率及丰富的功能性不饱和脂肪酸。香榧种仁含油率为42.67%~61.47%[2]。香榧种仁含有饱和脂肪酸和不饱和脂肪酸,其中以不饱和脂肪酸为主,主要为亚油酸、油酸和金松酸;饱和脂肪酸以山嵛酸和棕榈酸为主[4]。陈振德等[5]研究发现:榧属种子脂肪酸组成在种间无明显差异,榧属种仁不饱和脂肪酸含量占总脂肪酸含量的比例为76.1%~82.0%。将香榧与大豆Glycine max、花生Arachis hypogaea的油样进行比较,其脂肪酸成分如表1所示[6]。香榧种实中的棕榈酸、硬脂酸、油酸、亚油酸、花生一烯酸的含量与大豆、花生中的同类脂肪酸含量接近;香榧中亚麻酸、花生酸、木焦油酸含量略低于大豆、花生中的同类脂肪酸含量;而香榧中的山嵛酸和金松酸含量远高于大豆和花生中的含量;香榧总脂肪酸含量也略高于大豆和花生。同时,香榧油的折光率和皂化值与大豆油和花生油相近,碘价比花生油高。这说明香榧的脂肪酸营养价值高,是一种优质的植物油资源。
物种 脂肪酸含量/% 棕榈酸 硬脂酸 油酸 亚油酸 亚麻酸 花生酸 花生一烯酸 金松酸 香榧 7.45~10.24 3.24~3.45 26.00~35.52 39.60~41.90 0.33~0.65 0.18~0.39 0.66~0.94 9.13~12.96 大豆 10.20~12.15 3.24~4.15 17.29~46.84 33.43~57.80 3.86~8.76 0.26~0.38 0.18~0.30 0 花生 5.26~13.76 1.73~6.49 36.97~82.20 25.18~44.60 0.05~0.58 0.90~2.61 0.63~2.45 0 Table 1. Comparative analysis of fatty acids from T. grandis‘Merrillii’, G. max and A. hypogaea
香榧种实的油脂及其组成在不同发育阶段会发生变化。田荆祥等[10]研究发现:香榧种子在不同发育时期含油率有极显著差异,呈先逐渐增加后逐渐下降的趋势;香榧种实中不饱和脂肪酸总量随着种实成熟度提高而逐渐增加。王玫鹃等[11]探究了香榧种仁生长后期油脂、脂肪酸组分及典型脂溶性活性物质的变化趋势,发现香榧种实生长发育后期油脂含量由35.65%增长到54.50%,是油脂积累的关键时期。叶珊等[12]对香榧成熟后期的营养成分、脂肪酸组分变化进行研究,发现香榧裂果较青果含油率高5.6%。
香榧不同种源的油脂含量不同。朱海东等[13]对浙江诸暨、嵊州、磐安和安徽黄山等不同产地的香榧油的理化性质、脂肪酸、生育酚组成、多酚含量进行分析,发现各地区香榧脂肪酸组成几乎相同,但含量略有不同:以安徽黄山香榧油含量最高,为53.16%;浙江嵊州香榧亚油酸含量最高,达47.05%,浙江诸暨香榧油酸含量和单不饱和脂肪酸含量最高,分别为37.54%和38.03%。
香榧油脂含量高低与香榧生长环境密切相关。随着海拔高度的升高,香榧油脂含量变化并不显著,但香榧种实中的二十碳二烯酸、二十碳三烯酸、油酸和硬脂酸含量随之升高。香榧林土壤有机质含量、细菌多样性、土壤酶活性与香榧出仁率、种子形状、油脂及蛋白质含量均呈正相关[14−15]。HU等[16]研究发现:光合作用对香榧生物量积累和油脂合成产生重要的作用。ZHANG等[17]研究表明:施加小麦Triticum aestivum秸秆生物质炭在酸性土壤中会提高香榧种实的品质和营养成分。
-
陈振德等[3]研究发现:香榧种仁油脂可促进前列环素(prostacyclin,PGI2)合成,抑制血清血栓素(thromboxane,TXA2) 分泌,从而防治动脉粥样硬化。香榧种仁中的脂肪酸还可以降低血液中的三酰基甘油(triacylglycerol,TAG)水平。KHATTAB等[18]研究了日本榧Torreya nucifera的脂肪酸对大鼠Rattus norvegicus新陈代谢的作用,发现该脂肪酸可以有效降低大鼠肝脏及血清中的TAG水平,有利于促进大鼠的新陈代谢。香榧种仁油还具有抗氧化功能。CUI等[7]研究了香榧不同品种油脂的脂肪酸组成、抗氧化活性和酪氨酸酶抑制活性,发现野生型香榧种仁油有较强的抗氧化活性和酪氨酸酶抑制活性。
金松酸(sciadonic acid,SA)是香榧油的特殊脂肪酸,属于二十碳三烯酸(eicosadienoic acid,C20H34O2)。金松酸主要存在于裸子植物的种子[19−21]、叶[22−23]、木材[24]等器官中,极少数存在于被子植物的种子中。牛丽影等[25]在香榧油中发现金松酸占总含量的8.65%。金松酸对大鼠的血脂具有显著的调节作用,能抑制肝脏中脂肪酸合成酶(FASN)的活性,从而抑制恶性肿瘤产生[26]。金松酸还可通过促进甘油三酯分解代谢来抑制甘油三酯的合成代谢,使得HepG2细胞内甘油三酯的积累减少[27]。
-
油脂酸败是不饱和脂肪酸在储藏过程中,受环境、光照、氧气、水分、金属离子等因素的影响,被氧化成低分子脂肪酸的过程[28]。香榧种仁含有丰富的不饱和脂肪酸,在加工和储藏过程中极易发生氧化酸败,引起香榧种实品质下降。油脂过氧化值和油脂酸价是目前衡量香榧氧化酸败和品质优劣的重要指标[29]。
叶珊等[12]研究了不同成熟度和堆沤方式对香榧种仁品质的影响,发现在香榧假种皮开裂时采摘可明显提高种仁的可溶性蛋白含量和含油率,增加香榧种仁中的不饱和脂肪酸,有助于香榧炒制过程中的脱衣,从而提高香榧产品品质;对裂果采摘的种子进行1次堆沤,与传统2次堆沤相比,既能在时间上加快香榧后熟进程,又能达到一样的效果,保证香榧产品的优良品质。ZHOU等[30]研究发现:低温会通过增加去饱和酶(FAD2)生物合成和减少脂质过氧化来提高不饱和脂肪酸含量,从而提高香榧采后成熟期的出油率。ZHANG等[31]探究了温度和相对湿度对采后香榧油脂的交互影响,发现高湿度处理会导致香榧后熟过程提前;将温度设定在20 ℃,相对湿度为90%,可以延缓脂质氧化,提高油脂品质。
葛林梅等[28]研究发现:在香榧加工过程中,酸价和过氧化值均表现为不同程度的增加,香榧油脂发生水解氧化酸败。在氧化酸败的同时,香榧油脂组织中也存在例如多肽、酚等抗氧化物质,以抵御外界环境因素的影响。杨蕾等[32]对香榧种仁进行不同的加工方式,比较分析了香榧的香气物质和油脂品质,发现烘烤工艺更有利于保留香榧的特征香气,炒制香榧的酸败程度显著高于烘烤香榧,酸价高达0.767 mg·kg−1。罗凡等[33]研究了榨前处理对香榧油脂品质的影响,发现随热风处理时间的延长,香榧油的酸价呈先下降后略上升的趋势,拐点约在热风处理90 min时,但长时间高功率微波加热可能加速香榧油酸败,热风和微波处理均提高了香榧油的氧化诱导时间,在经过120和150 ℃热风的高强度加热后,氧化诱导时间均呈现2次上升,再次上升可能是因为产生了美拉德反应。
-
目前,香榧种仁油脂的提取方法主要有:水蒸气蒸馏法、有机溶剂提取法和超临界二氧化碳萃取法。水蒸气蒸馏法是指通过外部加热物料和水的混合物,利用精油不溶于水且沸点较低的特点,使水蒸气将精油带出,由冷凝管冷凝后收集[34]。该方法操作简便,但蒸馏过程中水或高温可能会破坏油脂成分。有机溶剂提取法是指通过有机溶剂的多次提取制备油脂,存在溶剂回收困难、有机溶剂残留的问题[35]。近年来一般采用超临界二氧化碳萃取法,通过对脂质的特殊溶解作用进行油脂制备,该方法具有操作简便、无溶剂残留等特点[36]。梁玉清等[37]用超临界二氧化碳萃取法和水蒸气蒸馏法提取香榧外种皮化学成分,发现超临界二氧化碳萃取法提取率为17.5%,产物为黄色膏状物;水蒸气蒸馏法提取率为7.7%,产物呈微黄色透明清澈油状。可见,超临界二氧化碳萃取法得率更高,成分比较全面,更适合萃取香榧外种皮。香榧种仁中油脂的研究内容及提取方法如表2所示。
研究内容 提取及分析方法 研究结果 文献来源 香榧种仁油脂分析 浸提法;气相色谱分析 香榧种仁含油量为54.39%,存放1 d后含油量略降 余象煜等[38] 国产榧属种仁油含量及脂肪酸测定 索氏抽提;气相色谱分析 种仁油脂含量42.67%~54.39%,脂肪酸组成以亚麻酸和油酸为主 陈振德等[5] 香榧种仁油脂肪酸和不皂化物组成分析 索氏抽提;气相色谱分析 香榧种仁油主要脂肪酸组成及含量,确定金松酸含量,并指出不皂化物组成 牛丽影等[25] 香榧种仁油脂理化性质及脂肪酸组成 浸提法;气相色谱-质谱分析 香榧油含量、酸值碘价等理化性质以及12种脂肪酸组成中主要脂肪酸含量 李红等[4] 香榧种仁油萃取及脂肪酸组成比较分析 超临界CO2流体萃取;气相色谱-质谱分析 萃取最佳工艺为:压力30 MPa、温度50 ℃、时间2 h,萃取率达16.2%;香榧种仁油主要含10 种脂肪酸 阙斐等[39] 香榧种仁超临界萃取物分析 超临界CO2流体萃取;气相色谱-质谱分析 超临界萃取脂肪油含量为16.21%,气相色谱-质谱分析确定脂肪酸主要组成 赵粼等[40] Table 2. Recent publications on the extraction and analysis of oils from T. grandis ‘Merrillii’ kernels
-
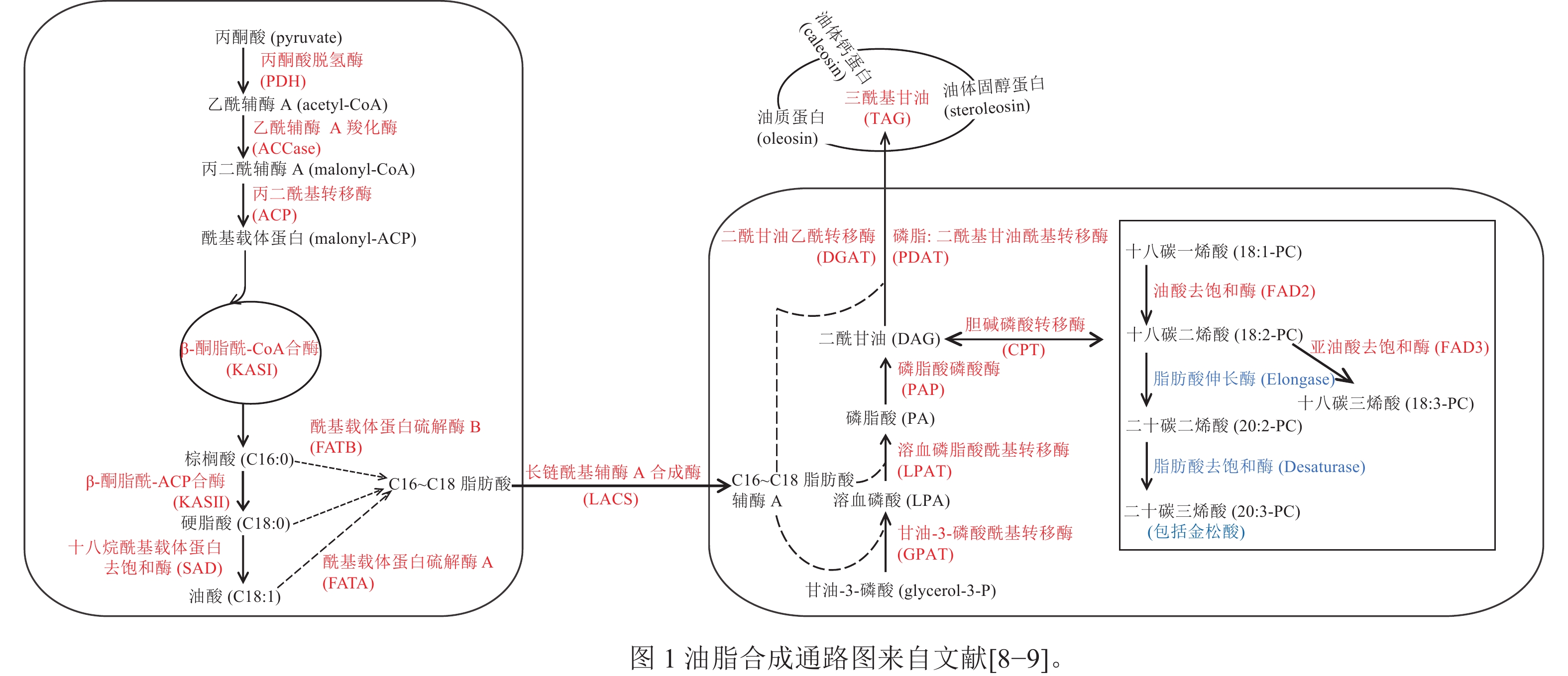
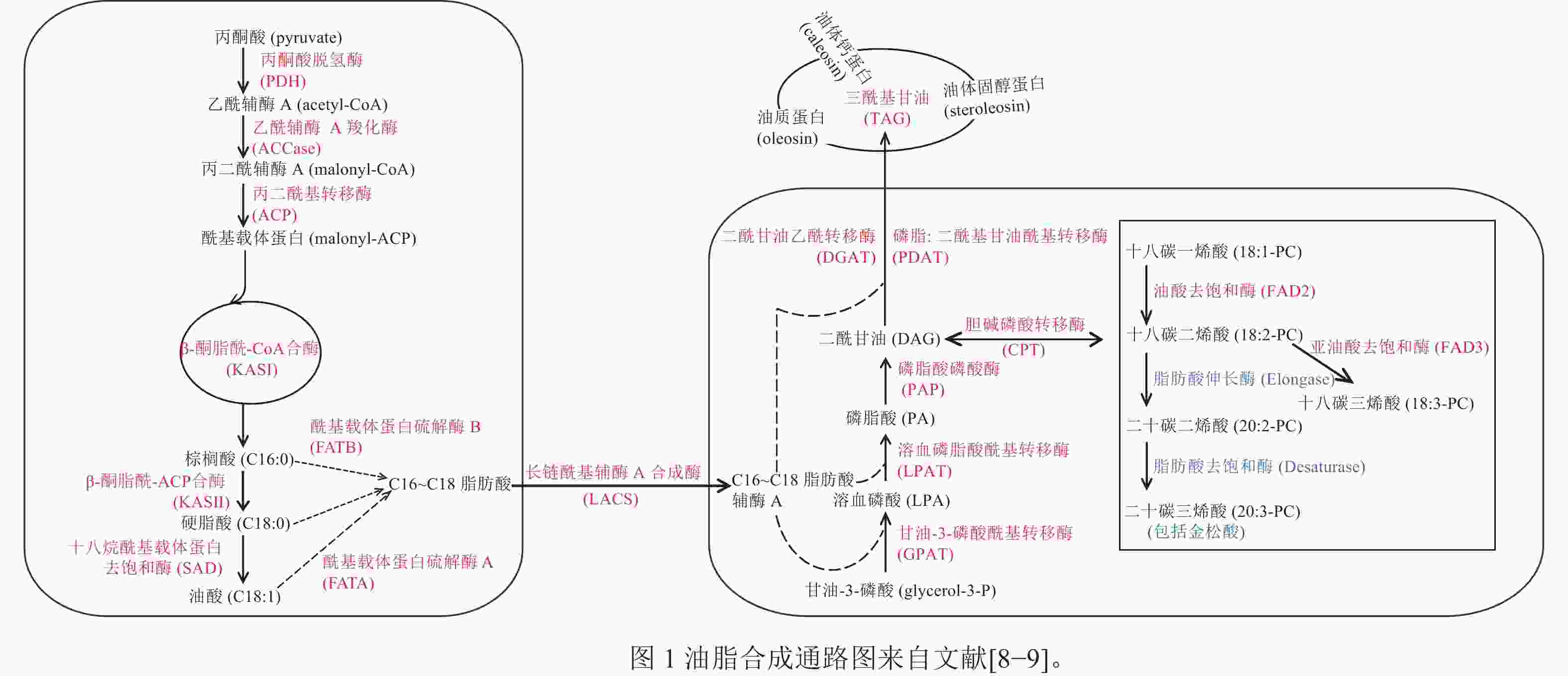
DING等[8]对10个不同的地方品种的香榧进行转录组测序,鉴定出175个与香榧油脂生物合成相关的基因,并提出了香榧油脂生物合成的代谢途径,如图1所示。在香榧基因组中,LOU等[9]比较分析了金松酸的合成途径及其关键合成酶。亚油酸和亚麻酸在C18Δ9-脂肪酸伸长酶(C18Δ9-elongase) 的作用下生成二十碳二烯酸和二十碳三烯酸;之后,二十碳二烯酸在C20Δ5-脂肪酸去饱和酶(C20Δ5-desaturase)的作用下生成金松酸。
DING等[8]研究发现:香榧PDH同源基因的BRD_TGR20275和TR56397-c0_g1_i1分别与油酸和二十碳二烯酸显著负相关,表明PDH可能会减少香榧的油脂积累。ACCase是油脂合成途径的初始步骤和限速步骤,目前,小麦、玉米Zea mays、拟南芥Arabidopsis thaliana等作物的ACCase已被鉴定[41]。DING等[8]对不同地方品种的香榧种实进行比较转录组分析,发现ACCase的BC基因和BCCP基因参与脂质生物合成。
GPAT是肯尼迪途径第1个反应的关键酶。郝静芳[42]发现GPAT7和GPAT9参与拟南芥种子中TAG的合成及一些不饱和脂肪酸的合成和调节。刘聪等[43]研究发现:甘蓝型油菜 Brassica napus的GPAT6可以改变拟南芥种子脂肪酸成分的比例。皮广静等[44]在研究花生油脂合成酰基转移酶基因表达与籽仁含油量相关性分析时发现:GPAT9在油脂积累的3个关键期(R4、R5、R6)的平均表达量与含油量显著正相关。香榧中也有GPAT基因,但目前香榧GPAT基因对油脂合成的调控作用以及它的生物学功能尚不清楚。
磷脂二酰甘油酰基转移酶(PDAT)和二酰甘油-O-酰基转移酶同源物(DGAT)是有助于拟南芥种子中TAG生物合成的2种主要酰基转移酶[45]。然而,这2种酶在TAG生物合成中的相对贡献尚不确定。DING等[8]研究发现:PDAT与香榧油脂含量的关系比DGAT更紧密,这表明PDAT对油积累的贡献大于DGAT。DGAT对于许多油籽的TAG生物合成也起到重要作用。JAKO等[46]在拟南芥中过表达AtDGAT,发现转基因拟南芥发育过程中的种子内DGAT表达比野生型提高了10%~70%,并且提高了种子含油量和种子的千粒重。DGAT2在TAG的组装中选择性的积累更多的不饱和脂肪酸。在紫苏Perilla frutescens和烟草Nicotiana tabacum中过表达DGAT2,可使不饱和脂肪酸含量升高,饱和脂肪酸含量下降[47]。
SAYANOVA等[48]研究发现:在转基因拟南芥植物中,C18Δ9-elongase与C20Δ5-desaturase的共表达导致水杨酸(SA)的产生。WU等[49]对圆榧T. grandis ‘Yuanfei’和细榧T. grandis‘Xifei’品种进行转录组测序,各找到2和1个C20Δ5-desaturase,1和2个C18Δ9-elongase;并将圆榧和细榧中的C18Δ9-elongase命名为TgELO1和TgELO2,发现与藻类中的OtELO1接近,并发现2个特征结构域(L-H-X-X-H和M-Y-X-Y-Y)。
在拟南芥中,Oleo1是种子油体蛋白最丰富的亚型,抑制其表达会导致脂质体变大[50]。CROWE等[51]研究发现:油体蛋白的表达在发育上受到反式激活剂ABI3的调节。PASARIBU等[52]研究表明:在大肠埃希菌Escherichia coli中过表达马尾松Pinus massoniana油体固醇蛋白,其形成的油滴比天然油体大,特别是对缺乏甾醇结合结构域的重组固醇蛋白。DING等[8]研究发现:在香榧发育过程中油体蛋白总体表达呈上升趋势;在烟草中过表达TgSLO1会导致油体尺寸变大和数量增加。
转录因子在调节脂质合成和积累方面发挥重要作用。转录因子WRI1在油脂积累方面作用显著。WRI1受到丙酮酸激酶(PKp-β1)、ACCase和KAS等下游基因的驱动[53−55]。LIU等[56]研究发现:在拟南芥中过表达WRI1基因可以增加拟南芥的种子质量和含油量。在香榧转录组中,TgWRI1与脂肪酸合成代谢通路关键酶的表达量呈负相关,但TgWRI1对油脂的调控作用有待进一步验证[8]。LBD基因家族在参与花青素和调节氮代谢方面起着重要作用,但在调节油脂合成的作用尚未报道[57]。LOU等[58]研究发现:TgLBD40可能通过调节赤霉素对油脂合成进行调控,通过在拟南芥中过表达TgLBD40,发现转基因种子含油量多于野生型。TgLBD40可以直接和TgDGAT1启动子结合,但需要与TgWRI1结合一起提高TgDGAT1的表达。
-
香榧含有丰富的油脂、蛋白质、维生素等营养物质,有巨大的经济价值和开发潜力。随着香榧种植面积和产量的增加,香榧种仁营养物质的有关研究需尽快推进,以适应市场多样化需求。同时研究香榧种仁营养物质也为提高香榧剩余副产品的再回收利用作出贡献。对于香榧油脂的研究,从最初香榧的脂肪酸组成,到香榧油脂的提取方法,再到香榧的分子调控机制,标志着对香榧油脂认识一直不断深入和完善。分子生物学的进步和各种新方法的出现,为进一步提高香榧油脂的提取效率和研究香榧油脂形成过程的分子机制提供了良好的条件和平台。如何在香榧油脂萃取工艺上减少香榧油脂的消耗,更加全面地反映香榧脂肪酸组成的问题仍需解答,需要深入研究香榧油脂的提取方法。今后可以通过改变适当的酶解条件,使油脂的释放率和产率得到提高,从而寻找新的萃取溶剂、调整萃取比例等方式提高油脂的提取效率。研究油脂合成代谢途径上调控基因的表达,以及其与转录因子之间的复杂网络关系,解析香榧油脂的分子调控机制,为选育高油含量的香榧品种提供基础。未来,随着香榧遗传转化体系的不断完善,将香榧油脂调控关键酶与香榧遗传转化技术有机结合,实现高含油量香榧优良品种的繁育,这对开发和利用香榧种仁种质资源具有十分重要的意义。
Research progress on synthesis and regulation mechanism of Torreya grandis‘Merrillii’ kernel oil
doi: 10.11833/j.issn.2095-0756.20230224
- Received Date: 2023-04-03
- Accepted Date: 2023-05-29
- Rev Recd Date: 2023-05-22
- Publish Date: 2023-08-20
-
Key words:
- Torreya grandis‘Merrillii’ /
- kernel oil /
- synthetic pathway /
- molecular mechanism /
- review
Abstract: Torreya grandis‘Merrillii’is one of the precious economic tree species with high nutritional and medicinal value. T. grandis‘Merrillii’ kernels are rich in nutrients such as oil, protein, and vitamins. The main components on T. grandis‘Merrillii’ kernel oil are fatty acids and their derivatives, which play an important role in reducing blood lipids, antioxidant and preventing cardiovascular and cerebrovascular diseases. In the past, studies on T. grandis‘Merrillii’ kernel oil mainly focused on the composition, extraction methods and functional characteristics. With the development of forest molecular biology, more and more studies have been conducted on T. grandis‘Merrillii’ kernel oil synthesis and regulation mechanism, including the excavation of anabolic pathway for oil synthesis, identification of key genes for oil accumulation and special fatty acid synthesis, and construction of regulatory networks. This study is to review the current research status of T. grandis‘Merrillii’kernel oil, including studies on fatty acid composition, oil quality, oil extraction methods, and oil synthesis regulation mechanism of T. grandis‘Merrillii’ kernels, so as to provide ideas and methods for the later development and utilization of T. grandis‘Merrillii’ kernel resources. [Ch, 1 fig. 2 tab. 58 ref.]
| Citation: | HE Ciying, LOU Heqiang, WU Jiasheng. Research progress on synthesis and regulation mechanism of Torreya grandis‘Merrillii’ kernel oil[J]. Journal of Zhejiang A&F University, 2023, 40(4): 714-722. DOI: 10.11833/j.issn.2095-0756.20230224 |









 DownLoad:
DownLoad: