-
指甲兰属Aerides是兰科Orchidaceae重要的观赏植物及药用植物,属下有30种,主要分布在印度、斯里兰卡、尼泊尔、不丹、缅甸、越南、老挝、泰国、马来西亚、菲律宾、印度尼西亚、巴布新几内亚,及中国南方亚热带区域[1]。中国共有多花指甲兰A. rosea、香花指甲兰A. odorata、小蓝指甲兰A. orthocentra、扇唇指甲兰A. flabellate和指甲兰A. flacata 等5种。指甲兰属为单轴分枝类兰花,与蝴蝶兰属Phalaenopsis、万代兰属Vanda、钻喙兰属Rhynchostylis等关系较近,都属于树兰亚科Epidendroideae万代兰族Vandeae指甲兰亚族Aeridinae,属间杂交亲和,可培育新品种[2]。据英国皇家园艺学会兰科登陆机构(RHS)记载,蝴蝶兰属与指甲兰属的远缘杂交种已有20余个。
多花指甲兰原生境花期为5—7月,果期为8月至次年5月,产于中国广西、贵州、云南,在不丹、印度、缅甸、老挝和越南也有分布,生于海拔320~1 530 m的山地林缘或山坡疏生的常绿阔叶林树干上。被《中国物种红色名录》记为极危(CR);被《中国生物多样性红色名录:高等植物卷》记为濒危(EN)。花色粉嫩、花量大、花型奇特、花香馥郁,是观赏价值极高的兰花,也可与蝴蝶兰进行远缘杂交,培育有香味的蝴蝶兰。
目前,对于多花指甲兰的研究较少,主要在组织培养、非共生萌发技术[3]、功能性成分分析[4]等方面。王清芸等[5]对其花粉活力和柱头可授性研究发现:开花6 d时的花粉活力最强,盛花期柱头可授性最高,但对其开花进程及繁育系统未进行系统研究。开展花部特征和繁育系统研究,不仅可以深入研究花朵的结构、传粉方式和媒介种类,还能够了解植物的生态适应机制,为杂交育种工作提供依据。以往兰科植物繁育系统研究[6-13]为本研究提供了参考。观察多花指甲兰开花进程,并进行繁育系统研究,可为该物种种质资源的保护提供有力保障,也为实现多花指甲兰的人工繁殖和产业化生产,及远缘杂交育种提供科学依据。
-
多花指甲兰种植于中国农业科学院蔬菜花卉研究所温室,该温室春秋季常开天窗;夏季开风机,水帘降温;冬季有暖气供暖。室温保持在26~30 ℃,相对湿度为65%~70%。取200株生长状况良好、无病虫害的5年生多花指甲兰进行试验。
-
随机选取盛花期全天开放的植株10株,测量株高、花序长、中萼片、侧萼片、花瓣、唇瓣、花序、花朵数及叶片数。按照DAFNI[14]的标准,选取10株植株进行开花进程观察,观察记录群体水平(群体内第1朵花至最后1朵花开放的日期)、单株水平(单株上第1朵花开放至最后1朵花凋谢的日期)、单花水平的开花进程(单朵花花瓣从两侧打开至花朵枯黄脱落的日期)。
-
选取初花期和末花期的花粉团,经配置的FAA固定液(100 mL FAA中含体积分数为50%的乙醇89 mL、冰乙酸6 mL、甲醛5 mL)处理24 h后,用乙醇溶液按梯度(体积分数分别为70%、80%、90%、95%、100%)脱水15 min,放入加有干燥剂的1.5 mL离心管中,用CO2临界点干燥仪(Leica CPD 300)干燥5 h,用双面胶将干燥后的植物样品粘在金属台上,待观察部位朝上。离子溅射仪(Hitachi E-1010)对金属台喷金、镀膜。在扫描电子显微镜(Hitachi S-4800)下观察和测量花粉团的表面纹饰、花粉外形态,并拍照。
筛选测定多花指甲兰花粉活力最适宜的方法:①醋酸洋红染色法。于每日9:00—10:00采集多花指甲兰各生长阶段的新鲜花粉,将花粉置于载玻片上,用解剖针将花粉团轻轻捣碎,滴入1~2滴的醋酸洋红染液后轻轻将其均匀分散开,盖上盖玻片,每次制片3张。静置10~15 min后,于倒置显微镜(Olympus BX53)下观察,选取6个不同视野进行拍照。花粉活力强的花粉会被染成红色,较淡的颜色被视为活力较弱。②氯化三苯四氮唑(质量浓度为1%的TTC)染色法。参照边子星[15]的方法:将新鲜的花粉置于2 mL的试管中,用解剖针将其轻轻捣碎,后滴入1滴质量浓度为1%的TTC染色液,黑暗培养24 h。摇晃试管,使得花粉分散均匀,用移液枪吸取含有花粉的TTC染液,置于载玻片上,用解剖针轻轻将花粉细胞均匀分散开,制片3张。放至倒置显微镜(Olympus BX53)下进行观察并拍照,选取6个不同的视野。以花粉细胞被染成红色为活力较强标准,白色为活力较弱标准。③花粉离体萌发法。参照欧阳英等[16]的方法:以质量浓度为5.000%蔗糖+质量浓度为0.050%硼酸+质量浓度为0.001%氯化钙+体积分数为5.000%椰汁为液体培养基。将花粉用解剖针取下置于双面凹载玻片上并散开,用滴管吸取上述培养液滴入含花粉的凹面里,后将载玻片放于垫有3~5张湿润滤纸的培养皿中,盖上盖子置于组培室(室内温度25 ℃,相对湿度80%以上)培养48 h后统计花粉萌发数。以花粉管长度超过花粉直径的2倍记为萌发。每阶段取3份样,制片后随机选取3个视野统计萌发数,重复3次。花粉萌发率=萌发的花粉数/视野内总花粉数×100%。
-
采用联苯胺-过氧化氢法[V(质量浓度为1%联苯胺)∶V(体积分数为3%过氧化氢)∶V(水)=4∶11∶22]测定各阶段柱头的可授性。用体视显微镜(Olympus SZ)观察并拍照。柱头周围反应液伴有大量的气泡产生表示柱头具有可授性,气泡越多表示可授性越强。
-
随机采取多花指甲兰成熟的花粉团,将其放置载玻片上碾碎,滴入碘-碘化钾浸染15 min,放置在倒置显微镜(Olympus BX53)下观察,花粉若经碘-碘化钾处理后显示红色说明花粉有脂质存在,处理后若呈蓝色或黑色则说明花粉有淀粉存在。
-
根据DAFNI[14]的标准计算OCI值。花朵直径<1 mm记为0;1~2 mm记为1;2~6 mm记为2;>6 mm则记为3。花药与柱头的空间位置在同一高度记为0,空间分离记为1。花药开裂时间和柱头可授粉期同步或雌蕊先熟记为0,雄蕊先熟记为1。三者之和为OCI。繁育系统的评判标准:OCI=0,为闭花受精;OCI=1,为专性自交;OCI=3,为自交亲和,有时需要传粉者;OCI≥4,为部分自交亲和,异交,需要传粉者。
-
随机选取初花Ⅱ期花朵进行人工控制授粉试验,设置6个处理(不去雄不套袋不授粉、不去雄套袋不授粉、去雄不授粉、人工自花授粉、人工同株异花授粉、人工异株授粉),检测多花指甲兰传粉方式。每个处理重复3次,共计20~30朵花。授粉30 d后统计结实率。结实率=结实数/授粉数×100%。
-
利用Excel 2016进行表格绘制,SPSS 25.0 进行数据处理、标准误计算及显著性分析(单因素方差分析、邓肯法),Photoshop 2021进行图片制作。
-
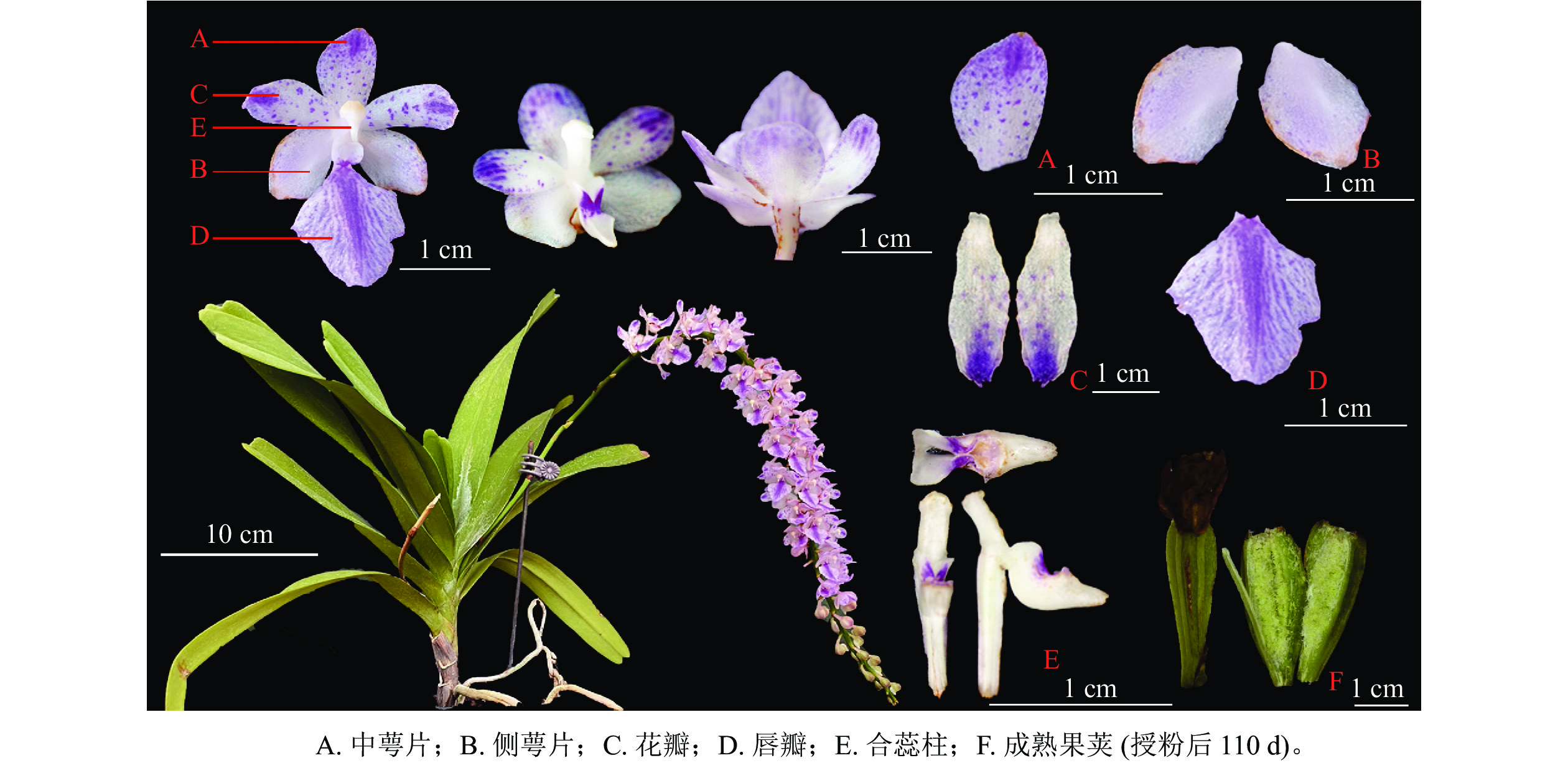
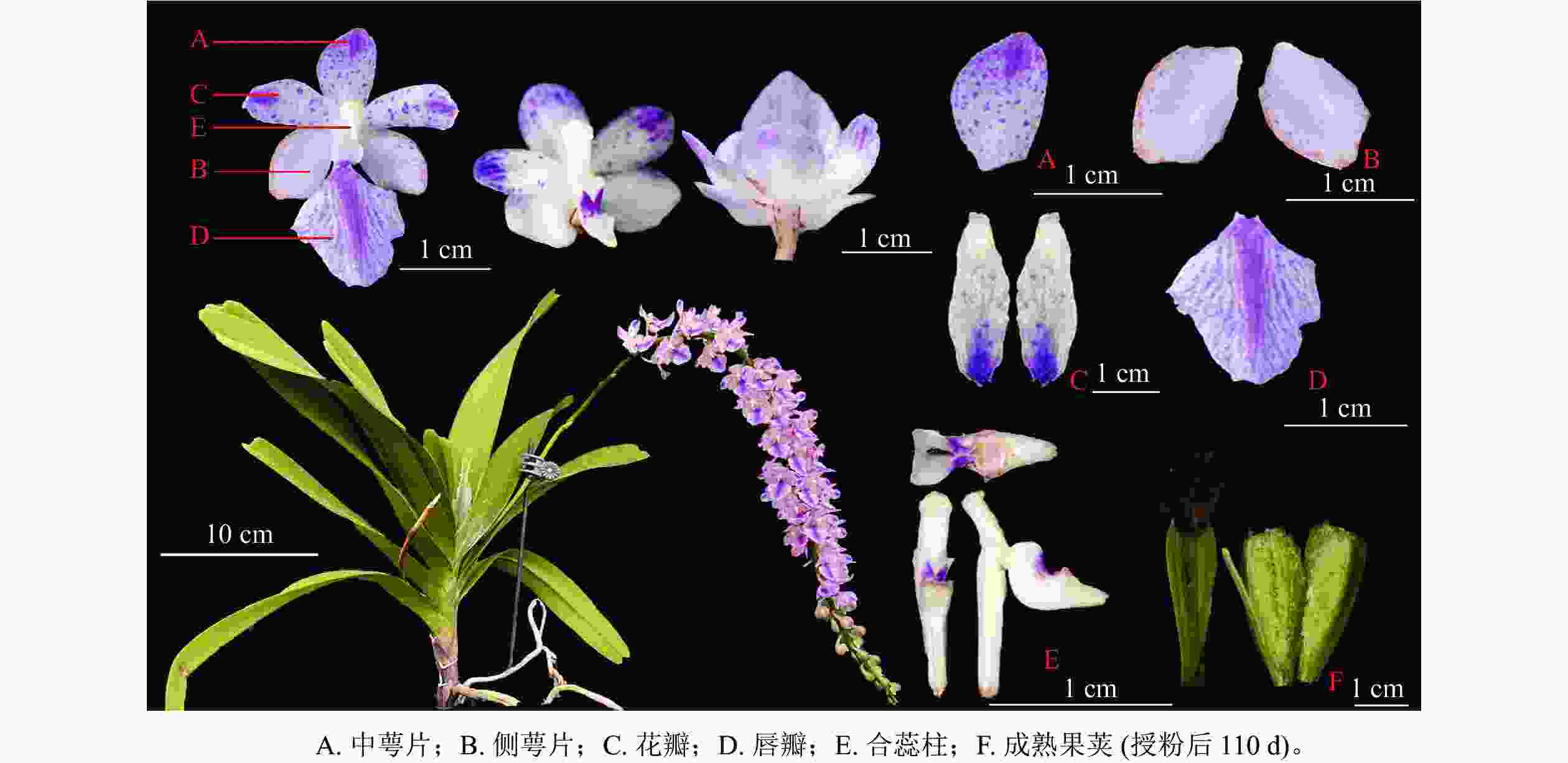
由图1可见:多花指甲兰有1~4个花梗,其总状花序长10~30 cm,花序形态似“狐尾”。茎粗壮,长约5~20 cm,花序叶腋生,花序轴较长,密生数十至近百朵花;花由1个中萼片、2个侧萼片、2个花瓣和1个唇瓣组成。子房棒状带纵棱,花梗和子房白色带淡紫色晕;中萼片和侧萼片呈卵圆形,花瓣披针形,花瓣与中萼片有粉紫色斑点。唇瓣上密布紫红色斑点,边缘稍具不整齐的锯齿。表1为多花指甲兰株高、最大叶长宽、花序长、中萼片和侧萼片的长宽、花瓣长宽及唇瓣的横纵径等花部表型性状。
植株
序号株高/
cm最大叶
长/cm最大叶
宽/cm花序
长/cm中萼片
长/cm中萼片
宽/cm侧萼片
长/cm侧萼片
宽/cm花瓣
长/cm花瓣
宽/cm唇瓣横
径/cm唇瓣纵
径/cm花序梗
粗/cm花朵横
径/cm花朵纵
径/cm花朵
数/个叶片
数/个1 43.0 21.0 4.0 27.6 1.1 0.5 1.3 0.7 2.6 1.5 1.2 1.1 0.5 3.5 3.5 67 13 2 33.0 22.0 3.0 20.0 1.2 0.5 1.1 0.6 2.5 1.2 1.2 1.6 0.6 2.7 4.0 46 12 3 32.0 19.5 3.0 23.0 1.0 0.7 1.1 0.5 3.0 2.1 1.3 1.5 0.4 3.0 3.3 58 10 4 26.0 27.9 3.7 14.6 0.8 0.8 0.8 0.6 3.0 1.7 1.3 1.6 0.6 2.5 3.0 19 13 5 29.0 23.5 4.3 14.1 0.7 0.8 0.8 0.4 3.1 1.8 1.2 1.4 0.3 3.0 3.8 26 12 6 27.0 15.8 3.1 14.3 0.8 0.6 1.2 0.5 3.0 1.3 1.6 1.5 0.2 3.4 3.5 26 10 7 28.0 16.5 2.6 14.7 0.6 0.6 1.3 0.7 2.0 2.1 1.6 1.5 0.2 3.6 3.0 19 7 8 21.5 20.0 4.0 14.6 1.0 0.6 0.7 0.4 1.6 1.7 1.1 1.5 0.3 2.8 2.7 31 11 9 23.5 21.0 2.9 13.0 1.2 0.5 0.8 0.7 2.7 2.0 1.3 1.6 0.3 2.8 3.0 25 11 10 25.5 22.0 2.5 14.7 0.8 0.8 1.2 0.6 2.7 2.1 1.1 1.4 0.2 3.0 3.0 20 12 平均值 27.31 20.92 3.31 17.06 0.92 0.65 1.04 0.58 2.62 1.75 1.29 1.47 0.35 3.03 3.28 33.70 11.13 标准误 2.31 1.08 0.20 1.53 0.06 0.04 0.07 0.04 0.48 0.11 0.06 0.04 0.05 0.11 0.13 5.45 0.69 Table 1. Phenotype of the A. rosea
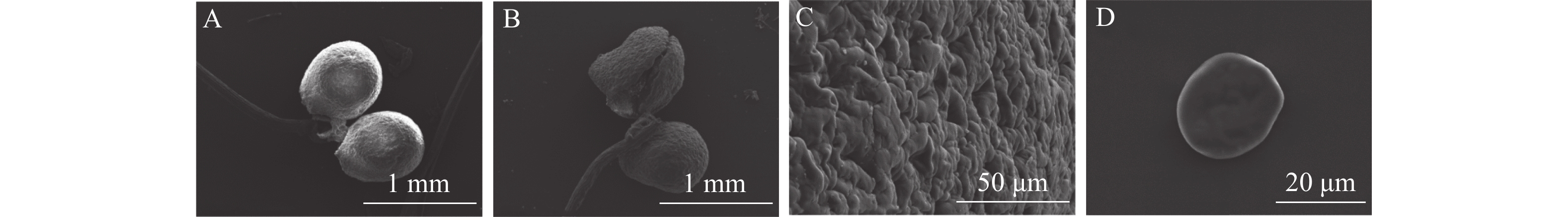
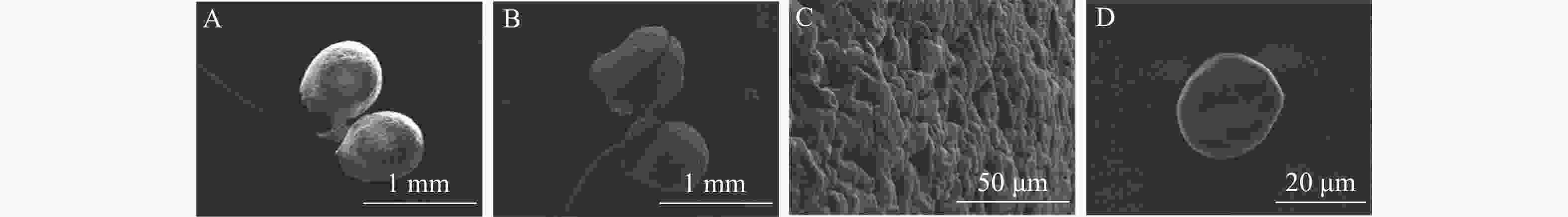
通过电子扫描显微镜观察(图2)可见:多花指甲兰在开花1~2 d花粉团比末花期花粉团更加饱满(图2A和B)。花粉团表面不平整,有极多凸起,由数万个花粉细胞紧密聚集而形成(图2C),花粉粒类球形(图2D),直径约200 μm。
-
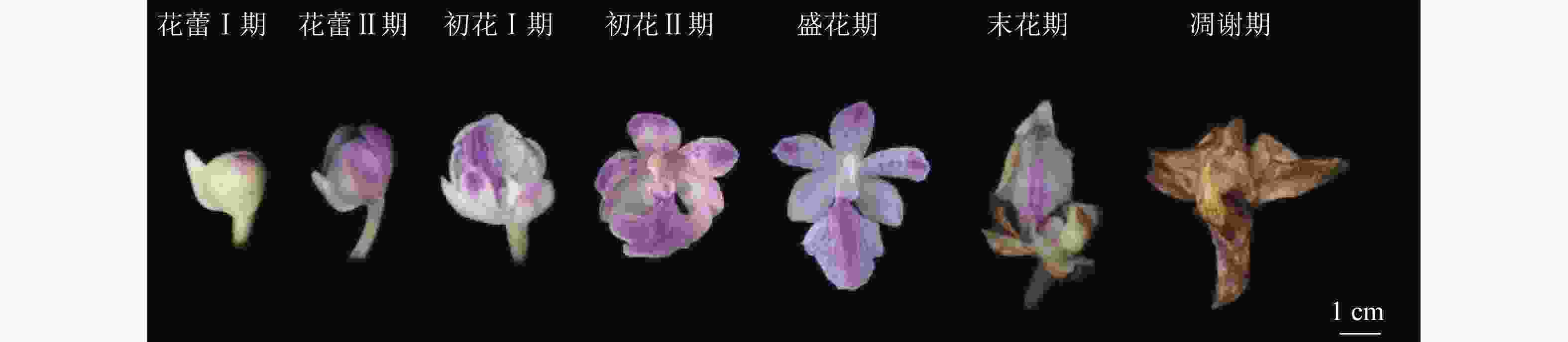
温室内,多花指甲兰在5月底零星开放,群体花期为5—7月,单花花期7~10 d,单株花期15 d。总状花序,花序的花朵从下至上开放,花序上第1朵花开放至整个花序的花全部开放约需10 d。按DAFNI[14]的标准将多花指甲兰的发育分为7个阶段(图3)。①花蕾Ⅰ期(开花前4~5 d)。花蕾未开放,呈不规则椭圆形,中萼片和唇瓣紧闭,可见距。②花蕾Ⅱ期(开花前1~3 d)。花蕾开始开放,中萼片和唇瓣开始分离,有紫色的花瓣露出。③初花Ⅰ期(开花1~2 d)。花蕾开始开放,唇瓣与花瓣分离,横径距离1.5 cm,柱头有黏液分泌。④初花Ⅱ期(开花3~4 d)。花蕾大部分开放,萼片微微卷曲,唇瓣向下竖起,花瓣和唇瓣纵径距离大于3.0 cm,能明显看到合蕊柱,柱头黏液量分泌达到最高。⑤盛花期(开花5~6 d)。花朵完全开放,花瓣完全打开,花粉团由鹅黄变成深黄。⑥末花期(开花7~8 d)。花朵枯萎,萼片卷曲,唇瓣凹陷,萼片和唇瓣的颜色变褐色。⑦凋谢期(开花9~10 d)。花朵完全失去水分,整体呈现褐色,花粉团变硬,花朵枯萎。
-
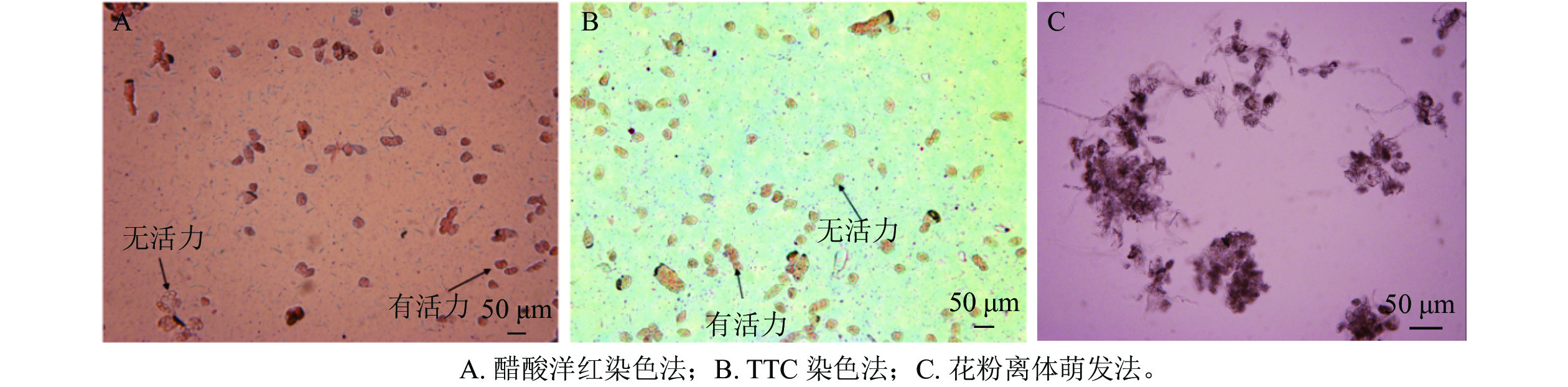
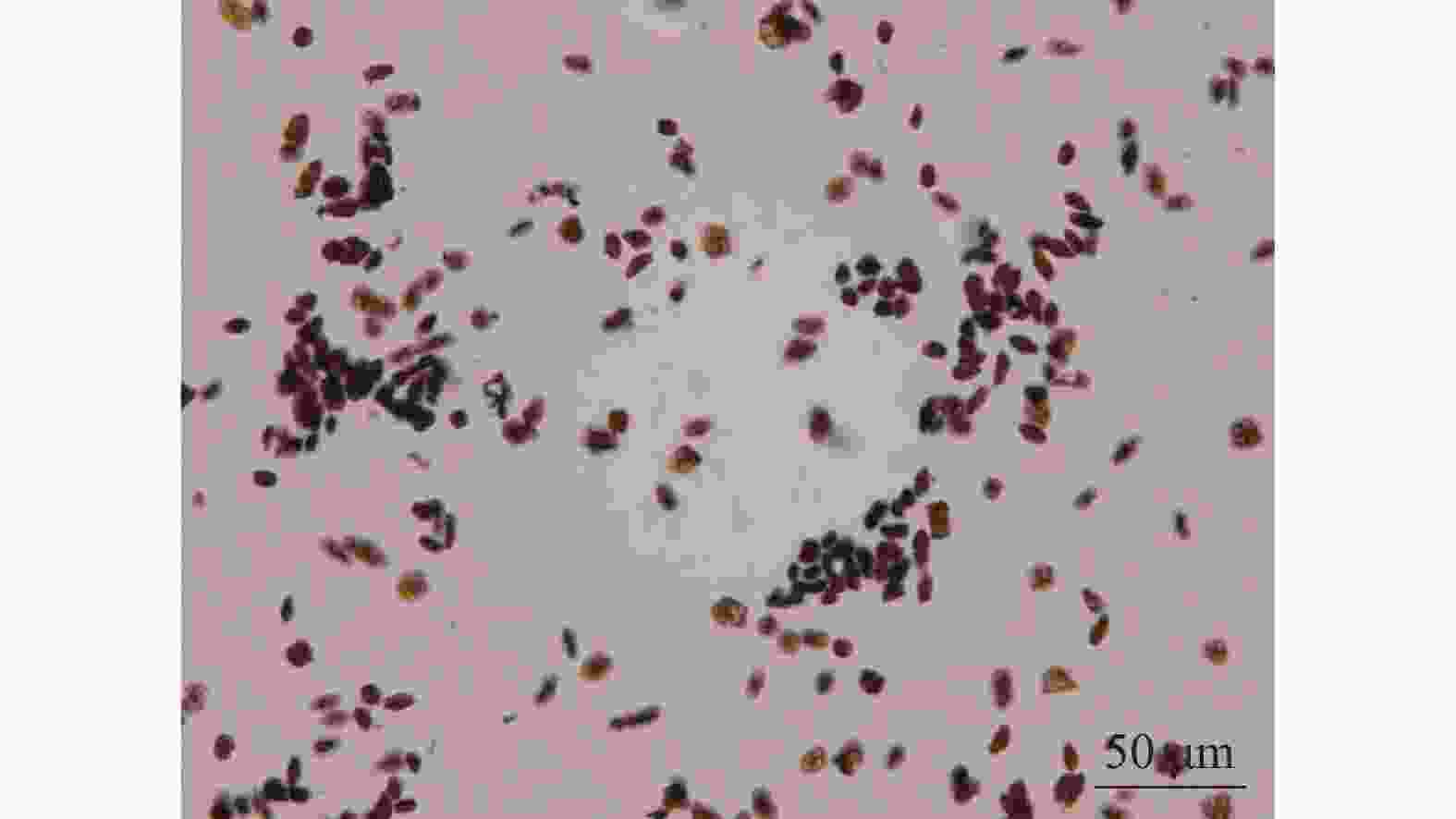
由图4可知:相对于醋酸洋红染色法,TTC染色法可明显区分有活性花粉,且TTC染色法所测得的结果略高于醋酸洋红染液测定的活力,花粉离体萌发法不能准确测定多花指甲兰花粉活力,其花粉管杂乱无章不易查数。因此选用TTC染色法测定多花指甲兰各时期花粉活力,由表2可以看出在盛花期花粉活力最高,可达74.57%,其次为初花Ⅱ期。
发育阶段 花粉活力/% 柱头可授性 花蕾Ⅰ期 14.92±0.69 f +/− 花蕾Ⅱ期 24.17±0.71 d +/− 初花Ⅰ期 33.72±9.92 c + 初花Ⅱ期 45.99±1.45 b ++ 盛花期 74.57±1.14 a ++ 末花期 20.92±0.83 e − 凋谢期 8.55±0.32 g − 说明:数值为平均值±标准误。不同的小写字母表示差异显著(P<0.01)。−表示柱头不具可授性,+/−表示部分柱头具可授性,+表示柱头具可授性,++表示柱头可授性较强。 Table 2. Test of pollen viability and stigma acceptability
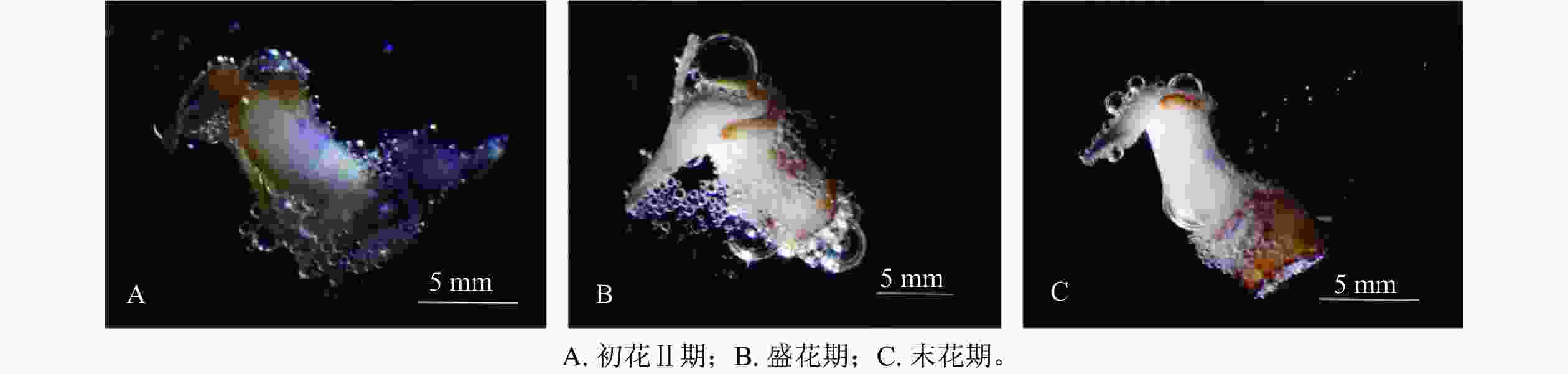
在对柱头可授性进行检测时发现(图5):在初花Ⅱ期和盛花期时柱头周围有大量的气泡产生,说明此阶段的可授性最强。故对多花指甲兰进行人工授粉时,应选择初花Ⅱ期或盛花期,盛花期授粉效率最高。授粉时,先将花粉用镊子取出,此时花粉团具有很强的黏性,易黏在柱头腔内,授粉成功后,通常在1周后即可看到子房开始膨大。
-
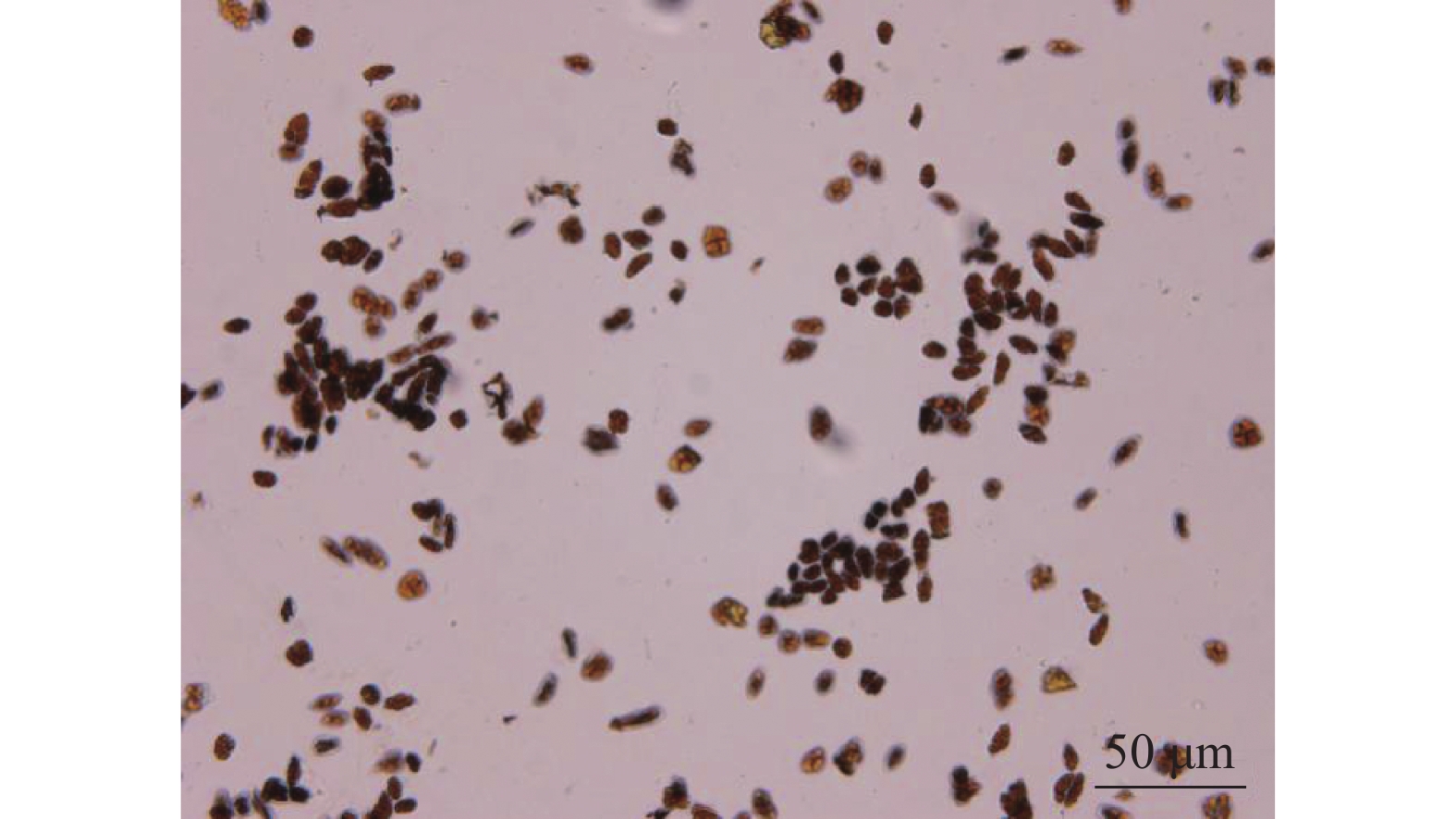
经碘-碘化钾对多花指甲兰的花粉进行染色后,在显微镜下可看到花粉会被染成红色(图6),表明多花指甲兰花粉中淀粉较少,脂质较多。以往研究表明[17]:兰科植物的花粉含有脂类,一般以花蜜和油类较多[18],而淀粉并不是经常存在,这与系统发育有关。有推断,脂质花粉营养丰富,更容易吸引昆虫进行传粉,而淀粉花粉有利于风媒传粉。
-
多花指甲兰花朵直径为2.5~3.6 cm,大于6 mm,分值为3;花粉成熟时聚成典型花粉团位于柱头上方,两者存在一定距离,无法接触,且花粉与柱头腔间的蕊喙发育,阻止自交授粉发生,分值为1;花粉和柱头同步成熟,分值为0。多花指甲兰的OCI为4,其繁育系统以异交为主,部分自交亲和,需要传粉者。
-
成功授粉后的花朵,5 d后可见柱头微微膨大,花瓣、唇瓣与萼片开始枯萎脱落;10 d左右子房膨大。授粉未成功的花朵在开花7 d左右花瓣开始枯黄,萎缩,约10 d后连同子房一起脱落。授粉成功的花朵比未授粉的花朵先枯萎。授粉30 d后统计果荚结实情况(表3)可知:多花指甲兰无论是否去雄、套袋,不进行人工授粉的结实率均为0,而人工自花授粉、人工同株异花授粉、人工异株授粉的结实率分别为85.71%、88.00%、84.00%,无显著差异。这表明多花指甲兰需要借助传粉媒介进行传粉,不存在自然自花授粉和无融合生殖现象。
处理方式 处理花/朵 结实数/朵 结实率/% 不去雄不套袋不授粉 22 0 0.00±0.00 b 不去雄套袋不授粉 30 0 0.00±0.00 b 去雄不授粉 30 0 0.00±0.00 b 人工自花授粉 21 18 85.71±0.12 a 人工同株异花授粉 25 22 88.00±0.08 a 人工异株授粉 25 21 84.00±0.32 a 说明:结实率为平均值±标准误。不同小写字母表示不同方法处理下结实率之间差异显著(P<0.05)。 Table 3. Percentage of fertile fruit in different pollination treatments
-
多花指甲兰有2枚侧萼片、1枚中萼片、2枚花瓣和1枚唇瓣,具有较高的观赏价值,是蝴蝶兰属不可多得的香花远缘杂交亲本。多花指甲兰的花期在5—7月,总状花序,开花时有香味,花自下而上循序开放,但时间差别不大。单株有1~4个花梗,数十朵花。单朵花自然状态下花期为7~10 d,整株花期约15 d。而王清芸等[5]对种植于云南省的多花指甲兰观察,发现其整株花期仅有10 d,说明温室栽培条件相比于露地条件,更适合多花指甲兰生长,可延长其花期。
花粉活力是决定授粉成功与否的关键,花粉活力的高低及柱头可授性的强弱与花朵开放时间有关。多花指甲兰的花粉为四分体结构,花粉进行离体萌发培养时,花粉团在培养液中自然散开,四合花粉粒均保持完整的膜结构,能够萌发,但经过物理碾压,花粉粒的膜结构易遭到破坏,对花粉粒的萌发造成一定的影响,从而加大记数时的误差,所以当尝试用花粉萌发法测定花粉活性,统计数据结果活性偏低,因此本研究并未采用花粉离体萌发法测定数据。本研究也比较了TTC和醋酸洋红2种染色液对多花指甲兰有活力花粉的染色状况。结果表明:2种染色液测定的花粉活力均随着花朵开放时间延长,呈先上升后下降的趋势,与其他兰花结果一致[12, 19]。但是TTC所测花粉活力略高于醋酸洋红,可见在温室条件下栽培的多花指甲兰更适合用TTC染色法测定花粉活力。采用联苯胺-过氧化氢法是检测柱头可授性较为简单常用的方法 [20−22],多花指甲兰同样适用此方法。开花时间的长短影响着柱头可授性的强弱,多花指甲兰在初花Ⅱ期和盛花期可授性达到最高强度,之后又逐步降低。多花指甲兰花粉与柱头同熟,在初花Ⅱ期和盛花期花粉活力与柱头的可授性都较强,因此,此时期开展授粉工作成功率最高。
植物的繁育系统是判断其种群能否延续的重要体系标准,通常是指对直接影响后代遗传组成的所有有性特征进行研究,人工授粉是最基本的研究方法。在对多花指甲兰进行人工控制授粉试验中,不去雄且不套袋、不去雄且套袋以及去雄不授粉的结实率均为0,但是人工自花授粉、人工同株异花授粉及人工异株授粉后,都有80%以上结实率。这些结果皆表明多花指甲兰有高度的自交和异交的能力。根据DANFI[14]的标准所得出的杂交指数也表明:多花指甲兰为自交和异交的混合交配模式,需要特定的传粉者。根据花粉组织化学染色结果表明:多花指甲兰花粉脂质含量较多,初步判断为虫媒传粉,但仍需后期野外验证。基于多花指甲兰繁育系统研究的结果,本研究在初花Ⅱ期和盛花期进行人工授粉,已经获得大量种苗。今后将在自然条件下对其进行人工授粉,提高繁殖效率,从而最大限度地保护多花指甲兰的种质资源。
-
多花指甲兰为总状花序,单花花期7~10 d,群体花期为5月初至7月底,开放时有香味。雌雄同株,且同步成熟,在盛花期(开花5~6 d)花粉活力与柱头可授性均达到最强,花粉活力高达74.57%。多花指甲兰花粉为近圆形四合花粉,主要由脂质组成。OCI为4,多花指甲兰不存在无融合生殖现象,繁育系统是需要传粉者传粉的自交和异交混合交配模式。
Flowering characteristics and breeding system of Aerides rosea
doi: 10.11833/j.issn.2095-0756.20230308
- Received Date: 2023-05-15
- Accepted Date: 2023-10-10
- Rev Recd Date: 2023-09-13
- Available Online: 2023-12-01
- Publish Date: 2024-02-20
-
Key words:
- Aerides rosea /
- flowering process /
- pollen viability /
- stigma acceptability /
- breeding system /
- hybridization index
Abstract:
| Citation: | ZHENG Pengrui, DONG Chunyan, CHEN Zhiguang, et al. Flowering characteristics and breeding system of Aerides rosea[J]. Journal of Zhejiang A&F University, 2024, 41(1): 124-131. DOI: 10.11833/j.issn.2095-0756.20230308 |










 DownLoad:
DownLoad: