-
土壤动物对土壤碳循环和土壤质量具有重要影响[1]。蚯蚓作为重要的土壤动物,呼吸产生的二氧化碳(CO2)是土壤呼吸的重要组成部分[2−3],其取食、掘穴、排泄等活动能改变土壤理化性质、微生物组成和活性及其他土壤动物的组成,影响地上植物生长,调节土壤有机碳的固定及矿化等生态过程,间接影响土壤温室气体的排放[4−6]。
土壤有机碳(SOC)在全球碳循环中扮演重要角色,保持或增加其稳定性是应对全球气候变化的关键措施之一[7]。引起土壤有机碳最初变化的主要为易分解、易矿化的活性有机碳,其对外界干扰导致的环境变化较为敏感[8],是预测土壤有机碳早期变化的重要指标[9]。蚯蚓主要从4个维度影响土壤有机碳动态和养分循环:蚯蚓活动、蚯蚓肠道、蚯蚓的排泄物和土壤的长期发育。蚯蚓的取食加强了植物残体分解中的生物过程,其生理活动又将有机质与矿质土混合,形成的土壤微粒富含有机质,为有机质提供物理保护的同时减缓有机质的周转,提高土壤潜在的碳固存能力[10],富含易碱解氮的蚓粪加快了周围凋落物的矿化过程[11]。有关蚯蚓活动对土壤CO2排放影响的Meta分析表明:接种蚯蚓后并未影响土壤有机碳储量,但土壤CO2排放量增加了33% [12]。也有研究表明:蚯蚓能够同时促进土壤有机碳的固定和CO2的排放,但培养时间超过750 d后会造成土壤全碳质量分数的降低[13]。LUBBERS等[14]同样发现:蚯蚓活动对土壤CO2排放的影响主要集中在试验初期,其作用效果会随着时间逐渐减弱[3],甚至相反[15]。JENNINGS等[16]研究表明:尽管蚯蚓对林地土壤碳库影响深远,但土壤总呼吸量不会因此显著升高,可能是由于蚯蚓所促进的异养呼吸与细根生物量降低的自养呼吸几乎相互抵消。因此,蚯蚓在特定的生态系统中,在有植物生长的状态下,通过取食凋落物等一系列活动对土壤活性有机碳及CO2排放的影响有哪些,值得进一步研究。
毛竹Phyllostachys edulis是中国亚热带地区最主要的竹种,约占总竹林面积的72.9%[17]。成熟竹林的地下鞭根系统可以将光合作用固定的碳持续输入土壤中[18],固碳趋势明显,固碳增汇潜力大,对平衡大气CO2具有重要作用[19]。当前对竹林进行经营(林地除草、垦复、施肥、覆盖和灌溉等)是中国竹产区竹林培育的普遍作业方式[20]。施肥在显著提高毛竹林生产力的同时,会消耗土壤更多的有机质[17−18],造成土壤碳的流失。
毛竹林生态系统为蚯蚓提供适宜的生境,毛竹林下凋落物可供蚯蚓取食;蚯蚓活动可以改善土壤性质,其粪便还能增加土壤肥力。然而蚯蚓活动如何影响毛竹林土壤CO2排放和活性有机碳仍不明晰。本研究以毛竹林土壤为研究对象,通过温室盆栽设置竹林生态微系统培养试验,研究蚯蚓活动对毛竹土壤中CO2排放及活性有机碳的影响。
-
供试土壤及凋落物均于2020年10月采自浙江省湖州市安吉县灵峰寺毛竹林基地。灵峰林场地处30°29′N,119°38′E,海拔为 150~200 m,竹林面积为108 hm2,其中毛竹面积为 86 hm2,毛竹立竹量为1.7亿株[21],过去10余年收获的生物产量平均为33.33 t·hm−2·a−1 [22]。土壤为种植多年的毛竹林土壤。采集0~20 cm层次土样,剔除石块、大中型土壤动物及根茎等残体后过2 mm筛。选取毛竹林内3种典型植物,分别为毛竹、金星蕨Parathelypteris glanduliger和紫金牛Ardisia japonica,按照毛竹林内面积占比(4.5∶2.0∶1.0)采摘。清洗后杀青并烘干,用多功能粉碎机(JP-400C)磨碎,过2 mm筛,混入0~20 cm土壤中,作为试验用凋落物。根据毛竹林土壤实际勘测,凋落物添加量为土壤质量的1%。供试土壤pH为5.43,含水量为20%,土壤有机碳质量分数为1.35 g·kg−1,可溶性有机碳为13.91 mg·kg−1,微生物生物量碳为42.31 mg·kg−1,全氮为1.03 g·kg−1。
毛竹苗购于某生态竹种农场,生物量为10~15 g·株−1。蚯蚓选用具有生殖环带的成熟威廉环毛蚓Pheretima tschiliensis,体质量为1.5~2.0 g·条−1。为减少试验误差,需在接种前1周将蚯蚓置于试验土壤中预培养,以排除肠道内容物。
-
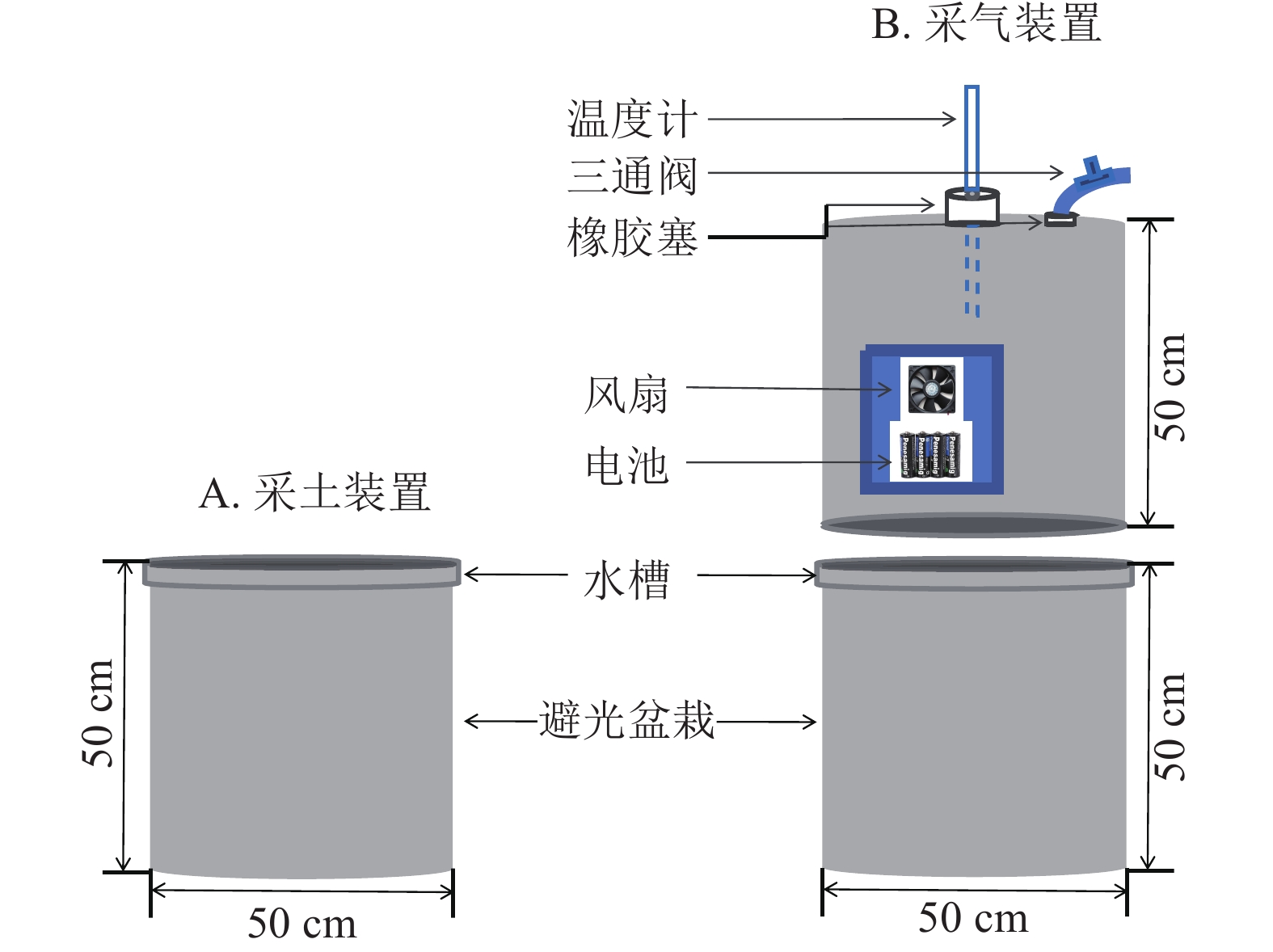
在盆栽装置中置入30 kg供试土壤,试验周期为85 d,共设3个处理:①盆栽内仅种植毛竹作为空白对照(ck);②毛竹盆栽内添加凋落物(MBL);③毛竹盆栽内同时添加凋落物及蚯蚓(MBLE)。每个处理4个重复,并做完全随机区组设计。为避免盆栽土壤采样后因容积改变对气体排量产生影响,同时设置采土(图1A)和采气(图1B) 2组。
选取竹径、竹高均相近的毛竹苗,每个盆内栽种1株。待长势稳定后,试验正式开始前1 d将9.6 kg试验用凋落物均匀混入MBL和MBLE盆栽土壤中(0~20 cm),选取大小均匀,体型相近的蚯蚓放入MBLE盆栽中,每盆5条。为防止蚯蚓逃逸,避免造成实验误差,每个盆内均装置尼龙网,并保证光照条件,满足植物正常所需。试验开始后第1、3、5、7、9、16、23、30、51、65、78、85天采集气体,共12次;第17、34、51、68、85天采集土样,共5次。
采土组中ck和MBL处理为随机取样,每个处理4盆,共设8盆;为避免采土过程中误伤蚯蚓,同时防止蚯蚓活动混匀原土壤和采土后补充的土壤,采取破坏性取样,MBLE处理共设20个盆栽装置(4个重复×5次破坏性取样),养殖100条蚯蚓。每个土样采集点都尽可能分布在毛竹周围,利用土钻取出盆内0~20 cm的土壤,每次土壤采集500 g。为避免采土后的缺口影响竹子后续正常生长,需要用毛竹土填满采样洞,并用聚氯乙烯小棒立于采样点表面做标记,避免同一位置重复采样。采集后的新鲜土样过2 mm筛后混合均匀并风干保存,用于测定其他基本理化指标。
为避免采土后原土壤减少和新加入的土壤造成气体排放不一致,故单独设计采气组。利用静态箱法收集CO2,静态箱材质为聚氯乙烯,规格为直径50 cm,高50 cm。外层贴铝箔纸,保温防晒;其内部设有风扇,用于混匀气体。盆栽装置的边缘设计凹槽,采气时,将静态箱嵌入凹槽内,而后向该槽中加入适量水,以确保形成1个密闭的空间。3组处理各设4个盆栽,共计12个盆栽和12个静态箱。每次采气时间为7:00—11:00,每个盆栽采集4个气样。采气具体操作为:用带有三通阀的医用注射器抽取20 mL气体打入真空瓶中,分别于封箱后0、10、20、30 min采集气体。采集后的气体瓶带回实验室用气相色谱仪(7890B)进行分析。每次采集气体时,用土壤温湿度仪测定土壤10 cm处的土壤体积含水量和土壤温度[23]。
-
土壤理化性质的测定参考《土壤农业化学分析方法》[24]。土壤pH采用pH计测定(水土质量比为2.5∶1.0);土壤有机质采用高温外加热重铬酸钾氧化-容量法测定,并用相关系数换算成土壤有机碳(SOC)质量分数;可溶性有机碳(DOC)使用总有机碳分析仪(Multi N/C 3100)测定(水土质量比为5∶1)。
土壤颗粒态有机碳(POC)参照CAMBARDELLA等[25]的测定方法:取风干土样20 g (过2 mm筛)和5 g·L−1的六偏磷酸钠[(NaPO3)6]溶液100 mL放入250 mL三角瓶中,90 r·min−1震荡18 h,完成后将混合液过53 μm筛冲洗至滤液澄清后,将筛上的土壤置于烧杯中,放至60 ℃烘箱中烘干12 h,得到颗粒土并进行称量。而后将颗粒土研磨过250 μm筛,利用重铬酸钾-浓硫酸外加热法(油浴)测定颗粒土中的有机碳质量分数。土壤颗粒态有机碳质量分数=颗粒土中有机碳质量分数×颗粒土比例,土壤矿物结合态有机碳(MAOC)质量分数=土壤有机碳质量分数−颗粒态有机碳质量分数。
土壤微生物生物量碳(MBC)使用氯仿-熏蒸浸提法[26−28]测定:土样做熏蒸和未熏蒸2种处理,用0.5 mol·L−1硫酸钾分别浸提后,使用总有机碳分析仪测定滤液中的碳质量分数,用熏蒸和未熏蒸样品间差值除以相应系数,即获得微生物生物量碳质量分数。
-
采用Excel 2016 处理数据,SPSS 18.0 对数据结果进行统计分析。使用ANONA单因素方差分析(Duncan检验法比较多个处理均值间差异,P<0.05),采用Spearman相关系数法分析数据的相关性(P<0.05)。利用Origin 8.0进行绘图。标准误没有特殊说明,则代表4个重复(n=4)的测量标准误。
-
试验结束时(第85天),采土组中剩余毛竹苗27株(共28株),存活率约为96.4%;剩余蚯蚓75条(共100条),存活率约为75.0%。MBL处理的毛竹生物量高于ck处理 18.1%;MBLE处理的毛竹生物量显著高于MBL处理20.3% (P<0.05),显著高于ck处理34.8% (P<0.05)。
MBLE处理毛竹生物量整体呈上升趋势(表1)。第17~34天毛竹生物量极显著增加34.3% (P<0.01),第51~68天毛竹生物量显著增加21.8% (P<0.05),第85天毛竹生物量比第17天增长了51.9%。
物种 处理 不同取样时间毛竹和蚯蚓生物量/g 0 17 34 51 68 85 d 毛竹 ck 11.17±1.05 Ab − − − − 24.58±1.16 Ba MBL 13.36±0.77 Ab − − − − 30.02±1.61 Ba MBLE 14.71±1.13 Ac 18.14±1.74 c 27.60±1.38 b 30.99±1.06 b 39.61±0.89 a 37.68±0.33 Aa 蚯蚓 MBLE 1.75±0.05 c 2.15±0.06 c 3.54±0.05 b 3.40±0.13 b 3.58±0.07 b 4.70±0.15 a 说明:ck. 盆栽内仅种植毛竹;MBL. 毛竹盆栽内添加凋落物;MBLE. 毛竹盆栽内同时添加凋落物和蚯蚓。−代表无数据。不同大写字母为同时间内不同处理间差异显著(P<0.05),不同小写字母为同处理不同时间间差异显著(P<0.05)。 Table 1. Biomass of Phyllostachys eduli and earthworm in different treatments
蚯蚓生物量同样整体呈上升趋势,第17~34天蚯蚓生物量极显著增加39.3% (P<0.01);第34~68天无显著差异,可能是期间有蚯蚓死亡,造成生物量增长幅度的降低;第68~85天蚯蚓生物量极显著增加23.8% (P<0.01),其生物量比试验开始时增长了62.8%。
-
不同处理的土壤CO2排放通量均有2次峰值(表2),ck分别在第16天和第51天,MBL处理分别在第7天和第16天,MBLE处理分别在第5天和第51天。第85天,MBL处理的CO2排放量最高,为34.75 mg·kg−1·h−1,比MBLE处理高25.2%,比ck高31.6%;MBLE处理的CO2排放量仅比ck高8.6%。
采样日期
(年-月-日)取样时间/d CO2排放通量/(mg·kg−1·h−1) CO2分段累积排放量/(mg·kg−1) ck MBL MBLE ck MBL MBLE 2021-01-05 1 18.68±1.97 Abcde 19.49±1.42 Afg 19.85±0.70 Ac 4.48±0.47 Ag 4.68±0.34 Aj 4.76±0.17 Ai 2021-01-07 3 12.73±1.91 Adef 23.89±4.36 Adefg 23.76±1.97 Abc 11.31±0.70 Bg 15.62±1.36 Aij 15.70±0.58 Ahi 2021-01-09 5 14.85±0.72 Bdef 17.22±2.08 Bg 54.13±4.58 Aa 18.18±0.83 Bg 24.68±1.31 Bij 38.04±2.40 Ahi 2021-01-11 7 10.09±1.47 Cf 69.60±2.10 Aa 30.64±3.01 Bbc 23.60±0.56 Bg 51.80±0.69 Ahi 55.56±2.66 Aghi 2021-01-13 9 15.50±1.09 Bdef 33.18±3.67 Abcdef 23.53±1.61 Bbc 30.39±0.37 Bf 72.10±0.68 Ah 67.71±2.79 Afg 2021-01-20 16 24.39±2.52 Bab 44.47±1.96 Ab 30.26±2.36 Bbc 64.97±2.38 Cf 138.68±4.36 Ag 113.70±3.80 Befg 2021-01-21 17 10.24±1.07 Bf 31.58±2.69 Abcdef 29.37±2.78 Abc 71.58±2.31 Cf 155.39±4.33 Afg 127.91±3.06 Bef 2021-01-27 23 11.70±1.41 Bef 20.94±2.23 ABefg 25.61±3.92 Abc 84.92±2.00 Cef 185.62±5.95 Aef 160.44±1.52 Bde 2021-02-03 30 16.54±2.02 Bbcdef 23.22±1.14 ABdefg 29.82±2.56 Abc 109.22±4.08 Be 222.99±7.23 Ae 207.51±4.35 Ad 2021-02-24 51 30.97±2.13 Ba 34.53±1.91 Bbcde 50.15±2.21 Aa 230.68±8.02 Cd 369.87±12.13 Bd 411.47±14.10 Ac 2021-03-10 65 19.70±0.56 Bbcd 25.63±2.35 ABcdefg 30.92±2.30 Abc 314.46±9.13 Cbc 469.87±17.16 Bc 545.36±19.28 Ab 2021-03-13 68 24.07±1.24 Bab 36.61±3.67 Abcd 34.93±1.97 Ab 335.99±8.80 Cb 501.06±16.22 Bc 577.45±20.33 Ab 2021-03-23 78 15.94±1.18 Bcdef 38.48±4.00 Abc 22.82±2.55 Bbc 383.02±8.38 Ca 591.40±11.34 Bb 645.29±22.10 Aa 2021-03-30 85 23.76±1.38 Babc 34.75±2.47 Abcd 25.99±0.85 Bbc 422.07±9.52 Ba 661.25±9.51 Aa 692.53±22.64 Aa 说明:不同大写字母为同时间内不同处理间差异显著(P<0.05),不同小写字母为同处理不同时间间差异显著(P<0.05)。 Table 2. Soil CO2 fluxes and phased cumulative CO2 emissions from different treatments
3组处理的土壤CO2分段累积排放量走势基本一致。第1~9天,3组处理间无显著性差异;第16~23天,MBLE处理的CO2累积排放量显著低于MBL处理(P<0.05);第51~78天,MBLE处理的CO2累积排放量高于MBL处理;第85天,凋落物的添加极显著提高了36.2%的土壤CO2排放量(P<0.01),凋落物及蚯蚓的添加极显著提升39.1%的土壤CO2排放量(P<0.01),其中蚯蚓的作用只增加了4.5%的土壤CO2排放量。
-
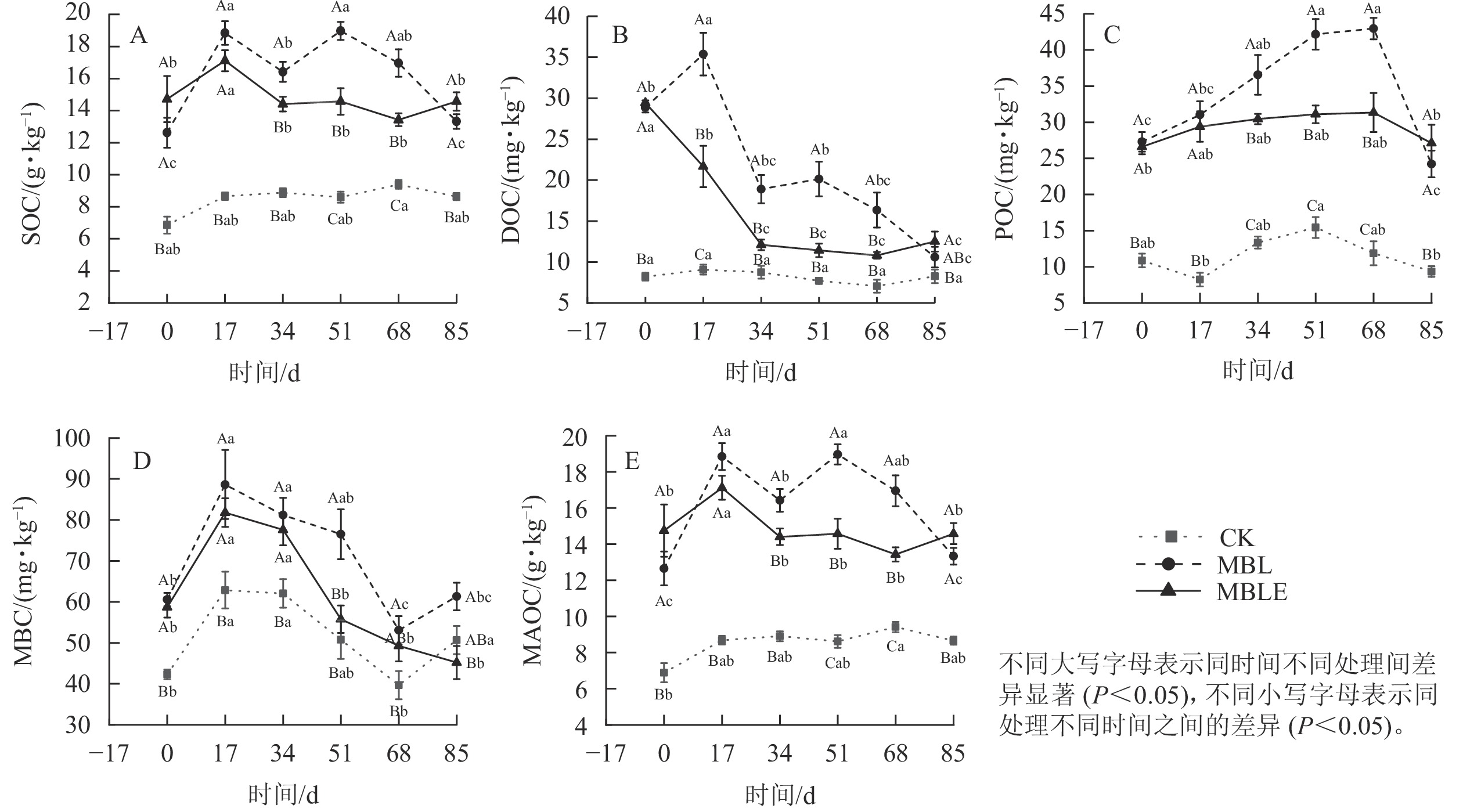
MBL处理的土壤有机碳质量分数在试验期间均显著高于ck处理(P<0.05, 图2A),并从第51天起持续降低,于第85天降至最低(13.36 g·kg−1),极显著高于ck处理35.1% (P<0.01)。MBLE处理则在第17天开始显著降低(P<0.05),至第34天后无显著变化。第85天,MBLE处理的有机碳质量分数仅高于MBL处理8.5%,无显著差异,但极显著高于ck处理40.6% (P<0.01)。
3组处理的土壤可溶性有机碳质量分数均在第17天达到峰值(图2B),MBL和MBLE处理均在第34天极显著降低(P<0.01)。MBL处理在第85天降到最低(10.60 mg·kg−1),高于ck处理21.5%。在试验结束时,MBLE处理的可溶性有机碳质量分数显著高于MBL处理15.2% (P<0.05),极显著高于ck处理33.9% (P<0.01)。
3组处理的土壤颗粒态有机碳质量分数变化趋势基本一致,均呈先升高后降低的趋势(图2C)。其中ck于第51天达到峰值(15.44 mg·kg−1);MBL和MBLE处理均于第68天达到峰值,而后MBL处理在第85天极显著降至最低,为24.23 mg·kg−1 (P<0.01)。试验结束时,MBLE处理的颗粒态有机碳质量分数仅高于MBL处理10.6%,无显著差异,MBLE处理极显著高于ck处理65.5% (P<0.01);MBL处理极显著高于ck处理61.4% (P<0.01)。
3组处理的土壤微生物生物量碳质量分数均于第17天达到最高(图2D)。ck和MBL处理变化趋势相同,均呈先降低后升高趋势,于第68天降至最低;MBLE处理的微生物生物量碳质量分数一直呈下降趋势,于第85天降至最低,显著低于ck处理24.4% (P<0.05),显著低于MBL处理26.3% (P<0.05)。
3组处理的土壤矿物结合态有机碳质量分数走势与土壤有机碳一致(图2E),其原因为土壤颗粒态有机碳质量分数相对土壤中有机碳极低,故土壤矿物结合态有机碳质量分数与土壤有机碳基本无差异。试验结束时,MBLE处理的土壤矿物结合态有机碳质量分数较MBL处理高8.5%,无显著差异。
-
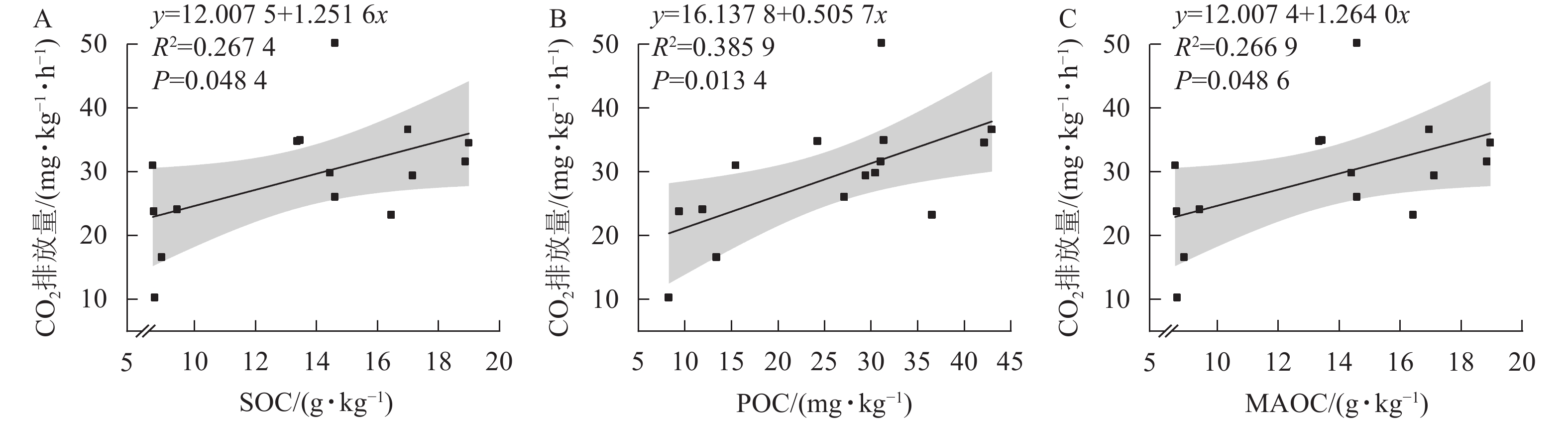
由土壤CO2排放量与土壤活性有机碳的相关性分析可知(图3),CO2排放量与土壤有机碳、颗粒态有机碳和矿物结合态有机碳呈显著相关(P<0.05)。
-
本研究发现:蚯蚓加入土壤第5天即可显著提高土壤CO2排放量。可能原因是蚯蚓呼吸产生CO2的同时,其携带的大量微生物进入土壤进行矿化作用,且土壤包含大量微生物和充足凋落物,蚯蚓可以通过刺激土壤微生物活性,加速土壤矿化[31−33],从而显著提高土壤CO2排放量和累积排放量。凋落物的单独添加同样加速土壤碳矿化(第7天),其原因可能为凋落物中的有机碳引起土壤微生物的正向激发效应,从而提升了土壤中碳的周转速率,加快土壤有机碳的矿化以及气体的释放[34]。MBL与MBLE处理的CO2排放通量几乎同时达到第1次峰值,且两者的土壤CO2累积排放量在第7~9天时基本一致。可能原因是本试验采用过2 mm筛的凋落物粉末,且与土壤混合均匀,有效提高了微生物与凋落物的接触表面积;而在自然界中,蚯蚓的破碎作用需要消耗大量时间才能减小凋落物尺寸,达到同样效果。
MBL处理的CO2排放通量于第16天达到第2次峰值,ck达到第1个峰值,此时MBL处理的CO2累积排放量开始显著高于MBLE处理,极显著高于ck。主要原因可能是蚯蚓在更新土壤微生物群落的同时还会取食微生物[35],造成了土壤中原微生物生物量的减少;且蚯蚓偏好取食土壤中更易利用的有机物颗粒[36],即蚯蚓的添加造成了土壤颗粒态有机碳质量分数的降低。与此同时,土壤CO2的排放与颗粒态有机碳质量分数呈显著正相关,这也是第85天MBLE处理的CO2总累积排放量显著低于MBL处理的原因之一。
MBLE处理与ck的CO2排放通量同时在第51天达到第2次峰值,此时MBLE处理CO2累积排放量反超MBL处理,并呈显著差异。在第17~68天,MBLE处理的土壤有机碳、可溶性有机碳、颗粒态有机碳、微生物生物量碳及矿物结合态有机碳质量分数均低于MBL处理,与此同时,MBLE处理的CO2排放通量均高于MBL处理,即蚯蚓活动在试验中期减少了土壤碳固定,增加了土壤碳矿化,但此期间MBL的CO2累积排放量高于MBLE处理。添加蚯蚓导致CO2排放通量增加与BORKEN等[3]研究结果相同,Lumbricina terrestris在为期120 d的野外试验初期显著促进了土壤CO2排放。WESSELLS等[37]在农业生态系统的研究中同样发现:蚯蚓对土壤呼吸的影响并非始终统一,但添加蚯蚓的样地的土壤呼吸比未添加或蚯蚓数量少的样地的土壤呼吸要高。通常认为:蚯蚓促进土壤CO2排放增加的主要原因是增强了微生物活性,但本研究中蚯蚓造成土壤微生物生物量碳质量分数降低,故蚯蚓造成土壤CO2排放通量增加的可能原因有:①蚯蚓呼吸直接产生CO2;②蚯蚓对微生物造成捕食压力,促进微生物对碳的利用效率[38−39];③蚯蚓活动消耗有机质促使碳矿化。第16~30天MBLE处理的CO2累积排放量比MBL处理低,可能原因是毛竹大幅生长利用碳,以及蚯蚓同化自身含碳物质,即蚯蚓的添加显著提高毛竹生物量。
试验末期(第68天后),MBL处理的土壤CO2排放通量逐渐趋于稳定,而MBLE处理的土壤CO2排放通量显著降低。第85天时,MBL处理的土壤CO2排放通量显著高于MBLE处理,两者的CO2累积排放量无显著差异。可能原因除了上述提到的蚯蚓取食造成颗粒态有机碳降低从而使土壤CO2排放减少外,还可能因为蚯蚓不停地取食凋落物及微生物,并通过其生理活动改变微生物生境,导致土壤微生物生物量碳的持续降低,使试验中期尚可通过增加对碳的利用效率的微生物无法保持土壤CO2的高排放量。随着时间推移,蚯蚓排泄出的粪便也逐渐积累,经过微生物的作用转化为有机质,并随其运动完成空间再分配,混合有机物及矿物土,促进了土壤的碳存储,故试验后期MBLE处理的土壤CO2排放量降低。
-
本研究证实了添加凋落物及同时添加凋落物和蚯蚓均可以显著提高土壤活性有机碳质量分数。凋落物作为外源有机碳引入土壤,促进了凋落物有机碳向土壤有机碳的转化与更新,从而有效提高土壤有机碳活性[40]。本研究同时证实了蚯蚓活动对土壤碳的影响随时间变化而变化。
本研究表明:添加蚯蚓后的第1个月土壤有机碳、可溶性有机碳和矿物结合态有机碳减少,第3个月则开始增加。前期结果与HALE等[31]的研究结果类似,该研究表明:蚯蚓活动显著降低了森林地表物厚度和枯枝落叶层土壤中碳质量分数,加快了森林地表物的周转;EDWARDS等[32]的研究也表明:Allolobophora caliginosa和Octolasion cyaneum每年能消耗0~15 cm土层中4%~10%的土壤和10%的有机质。可能原因是蚯蚓吞食土壤颗粒的同时也会取食凋落物用于同化自身的含碳物质,其生命活动需要消耗大量有机质,导致环境中的土壤有机碳质量分数降低[13]。随着时间推移,蚓粪在微生物的作用下转化为土壤有机质,并通过蚯蚓运动完成蚓粪的空间再分配[33],使有机质与矿质土混合,逐渐形成富含有机质的表层土壤,为有机质提供物理保护。与此同时,蚯蚓运动轨迹可以促进土壤孔隙度、通气性和入渗率的提高[41],分泌的黏液及其他代谢产物又是活性高、易被微生物降解的有机质[42],进而减缓有机质的周转, 提高土壤潜在固碳能力,改变土壤理化结构(如透气性、pH等)。因此,土壤有机碳、可溶性有机碳和矿物结合态有机碳质量分数在试验第2个月时下降趋势渐缓,直至第3个月开始上升。MBLE处理的前2个月内土壤颗粒态有机碳均显著低于未添加蚯蚓的MBL处理,可能原因是蚯蚓更易取食富含有机颗粒的土壤,这与KOUTIKA等[36]的研究结论相符,即蚯蚓的取食更容易利用土壤中的有机物颗粒。
目前,关于蚯蚓活动对微生物生物量碳的变化及微生物活性的影响没有相对统一的结论。本研究表明: MBLE处理的土壤微生物生物量碳质量分数在3个月的试验期内一直持续降低,且从试验中期起显著低于未添加蚯蚓的MBL处理。这一结果与BOHLEN等[43]、FROUZ等[44]和张宝贵等[45]的研究结果类似,即蚯蚓的生理活动降低了土壤微生物生物量碳。在北美的森林中,欧洲蚯蚓的入侵会致使土壤总的微生物生物量锐减约42%[46];MCLEAN等[47]的研究也同样显示:蚯蚓的存在使得微生物生物量碳减少。造成本研究中MBLE处理土壤微生物生物量碳质量分数锐减的可能原因为:一是微生物作为蚯蚓的第二食物来源,蚯蚓肠道消化导致土壤微生物总量的降低;二是蚯蚓与微生物竞争资源导致其数量降低;三是蚯蚓的一系列生理活动对土壤理化性质产生影响,从而改变微生物所处的微环境,直接或间接地影响土壤微生物生存。
-
蚯蚓活动显著提高了毛竹生物量,其活动对土壤CO2排放及活性有机碳的影响均具有一定的时效性。试验前期(<51 d),蚯蚓活动加快土壤碳矿化,显著提高土壤CO2的排放通量,降低土壤活性有机碳质量分数;试验后期(>51 d),蚯蚓活动逐渐减少碳矿化,土壤CO2的排放通量显著降低,提高土壤活性有机碳质量分数。在为期85 d的试验中,蚯蚓活动未显著改变土壤CO2累积排放量,但显著降低了土壤微生物生物量。本研究加强了蚯蚓活动影响毛竹林土壤温室气体排放和土壤活性碳的认知,为蚯蚓活动应对全球气候变化及影响毛竹林土壤活性有机碳变化过程中的潜在贡献提供基础数据。但由于温室盆栽试验空间受限,添加的凋落物量仅够蚯蚓取食3个月,故整体试验周期偏短,得出的结论只是栽植盆内蚯蚓对土壤CO2排放及活性有机碳的阶段效应,对于真实毛竹林环境中可能产生的更长期效应的推断存在一定的局限性。未来可开展野外长期试验,以揭示盆栽和野外试验结果的异同和内在机制。
Responses of CO2 emissions and labile organic carbon to earthworm activities in Phyllostachys edulis forest soil
doi: 10.11833/j.issn.2095-0756.20230369
- Received Date: 2023-06-20
- Accepted Date: 2024-01-20
- Rev Recd Date: 2023-12-12
- Available Online: 2024-05-22
- Publish Date: 2024-05-22
-
Key words:
- earthworm activity /
- labile organic carbon /
- Phyllostachys edulis /
- soil CO2 emission
Abstract:
| Citation: | TIAN Xiaoqingfan, XIAO Xiangqian, QIU Yufeng, et al. Responses of CO2 emissions and labile organic carbon to earthworm activities in Phyllostachys edulis forest soil[J]. Journal of Zhejiang A&F University, 2024, 41(3): 486-495. DOI: 10.11833/j.issn.2095-0756.20230369 |











 DownLoad:
DownLoad: