-
湿地是地球上物种最丰富、生产力最高、生态系统服务功能最强的生态系统,被誉为“地球之肾”,在维持物种多样性、净化水质、调节生态系统平衡等方面发挥重要作用[1−2]。近年来,全球变化及人为干扰导致湿地大面积退化[3],引起湿地结构、功能及生态过程的一系列变化,并影响土壤质地、结构、养分状况、酸碱性及溶氧量,最终对土壤微生物群落组成、结构及多样性产生一系列的调控作用[4]。
细菌作为湿地生态系统中的重要组成部分,主要参与土壤形成、凋落物分解、养分供应及生态系统养分循环[5],能够作为土壤生态系统变化的预警指标[6],对湿地生态系统结构及功能的维持与稳定起着不容忽视的作用。前人研究表明:高寒湿地和鄱阳湖湿地退化,导致土壤蓄水保肥能力降低、养分流失、碳氮转化速率减慢,显著抑制土壤细菌群落多样性[7−8]。但也有一些研究表明:人为干扰引起湿地排干、水分流失、土壤酸化及土壤养分供给改变,能够导致湿地土壤细菌多样性增加[9−10]。另外,三江平原湿地退化引起的土壤酸碱度及含水量变化仅影响土壤细菌群落组成,而对细菌多样性无显著影响[11]。可见,土壤细菌群落对湿地土壤理化性质变化的响应,存在不确定性。这种不确定性可能与全球变化、区域气候、湿地类型及人为活动干扰密切相关。因此,探明“不同退化阶段—土壤理化环境—细菌群落结构和多样性”之间的耦合关系,对于理解全球气候变化和人为干扰引起的湿地退化对土壤细菌群落的影响机制,具有十分重要的科学意义。
纳帕海高原湿地地处青藏高原香格里拉县内,其特殊的闭合—半闭合地形孕育着丰富的生物多样性,是全球生物多样性保护的重点区域[12]。近20多年来,在喀斯特作用和人为干扰的叠加影响下,该区湖水外泄,湖面面积大幅度减小,沼泽湿地逐渐旱化为沼泽化草甸和草甸,导致湿地水文和理化环境发生改变,进而影响土壤细菌群落结构及多样性[13]。本研究选取纳帕海不同退化阶段高原湿地类型(沼泽湿地、沼泽化草甸和草甸)为研究对象,运用Illumina高通量测序技术,揭示不同退化阶段湿地的土壤细菌群落结构及多样性干湿季变化特征,并分析细菌群落结构及多样性与土壤理化性质变化之间的关系,从而阐明土壤细菌群落对纳帕海高原湿地退化过程的响应规律,以期为理解人为干扰及全球气候变化加剧背景下高原退化湿地的土壤微生物多样性保育提供关键数据支撑。
-
纳帕海湿地(27°49′~27°55′N,99°37′~99°43′E)地处滇西北横断山区香格里拉县,面积为3100 hm2,海拔为3260 m[12],是中国典型的高原季节性湿地,属于冷凉湿润的高原气候[14]。该区域年平均气温为5.4 ℃,最热月平均气温为13.2 ℃,最冷月平均气温为−3.8 ℃;干湿季节分明,雨季(5—10月)降雨量高达495.9 mm;干季(11月至翌年4月)降雨量仅占全年的20%[13]。在人为和自然因素的共同作用下,沼泽湿地(常年淹水)逐步向沼泽化草甸(季节性淹水)和草甸(无积水)退化。
-
于2015年1月(干季)和8月(湿季),在每种退化湿地样带中分别随机布设3个10 m×10 m样地(表1),每个样地内按对角线法布设5个采样点(4个顶角和1个中心),分别采集各点样品并混合为1个土样,共采集18份土壤样品。去除各样点地表2 cm厚的覆盖物,然后用土钻钻取0~20 cm土层土样,去除石砾、残根后混合,并用四分法取适量土壤装入无菌自封袋,贴好标签装入便携式冰箱尽快带回实验室(沼泽湿地常年淹水,用特质采样器采样[15])。将带回的土样约100 g用于测定土壤自然含水率,约1 kg经自然风干、磨细过100目和10目筛后用于测定土壤基本性质,约200 g于−70 ℃下冷冻保存,用于土壤DNA提取和细菌高通量测序。
湿地类型 经度(N) 纬度(E) 积水深度/cm 优势植物 沼泽湿地(SW) 27°50′43.46″ 99°39′07.86″ 8.5~23.0 杉叶藻Hippuris vulgaris、狐尾藻Myripophyllum spicatum、篦齿眼子菜
Potamogeton pectinatus沼泽化草甸(SM) 27°50′43.46″ 99°38′34.60″ −19.3~5.5 矮地榆Sanguisorba filiformis、发草Deschampsia caespitosa、无翅薹草
Carex pleistoguna、斑唇马先蒿Pedicularis longiflora var. tubiformis草甸(M) 27°49′56.13″ 99°38′55.26″ −154.0~−123.0 大狼毒Euphorbia jolkinii、剪股颖Agrostis matsumurae Table 1. Basic information of the sampling sites
-
土壤理化性质测定参照鲍士旦[16]方法,其中:土壤自然含水率采用烘干法;pH采用电位法(水土比为1.0∶2.5);有机质采用重铬酸钾氧化-外加热法;全氮采用硫酸-高氯酸消化开氏定氮法;全磷采用硫酸-高氯酸消煮-钼锑抗比色法;全钾采用氢氧化钠熔融-火焰光度法;速效氮采用碱解扩散吸收法;速效磷采用0.030 mol·L−1氟化铵-0.025 mol·L−1盐酸浸提钼蓝比色法;速效钾采用1.000 mol·L−1中性醋酸铵浸提火焰光度法。
-
用Soil DNA KIT试剂盒提取土壤总DNA,操作步骤参照试剂盒说明书。每份混合土样各提取3个DNA,充分混合后送往上海生工生物有限公司完成细菌高通量测序。利用引物341F[CCCTACA2CGACGCTCTTCCGATTG(barcode)CCTACGGGGGAG]和805R[GACTGGAGTTCCTTGGCACCCGAGAATTCCAGACTATATC]对细菌V3~V4区进行扩增,扩增过程分2轮。第1轮:10×PCR缓冲液5.0 μL,10 mmol·L−1dNTPs 0.5 μL,DNA模板10 ng,上游、下游引物各0.5 μL,Plantium Taq (5×16.67 mkat·L−1) 0.5 μL;扩增条件为:94 ℃预变性3 min,5个循环(94 ℃变性30 s、45 ℃退火20 s、65 ℃延伸30 s),20个循环(94 ℃变性20 s、55 ℃退火20 s、72 ℃延伸30 s),72 ℃延伸5 min。第2轮:DNA模板为20 ng,其他反应体系与第1轮一致;扩增条件为:95 ℃预变性30 s,5个循环(95 ℃变性15 s、55 ℃退火15 s、72 ℃延伸30 s),72 ℃延伸5 min。PCR扩增结束后,将纯化质检合格的扩增产物按1∶1等量混合,利用Miseq台式测序仪2×300 bp双端测序(paired-end)[13]。
-
实验数据用Excel 2007整理。数据分析前用SPSS 26进行正态分析和方差齐性检验(P<0.05)。采用单因素方差分析(one-way ANOVA)比较各样地变量之间的差异显著性,成对样本t检验比较干湿季之间的差异显著性。利用Mothur软件将相似性大于97%的序列归为同一种可操作分类单元(OTU),并计算Alpha多样性指数:丰富度指数(Richness)、香农指数 (Shannon)、艾斯指数(ACE)、赵氏指数(Chao1)、辛普森指数(Simpson)[13]。以理化因子为环境变量,细菌群落相对丰度为物种数据,采用Mantel分析理化因子对细菌群落结构的影响。
-
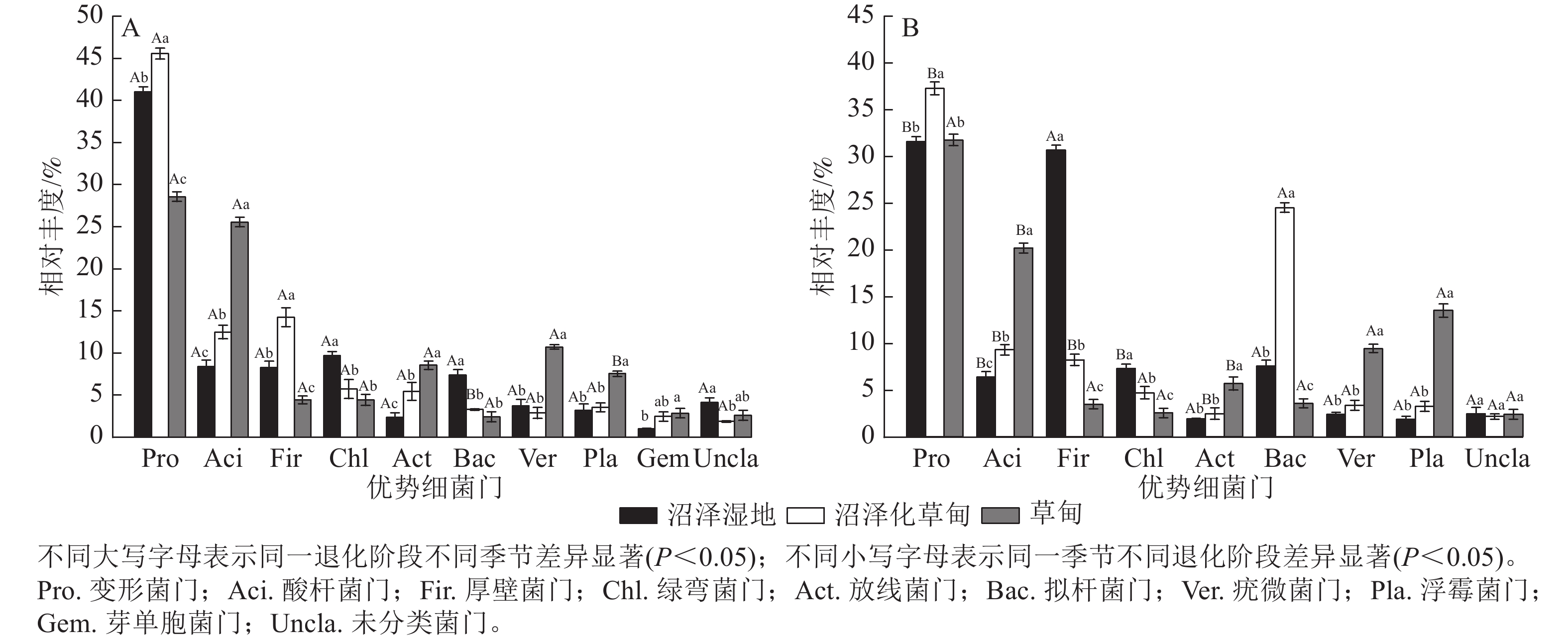
高通量测序结果显示:在干季和湿季共检测到相对丰度>1% 的细菌门主要有变形菌门Proteobacteria、酸杆菌门Acidobacteria、厚壁菌门Firmicutes、绿弯菌门Chloroflexi、放线菌门Actinobacteria、拟杆菌门Bacteroidetes、疣微菌门Verrucomicrobia、浮霉菌门Planctomycetes和未分类细菌门。其中,变形菌门是纳帕海高原湿地优势菌门,相对丰度高达35.92%,芽单胞菌门Gemmatimonadetes为干季特有菌门(图1)。
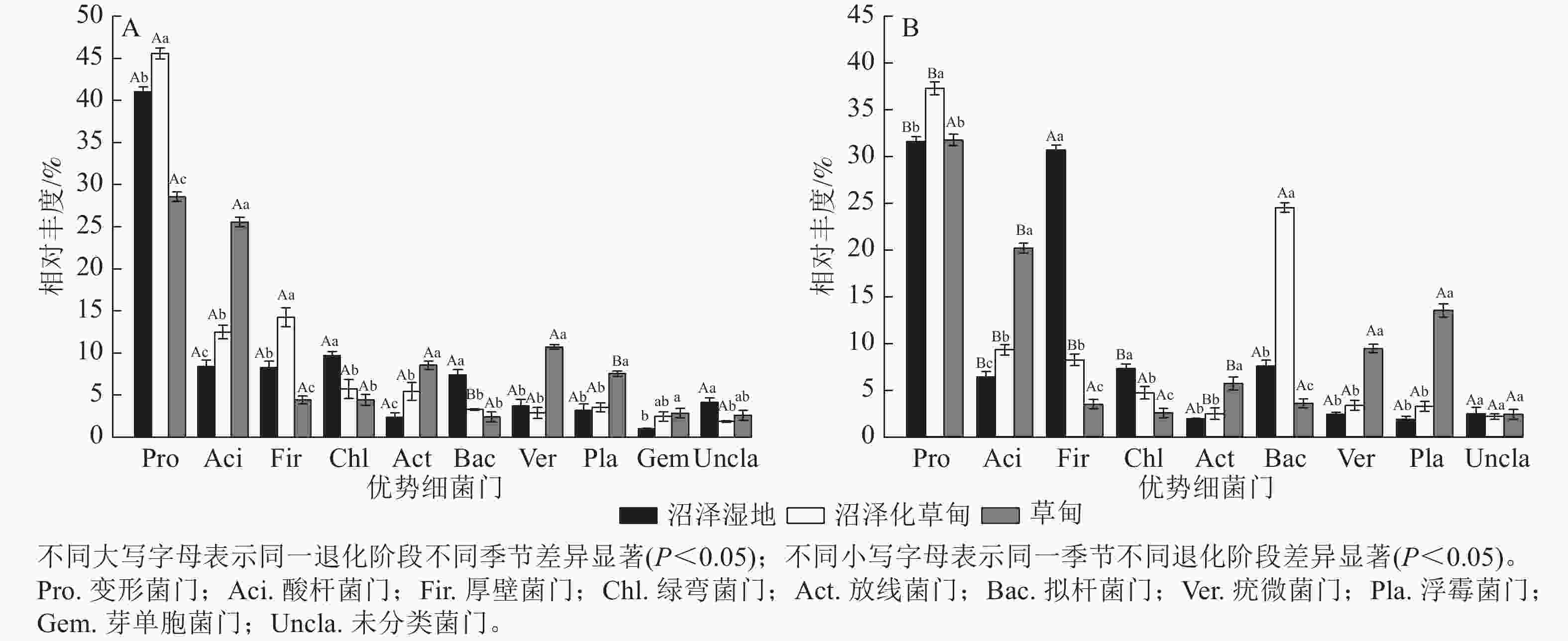
Figure 1. Composition of dry (A) and wet (B) season bacteria phylum in soil at different degradation stages
不同退化阶段土壤细菌门相对丰度差异显著(P<0.05)。与沼泽湿地相比较,在干季,沼泽化草甸的变形菌门、酸杆菌门和厚壁菌门相对丰度显著增加(P<0.05),分别增加11.04%、49.10%和72.31%,绿弯菌门和拟杆菌门相对丰度分别减少40.89%和55.50%;草甸的酸杆菌门、放线菌门、疣微菌门、浮霉菌门和芽单胞菌门相对丰度分别增加205.38%、260.76%、188.17%、135.31%和182.18%,变形菌门、厚壁菌门和拟杆菌门相对丰度分别减少30.34%、46.55%和67.16%。在湿季,沼泽化草甸的变形菌门、酸杆菌门和拟杆菌门相对丰度分别增加17.98%、45.84%和223.54%,厚壁菌门和绿弯菌门相对丰度分别减少73.17%和35.39% (P<0.05);草甸的酸杆菌门、放线菌门、疣微菌门和浮霉菌门相对丰度分别增加216.33%、194.30%、294.56%和624.73%,厚壁菌门和拟杆菌门相对丰度分别减少88.63%和52.65%。
不同退化阶段土壤细菌门相对丰度干湿季节存在显著差异(P<0.05)。沼泽湿地的变形菌门、酸杆菌门和绿弯菌门相对丰度为干季大于湿季,湿季分别减少了23.15%、23.89%和24.53%;厚壁菌门为湿季大于干季,是干季的3.70倍。沼泽化草甸的变形菌门、酸杆菌门、厚壁菌门和放线菌门相对丰度在湿季分别减少了18.35%、25.56%、42.39%和54.50%;拟杆菌门相对丰度在湿季显著增加(P<0.05),是干季的7.46倍。草甸的酸杆菌门相对丰度在湿季减少了21.17%;浮霉菌门相对丰度在湿季增加了1.79倍。
-
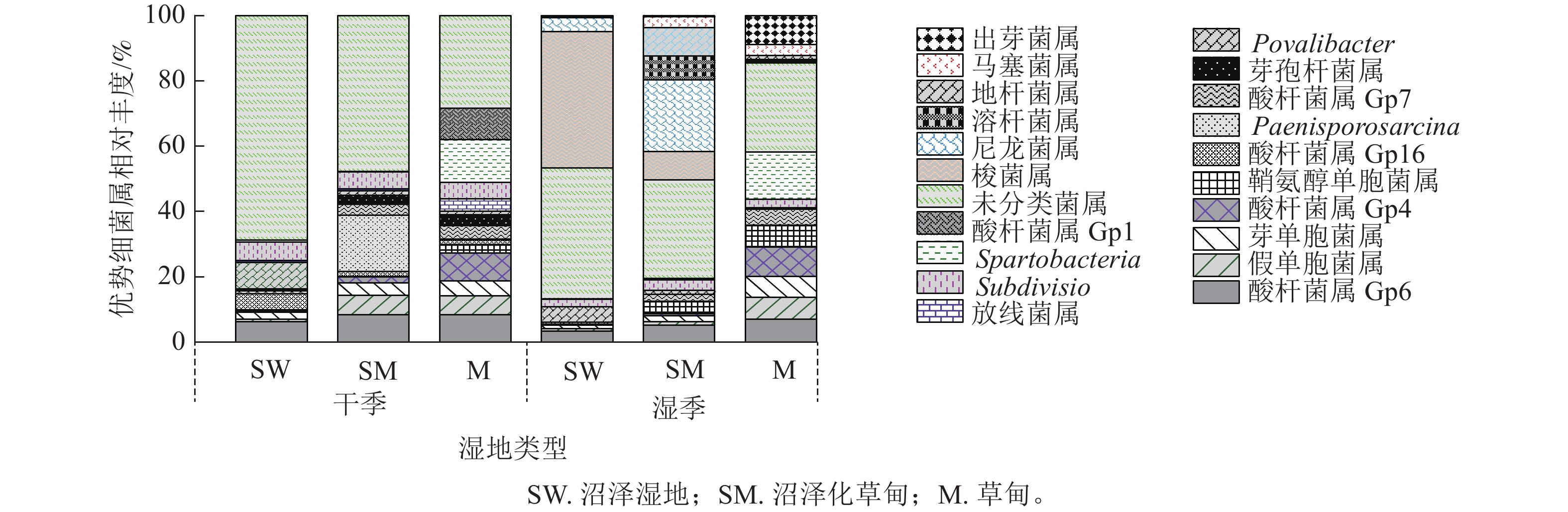
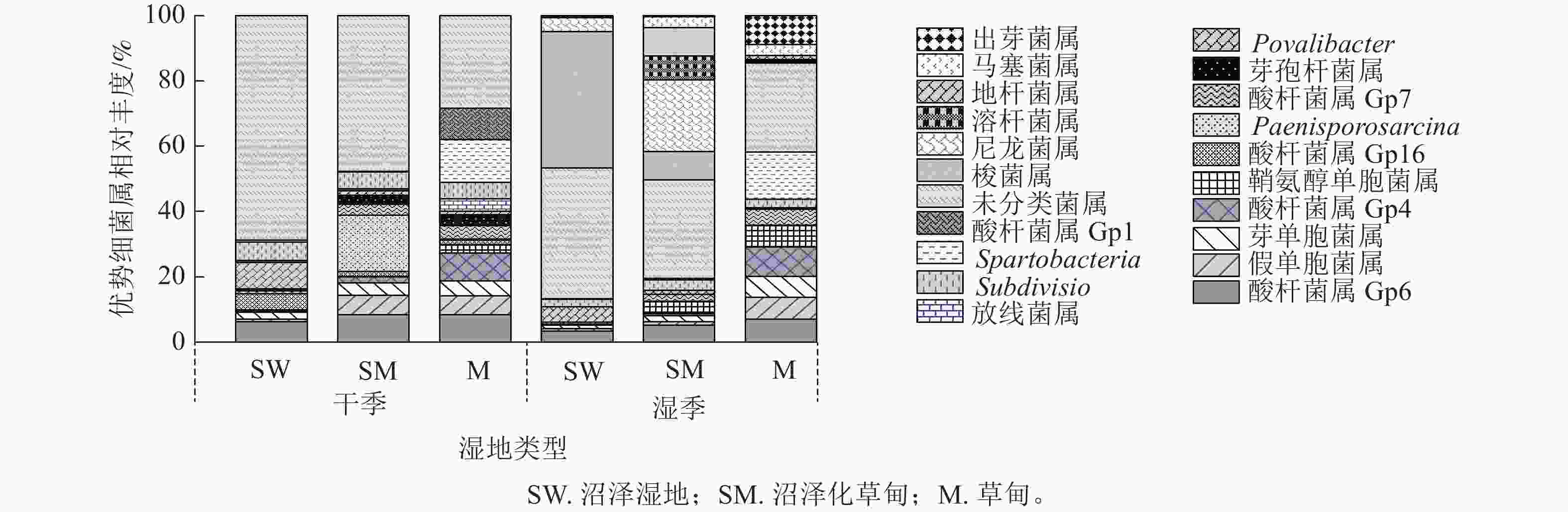
在属水平上,共检测到相对丰度>0.01%的细菌属有酸杆菌属(Gp4、Gp6、Gp7)、假单胞菌属Pseudomonas、芽单胞菌属Gemmatimonas、鞘氨醇单胞菌属Sphingomonas、Povalibacter、Subdivision、Spartobacteria和未分类菌属。除此之外,酸杆菌属(Gp1、Gp16)、Paenisporosarcina、芽孢杆菌属Bacillus和放线菌属Gaiella为干季特有菌属;梭菌属Clostridium、尼龙菌属Flavobacterium、溶杆菌属Lysobacter、地杆菌属Pedobacter、马塞菌属Massilia和出芽菌属Gemmata为湿季特有菌属。未分类菌属为纳帕海高原湿地优势菌属,相对丰度高达20.64% (图2)。
湿地退化显著影响土壤细菌属相对丰度(P<0.05)。与沼泽湿地相比较,在干季,沼泽化草甸的假单胞菌属、Paenisporosarcina属相对丰度显著增加(P<0.05),分别增加9.34、455.50倍,Povalibacter属相对丰度显著减少77.29% (P<0.05);草甸的酸杆菌属(GP16)、假单胞菌属、Spartobacteria属相对丰度分别增加409.35、9.54和30.86倍,Povalibacter属和未分类菌属相对丰度分别减少80.06%和41.25%。在湿季,沼泽化草甸的尼龙菌属、溶杆菌属、地杆菌属相对丰度分别增加5.87、228.50和197.98倍,梭菌属相对丰度显著减少76.28% (P<0.05);草甸的出芽菌属相对丰度显著增加116.75倍(P<0.05),未分类菌属和梭菌属相对丰度分别减少33.49%和99.34%。
不同退化阶段的共有菌属因干湿季节变化而存在差异。沼泽湿地和沼泽化草甸的未分类菌属相对丰度均为干季大于湿季,在湿季分别减少21.59%和26.61%。沼泽化草甸中的假单胞菌属相对丰度在湿季减少79.75%;鞘氨醇单胞菌属相对丰度在湿季增加了95.78%。
-
由表2可见:湿地退化显著影响土壤细菌群落多样性(P<0.05)。在干季,沼泽化草甸和草甸的丰富度指数、香农指数、艾斯指数和Chao1指数较沼泽湿地显著增加(P<0.05),沼泽化草甸与草甸间差异不显著(P>0.05);在湿季,沼泽化草甸和草甸的丰富度指数、香农指数、艾斯指数和Chao1指数较沼泽湿地也显著增加,且沼泽化草甸显著高于草甸(P<0.05)。不同退化阶段土壤细菌群落多样性指数在季节变化上存在差异。沼泽湿地和沼泽化草甸的丰富度指数、艾斯指数和Chao1指数均为湿季大于干季;草甸的丰富度指数、香农指数、艾斯指数均为干季大于湿季,辛普森指数相反,且差异显著(P<0.05)。
湿地
类型季节 丰富度
指数香农
指数艾斯
指数Chao1
指数辛普森
指数沼泽
湿地干季 4056 Bc 6.16 Ab 5668.94 Bb 5368.14 Bb 0.0135 Aa 湿季 4201 Ac 6.20 Ac 6095.66 Ac 5789.86 Ac 0.0118 Aa 沼泽化
草甸干季 5352 Bb 6.68 Aa 7046.88 Ba 6631.88 Ba 0.0061 Ab 湿季 5697 Aa 7.14 Aa 8095.30 Aa 8121.55 Aa 0.0049 Ab 草甸 干季 5451 Aa 6.83 Aa 7059.57 Aa 6655.28 Aa 0.0041 Bc 湿季 4915 Bb 6.27 Bb 6673.81 Bb 6398.10 Ab 0.0130 Aa 说明:表中数据为平均值。不同大写字母表示同一退化阶段不同季节差异显著(P<0.05);不同小写字母表示同一季节不同退化阶段差异显著(P<0.05)。 Table 2. Diversity index of soil bacterial community at different degradation stages
-
由表3可知:湿地退化显著改变土壤理化性质(P<0.05)。湿地退化使土壤含水量以及有机质、全氮和速效氮质量分数显著减少(P<0.05)。干季的沼泽化草甸分别减少77.77%、41.16%、46.53%和33.91%,草甸分别减少80.93%、64.81%、87.31%和50.79%;湿季的沼泽化草甸分别减少32.30%、25.01%、51.34%和7.32%,草甸分别减少78.31%、61.99%、70.62%和39.18%,且土壤逐渐酸化。土壤磷、钾养分及碳氮比的变化趋势有所差异。较沼泽湿地,在干季,沼泽化草甸土壤全磷、全钾、速效磷、速效钾质量分数分别增加46.88%、9.39%、15.34%和27.38%;草甸土壤全磷质量分数及碳氮比分别增加14.06%和176.80%,全钾、速效磷及速效钾质量分数分别减少31.41%、42.92%和40.97%。在湿季,沼泽化草甸土壤全钾质量分数和碳氮比分别减少14.35%和63.88%,速效磷及速效钾质量分数分别增加66.15%和108.66%;草甸土壤全磷、速效磷、速效钾质量分数及碳氮比分别减少30.09%、33.57%、49.88%和37.97%,全钾质量分数显著增加51.48% (P<0.05)。
湿地类型 干湿季 含水量/% 有机质/(g·kg−1) 全氮/(g·kg−1) 全磷/(g·kg−1) 全钾/(g·kg−1) 沼泽湿地 干季 106.15±0.47 Ba 138.20±4.29 Aa 9.22±0.20 Ba 0.64±0.01 Ac 10.76±0.36 Ab 湿季 117.15±0.60 Aa 144.40±2.52 Aa 11.98±0.29 Aa 0.57±0.01 Aa 9.13±0.20 Ab 沼泽化草甸 干季 23.60±1.52 Bb 81.31±1.45 Bb 4.93±0.31 Ab 0.94±0.02 Aa 11.77±0.29 Aa 湿季 79.31±0.91 Ab 108.28±1.37 Ab 5.83±0.31 Ab 0.56±0.02 Ba 7.82±0.15 Bc 草甸 干季 20.24±1.04 Bb 48.63±6.60 Bc 1.17±0.04 Bc 0.73±0.01 Ab 7.38±0.18 Ac 湿季 25.41±0.50 Ac 54.89±2.13 Ac 3.52±0.05 Ac 0.37±0.02 Bb 13.83±0.13 Aa 湿地类型 干湿季 碳氮比 pH 速效氮/(mg·kg−1) 速效磷/(mg·kg−1) 速效钾/(mg·kg−1) 沼泽湿地 干季 8.75±0.37 Bb 7.92±0.01 Aa 627.75±2.29 Aa 6.78±0.16 Ab 176.76±0.93 Bb 湿季 14.59±0.55 Aa 7.87±0.02 Aa 494.61±7.02 Ba 7.15±0.20 Ab 297.36±9.03 Ab 沼泽化草甸 干季 9.81±0.47 Ab 6.97±0.04 Bb 414.85±1.37 Bb 7.82±0.14 Ba 225.16±1.29 Ba 湿季 5.27±0.13 Bc 7.82±0.11 Aa 458.39±3.36 Ab 11.88±1.21 Aa 620.46±4.70 Aa 草甸 干季 24.22±3.30 Aa 5.92±0.12 Ac 308.92±1.36 Ac 3.87±0.14 Bc 104.35±1.44 Ac 湿季 9.05±0.35 Bb 5.65±0.08 Ab 300.84±3.44 Ac 4.75±0.05 Ac 149.04±8.64 Ac 说明:表中数据为平均值±标准误。不同大写字母表示同一退化阶段不同季节差异显著(P<0.05);不同小写字母表示同一季节不同退化阶段差异显著(P<0.05)。 Table 3. Soil physical and chemical characteristics at different degradation stages
干湿季节变化显著影响土壤理化性质的变化规律(P<0.05)。沼泽湿地土壤含水量以及全氮、碳氮比、速效钾质量分数均为湿季大于干季,湿季分别增加10.36%、29.93%、40.03%和68.23%;速效氮在湿季显著减少21.21%(P<0.05)。沼泽化草甸土壤含水量以及有机质、速效氮、速效磷和速效钾质量分数在湿季比干季分别增加236.06%、33.17%、10.50%、51.92%和175.56%;全磷、全钾质量分数和碳氮比在湿季分别减少40.43%、33.56%和46.28%;湿季pH升高,土壤偏碱性。草甸土壤含水量以及有机质、全氮、速效磷质量分数在湿季比干季分别增加25.54%、12.87%、200.85%和22.74%;全磷质量分数和碳氮比分别减少49.32%和62.63%。
-
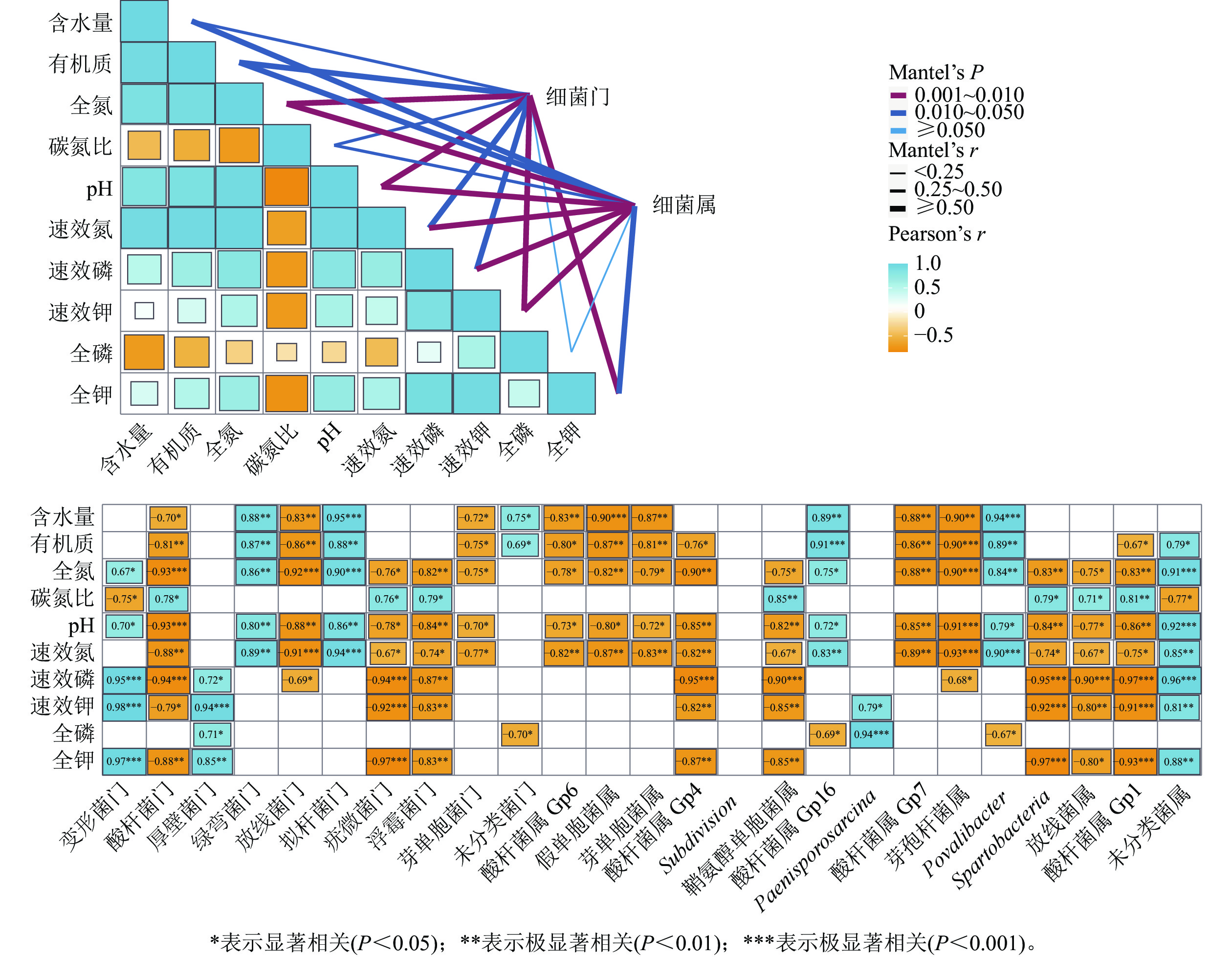
干季土壤理化因子与土壤细菌群落组成的Mantel分析结果如图3所示。门水平上,土壤全氮、全钾、速效钾质量分数及pH和土壤细菌门的曼特尔显著值最小(P<0.01),说明土壤pH以及氮和钾质量分数的高低是调控干季纳帕海不同退化阶段湿地土壤细菌群落结构的主要理化因子。其中,全氮质量分数和pH与酸杆菌门、放线菌门、疣微菌门、浮霉菌门、芽单胞菌门呈显著负相关(r=−0.93~−0.70,P<0.05),与变形菌门、绿弯菌门、拟杆菌门呈显著正相关(r=0.67~0.90,P<0.05)。全钾和速效钾质量分数与变形菌门、厚壁菌门呈极显著正相关(r=0.85~0.98,P<0.01),与酸杆菌门、疣微菌门、浮霉菌门呈显著负相关(r=−0.97~−0.79,P<0.05)。
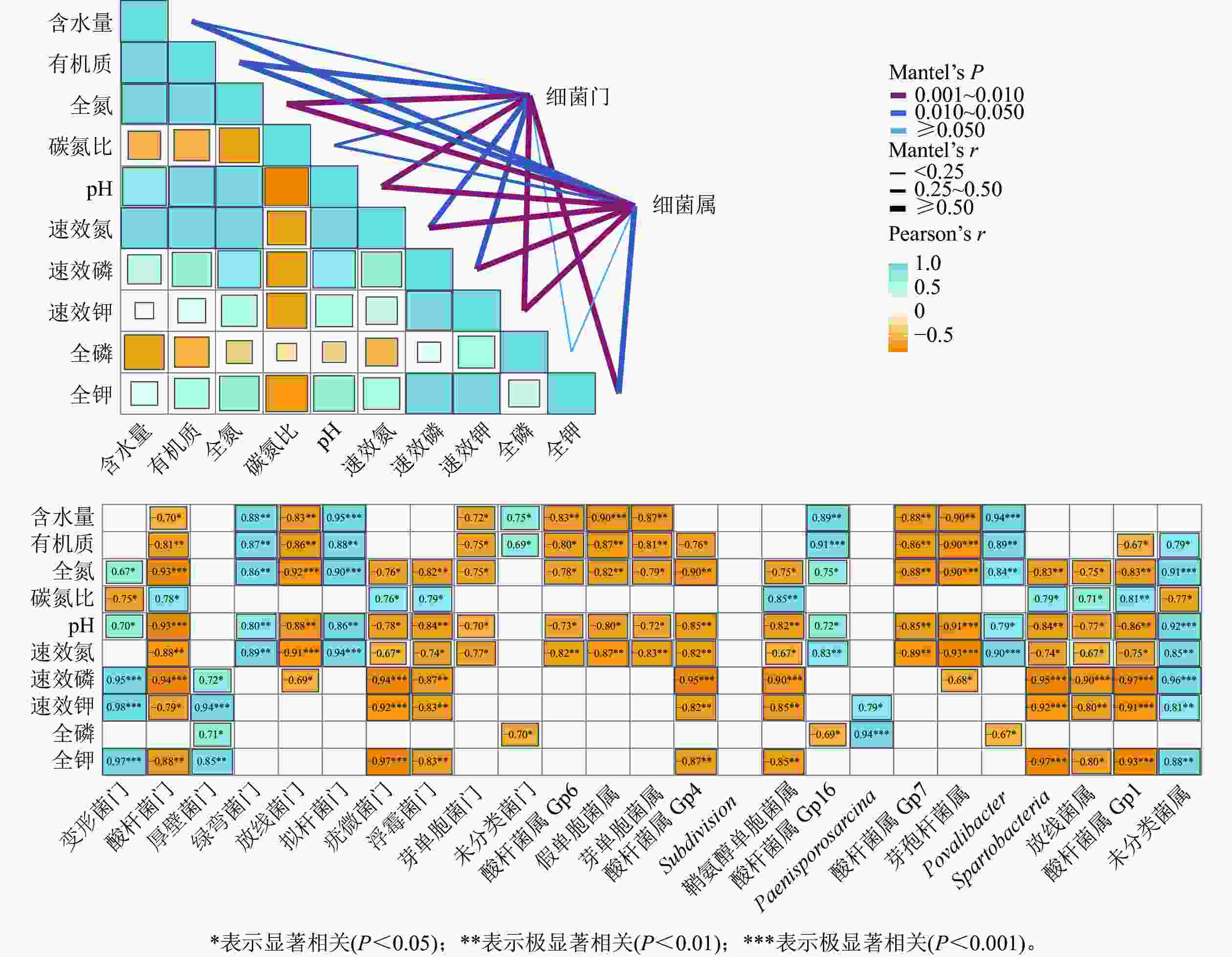
Figure 3. Mantel test analysis of the relationship between soil physical and chemical factors and bacterial community structure in dry season
属水平上,土壤氮、磷、钾质量分数以及pH的高低是调控干季纳帕海不同退化阶段湿地土壤细菌群落组成的主要理化因子。其中,全氮、速效氮质量分数以及pH与酸杆菌属(Gp1、Gp4、Gp6、Gp7)、假单胞菌属、芽单胞菌属、鞘氨醇单胞菌属、芽孢杆菌属、Spartobacteria、出芽菌属呈显著负相关(r=−0.93~−0.67,P<0.05),与Gp16属、Povalibacter、未分类菌属呈显著正相关(r=0.72~0.91,P<0.05)。速效磷、速效钾质量分数与酸杆菌属(Gp1、Gp4)、鞘氨醇单胞菌属、Spartobacteria、出芽菌属呈极显著负相关(r=−0.97~−0.80),与未分类菌属呈极显著正相关(r=0.81~0.96,P<0.01)。
-
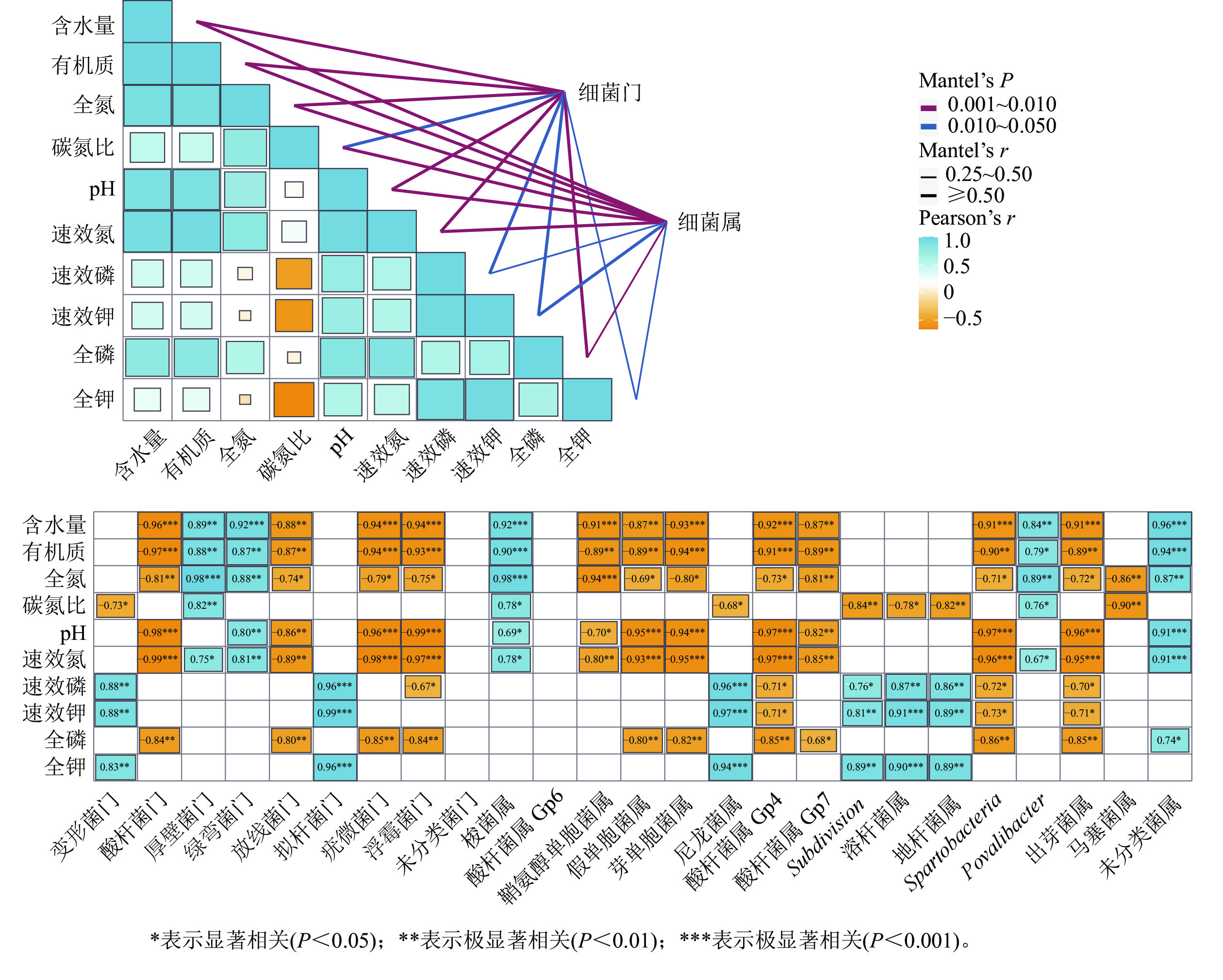
湿季土壤理化因子与土壤细菌群落组成的Mentel分析结果如图4所示。门水平上,有机质、氮和磷质量分数及含水量、pH的高低是调控纳帕海不同退化阶段湿地土壤细菌群落结构的主要理化因子。其中,含水量、pH以及有机质、全氮、全磷、速效氮质量分数与酸杆菌门、放线菌门、疣微菌门和浮霉菌门呈显著负相关(r=−0.98~−0.74,P<0.05),有机质、全氮、速效氮质量分数以及含水量与厚壁菌门、绿弯菌门呈显著正相关(r=0.75~0.96,P<0.05)。
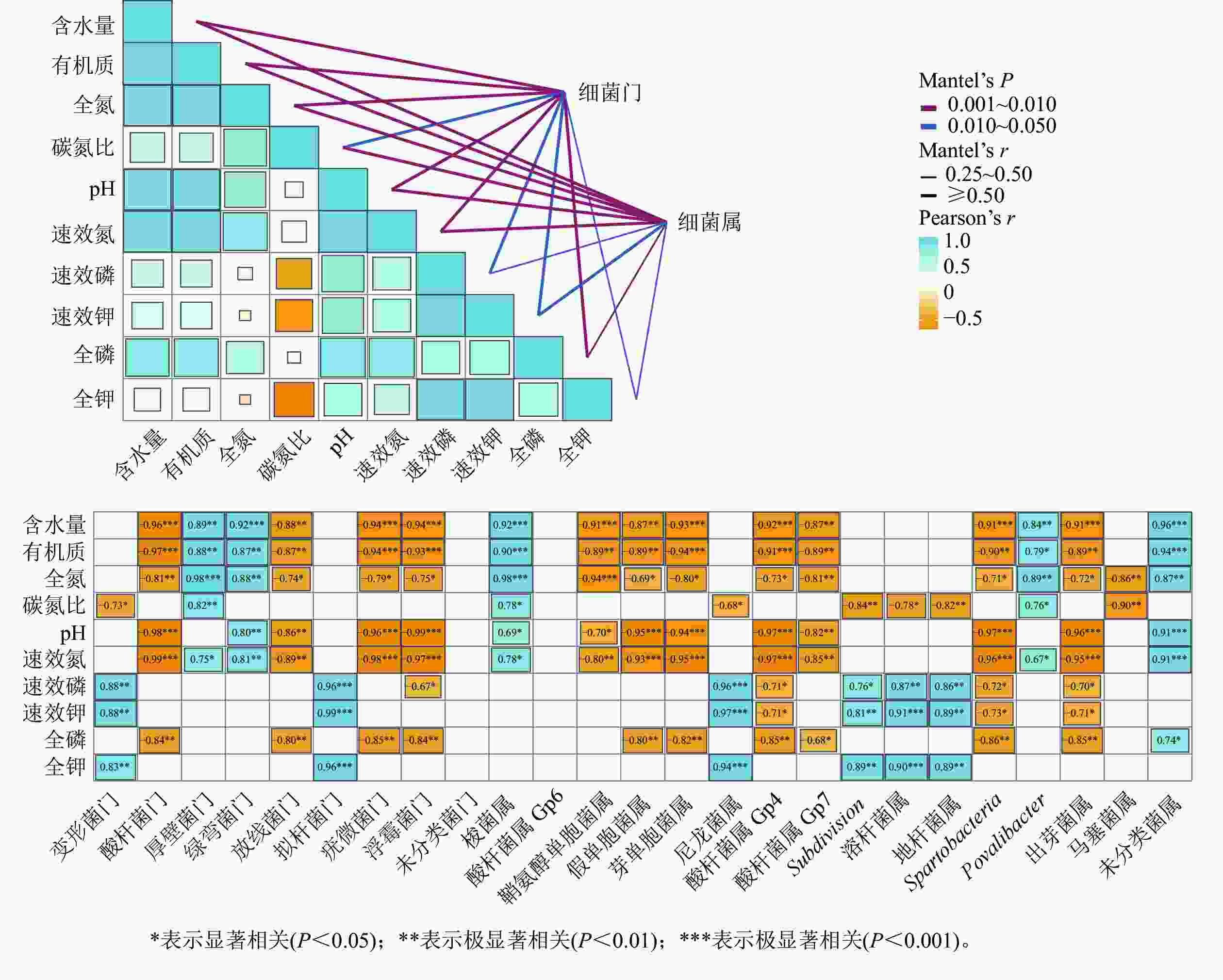
Figure 4. Mantel test analysis of the relationship between soil physical and chemical factors and bacterial community structure in wet season
属水平上,有机质、氮、磷质量分数以及pH、含水量、碳氮比是调控纳帕海不同退化阶段湿地土壤细菌群落组成的主要理化因子。其中,有机质、全氮、全磷、速效氮质量分数以及含水量、pH 与假单胞菌属、芽单胞菌属、Gp4属、Gp7属、Spartobacteria、出芽菌属呈显著负相关(r=−0.97~−0.71,P<0.05),与梭菌属、Povalibacter、未分类菌属呈显著正相关(r=0.79~0.96,P<0.05);碳氮比与尼龙菌属、Subdivision、溶杆菌属、地杆菌属、马塞菌属呈显著负相关(r=−0.90~−0.68,P<0.05),与梭菌属和Povalibacter呈显著正相关(r=0.76~0.78,P<0.05)。
-
对土壤理化因子与细菌多样性指数进行Pearson相关性分析,结果如表4所示。在干季,丰富度指数、香农指数、艾斯指数、Chao1指数与有机质、全氮、速效氮质量分数以及pH、含水量呈极显著负相关(r=−0.99~−0.83,P<0.01)。可见:土壤有机质、全氮、速效氮质量分数以及pH、含水量是影响干季土壤细菌多样性的主控因子,且对细菌多样性起抑制作用。
项目 干季 湿季 丰富度指数 香农指数 艾斯指数 Chao1指数 辛普森指数 丰富度指数 香农指数 艾斯指数 Chao1指数 辛普森指数 含水量 −0.99** −0.92** −0.99** −0.99** 0.98** − − − − − 有机质 −0.87** −0.89** −0.85** −0.85** 0.93** − − − − − 全氮 −0.91** −0.90** −0.89** −0.89** 0.96** −0.67* − − − − 全磷 − − 0.71* 0.70* − − − − − − 全钾 − − − − − 0.72* 0.88** 0.83** 0.85** −0.84** 碳氮比 − − − − −0.70* −0.96** −0.79* −0.91** −0.90** − pH −0.86** −0.83** −0.83** −0.83** 0.91** − − − − − 速效氮 −0.96** −0.93** −0.95** −0.94** 0.99** − − − − − 速效磷 − − − − − − 0.88** 0.79* 0.81** −0.90** 速效钾 − − − − − 0.68* 0.91** 0.82** 0.84** −0.91** 说明:*表示显著相关(P<0. 05);**表示极显著相关(P<0. 01);−表示不相关(P>0.05)。 Table 4. Correlation analysis between main soil physical and chemical factors and bacterial community diversity
在湿季,香农指数、艾斯指数、Chao1指数与土壤全钾、速效钾、速效磷质量分数呈显著正相关(r=0.79~0.91,P<0.05),与碳氮比呈显著负相关(r=−0.91~−0.79,P<0.05),是影响湿季土壤细菌多样性的主控因子。其中,全钾、速效钾、速效磷质量分数对细菌多样性起促进作用,而碳氮比起抑制作用。
-
土壤细菌作为微生物群落中数量最丰富、种类最多、生物量最大的功能类群,能够对高原湿地退化引起的土壤微域环境变化产生敏感响应[17]。变形菌门是纳帕海湿地的主要优势类群,与李金业等[18]、李玉倩等[19]研究结果一致。变形菌门的生态幅广、适宜能力强,能在不同退化湿地环境中形成较为稳定的生态位,但其喜弱碱特性会影响其相对丰度的变化[20]。本研究中,在轻度退化的弱碱沼泽化草甸土壤中变形菌门相对丰度显著增加。酸杆菌门、放线菌门、疣微菌门、浮霉菌门和芽单胞菌门相对丰度均随湿地退化程度的加深而增加。酸杆菌属嗜酸菌、寡营养类群,其胞外多糖与补偿溶质的产生与积累使其更适应于含水量低、酸性较强、养分较低的退化草甸土壤[21];放线菌门、疣微菌门Spartobacteria属和浮霉菌门等属好气性细菌,喜欢通气良好的环境,湿地退化导致土壤含水量减少,土壤孔隙度、通气状况得到改善,促进其相对丰度的显著增加[22];疣微菌门是高寒沙化草甸的特有菌群,主要通过磷来维持其群落机制和养分平衡[23],故在磷分较丰富的退化草甸土壤中相对丰度较高。本研究发现:湿地退化会抑制绿弯菌门的生长繁殖,这是因为该菌门属兼性厌氧菌,在养分匮乏的条件下进行光能自养,但仍以无光或有光且缺氧条件下的化学能或光能异养生长为主[24],湿地退化导致土壤水分减少而抑制其相对丰度增加。综上所述,湿地退化过程中土壤含水量减少,使得土壤通气透水性得到改善,促进好气性细菌类群大量繁殖。
干湿季节交替通过调节水分、温度以及细菌对土壤养分的利用关系从而改变土壤细菌群落组成[25]。本研究发现:厚壁菌门在沼泽湿地中为湿季大于干季,而在沼泽化草甸中为干季大于湿季。厚壁菌属厌氧快速生长型菌群,具有固碳作用,大多存在于动物肠道中[26]。湿季雨水冲刷,厚壁菌门随动物粪便流入沼泽湿地,相对丰度增加,同时动物粪便的输入可直接刺激其相对丰度的增加;而沼泽化草甸的有机底物相对较低,干季丰富的凋落物为厚壁菌门提供充足碳源,相对丰度较湿季增加。梭菌属为湿季特有菌属,在沼泽湿地中占优势,相对丰度高达22.60%。该菌属是来自厚壁菌门的专性厌氧铁还原菌,在严格厌氧条件下才能生存,主要通过还原铁获取生长能量[27],沼泽湿地常年淹水,湿季适宜的温度和充足的有机底物可促进其快速生长;而在干季,养分较少、温度相对较低,有利于厌氧寡营养绿弯菌门聚集[28]。拟杆菌门(尼龙菌属)在湿季沼泽化草甸显著增加,这主要与其需氧特性和水生环境的生物学特性有关[29]。酸杆菌门具有降解植物残体多聚物的能力[21],干季植物枯死,凋落物的分解为酸杆菌的繁殖及降解提供更好的养分条件[30],故相对丰度呈现干季大于湿季的变化趋势。芽单胞菌门在干季相对丰度较低,在湿季则未检测到,进一步表明了芽单胞菌属好氧菌,适宜生存于较为干燥的环境中[31−32]。因此,干湿季更替显著影响好养厌养、需氧厌氧细菌群落分布格局。
-
高原湿地退化通过影响水热条件、土壤结构、土壤养分,进而影响土壤细菌群落多样性[33]。本研究中,沼泽化草甸和草甸土壤的丰富度指数、香农指数、艾斯指数和Chao1指数显著高于沼泽湿地,说明湿地退化会促进细菌多样性的增加。这可能是沼泽湿地有机底物常年积累,但由于其土壤处于厌氧状况,不利于微生物对养分的矿化,难为大多数细菌提供直接能量来源[34],因此,细菌多样性较沼泽化草甸和草甸细菌低。另一方面,湿地在退化过程中,土壤孔隙度、通气状况得到改善,碱性减弱、凋落物分解加快,农药化肥的残留以及牛粪的输入为细菌生命活动提供物质源泉[35],有利于好氧喜酸细菌大量繁殖,从而导致细菌多样性较沼泽湿地高。另外,湿地退化导致植被类型呈现挺水植物—湿中生植物—旱生植物的演替格局[12],地上凋落物、根系分泌物的增加直接为土壤细菌提供可利用的碳氮及其他养分,细菌多样性增加[13]。
干湿季节更替引起的降雨量和温湿度变化,可能影响土壤理化性质及酶活性变化,进而调控土壤细菌群落多样性的干湿季变化[36]。本研究中,沼泽湿地和沼泽化草甸土壤细菌的丰富度指数、艾斯指数湿季显著高于干季,而草甸则为干季显著高于湿季。原因可能是沼泽湿地和沼泽化草甸淹水较多导致通气透水性差,抑制了需氧细菌对有机质的降解[24]。但在湿季,由于温度升高,细菌酶活性增强,溶解氧降低[37],刺激细菌大量繁殖,导致湿季细菌多样性高于干季。相较于沼泽湿地和沼泽化草甸,草甸土壤含水量低,通气透水性好,为需氧细菌提供良好的微氧环境[34]。特别是湿季放牧和旅游增加,土壤细菌多样性会因牲畜和游客践踏引起的土壤板结和理化性质变化,而导致细菌多样性湿季低于干季[38]。因此,干湿季节更替使得纳帕海不同退化阶段土壤细菌群落多样性存在差异。
-
湿地退化过程中土壤水分状况变化,直接或间接导致土壤环境厌氧-需氧界面通气性、酸碱性和养分状况的改变,进而显著影响土壤细菌群落结构及多样性[39−40]。纳帕海高原湿地不同退化阶段土壤含水量是影响干季土壤细菌群落结构和多样性变化的主要因子,由于沼泽湿地—沼泽化草甸—草甸演替过程中,土壤含水量减少,土壤质地疏松和溶解氧增加,有利于土壤养分分解,从而促进需氧菌的繁殖,细菌多样性增加。牛佳等[41]指出:水分是影响土壤细菌群落结构的主要因子,通过调节土壤酸碱度及养分分布格局,进而影响细菌群落结构组成。本研究中,土壤含水量与全氮、有机质、速效氮质量分数以及pH呈显著正相关,并随湿地退化而显著减少。pH高低决定整个湿地生态系统元素循环反应体系的酸碱度[42],显著影响细菌群落组成。湿地退化过程中,土壤酸化使得土壤碳氮磷养分有效性发生改变[43],从而影响土壤细菌群落分布格局。Mantel分析结果显示:pH同土壤有机质、全氮、速效氮质量分数以及含水量显著促进酸杆菌门、放线菌门、浮霉菌门和疣微菌门相对丰度增加,而抑制变形菌门、绿弯菌门和拟杆菌门相对丰度增加。原因是变形菌门、绿弯菌门和拟杆菌门属于固碳微生物,具有好氧喜弱碱特性[44],湿地退化,碳氮质量分数降低不利于细菌生长繁殖。这与林春英等[7]在高寒沼泽湿地退化研究中得出的结论相似。
不同退化阶段土壤碳氮比增加对湿季细菌多样性增加起抑制作用。李杰[45]研究得出:碳氮比通过调节微生物分解进程进而调节土壤养分有效性,高碳氮比抑制土壤微生物活性从而缓解有机质分解,而低碳氮比加快微生物对有机质的分解、转化。本研究中,高原湿地退化过程中,土壤碳氮比降低,细菌可利用养分转化速率加快[46],细菌多样性增加;但湿地退化引起的土壤有机质减少也会导致土壤细菌可利用碳源减少,使得绝大部分共养厌氧细菌相对丰度下降(厚壁菌门、变形菌门)。Mantel分析结果显示:土壤含水量及碳、氮质量分数减少,显著抑制厚壁菌门、绿弯菌门和梭菌属相对丰度的增加;磷促进变形菌门、拟杆菌门(尼龙菌属)和地杆菌属相对丰度的增加。原因是湿地退化,土壤碳氮质量分数及含水量减少,厌氧需养菌受到抑制[47];同时,湿地退化引起的土壤酸化可刺激铁铝氧化物释放磷元素,进而促进喜磷细菌(变形菌门、拟杆菌门以及尼龙菌属和地杆菌属)大量繁殖。综上所述,湿地退化引起土壤养分状况、含水量及酸碱度改变,通过影响土壤细菌的养分需求和代谢过程,进而调控纳帕海高原湿地土壤细菌多样性。
-
纳帕海高原湿地退化显著影响土壤理化环境的时空异质性,进而调控土壤细菌群落结构及多样性。湿地退化引起土壤有机质、氮质量分数以及水分、pH减小,导致酸杆菌门、放线菌门、浮霉菌门、疣微菌门相对丰度显著增加,绿弯菌门相对丰度显著减少及变形菌门、厚壁菌门(梭菌属)和拟杆菌门(尼龙菌属)干湿季差异,进而导致退化湿地土壤细菌多样性较沼泽湿地显著增加。因此,湿地退化导致土壤水分、酸碱度及土壤养分供给状况发生改变,从而显著影响土壤细菌群落结构及多样性。
Response of soil bacterial community to wetland degradation in the Napahai Plateau
doi: 10.11833/j.issn.2095-0756.20230331
- Received Date: 2023-05-25
- Accepted Date: 2023-09-08
- Rev Recd Date: 2023-09-01
- Available Online: 2023-10-11
- Publish Date: 2024-04-01
-
Key words:
- Napahai /
- wetland degradation /
- soil bacteria /
- community diversity /
- dry and wet seasons
Abstract:
| Citation: | ZHAO Dingrong, LU Mei, ZHAO Xuyan, et al. Response of soil bacterial community to wetland degradation in the Napahai Plateau[J]. Journal of Zhejiang A&F University, 2024, 41(2): 406-418. DOI: 10.11833/j.issn.2095-0756.20230331 |









 DownLoad:
DownLoad: