-
园艺植物是指提供人类食用或观赏的植物,包括果树、蔬菜、观赏植物等,具有较高的经济价值和美化用途[1]。在现代社会中园艺植物产品已成为人们生活中不可缺少的部分,且市场需求在逐年增加。目前,园艺植物生产面临育种周期长、选择范围有限等问题,已经不能满足日益增长变化的市场需求[2]。研究园艺植物生长发育的内在机制对解决上述问题至关重要,并可有效提高其产量和质量。园艺植物通过光合作用产生的碳水化合物,需经过复杂的方式运输到库器官(如根、茎、嫩叶、果实),具体包含有机同化物在源端韧皮部的装载、经韧皮部长距离运输、库器官韧皮部的卸出、韧皮部后运输等一系列过程[3−4]。其中,韧皮部卸载对光合同化物在器官之间的运输和分配有着重要作用,是决定园艺植物产量和生产力的重要因素[5]。韧皮部卸载指在韧皮部运输的同化物从筛分子伴胞复合体(SE-CC)卸出的筛分子卸载和韧皮部短距离后运输2个密切相关的过程,是目前植物研究的热点领域之一。简言之,韧皮部卸载即光合同化物从维管束韧皮部转移到库细胞以促进植物生长发育和能量储存的过程[6−7]。
韧皮部运输的主要糖成分是研究韧皮部卸载的重要基础,植物体内糖的转运不仅对植物的生理活动如光合作用和碳分配等有直接影响,还影响植物的营养发育和花芽分化等过程[8]。本研究从韧皮部运输的主要糖分形式、韧皮部卸载方式、韧皮部卸载的研究方法及对园艺植物的影响等4个方面对园艺植物韧皮部卸载研究进行评述,旨在为后续研究提供参考和借鉴。
-
在研究同化物卸载途径前,首先应该清楚韧皮部运输同化物的主要形式。还原糖类在运输过程中极易被氧化,因此,能进行韧皮部长距离运输的糖类为非还原糖或糖醇。大多数高等植物以蔗糖作为光合产物的主要运输形式[9],但自然界也存在以其他形式的糖作为光合产物主要运输形式的植物,如约5%的植物以棉子糖系列寡糖或者山梨醇为主,园艺植物中如黄瓜Cucumis sativus、西瓜Citrullus lanatus等葫芦科Cucurbitaceae植物以棉子糖为主[10−11],蔷薇科Rosaceae以山梨醇为主[12]。需注意区分同化物的储藏形式和运输形式,如西瓜尽管以棉子糖系列寡糖为主要运输糖分,但果实储存糖分则是以蔗糖为主[11]。
-
虽然韧皮部卸载包括筛分子卸载和韧皮部后运输2个主要过程[6],但是不同的园艺植物在不同的发育时期以及不同的组织器官中,韧皮部卸载的方式也存在很大差别[13]。韧皮部卸载方式主要包括共质体途径、质外体途径或两者交替途径。其中共质体途径又可称为胞间转运,而质外体途径又可称为质膜转运[7]。
-
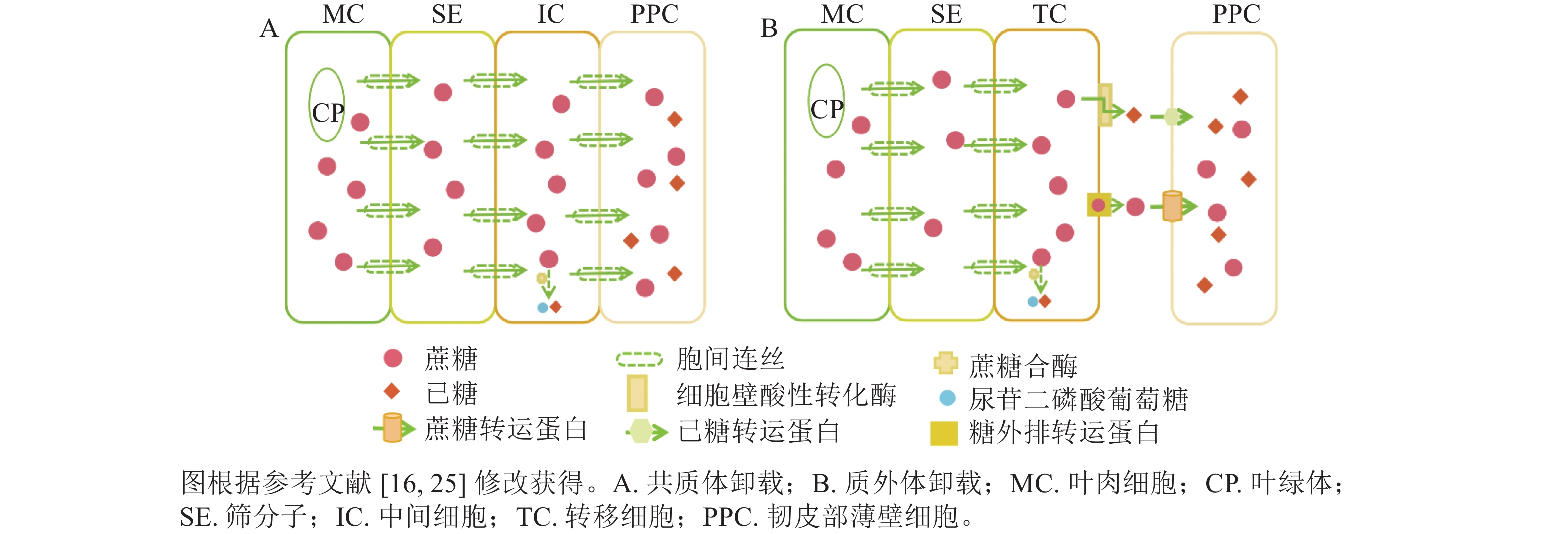
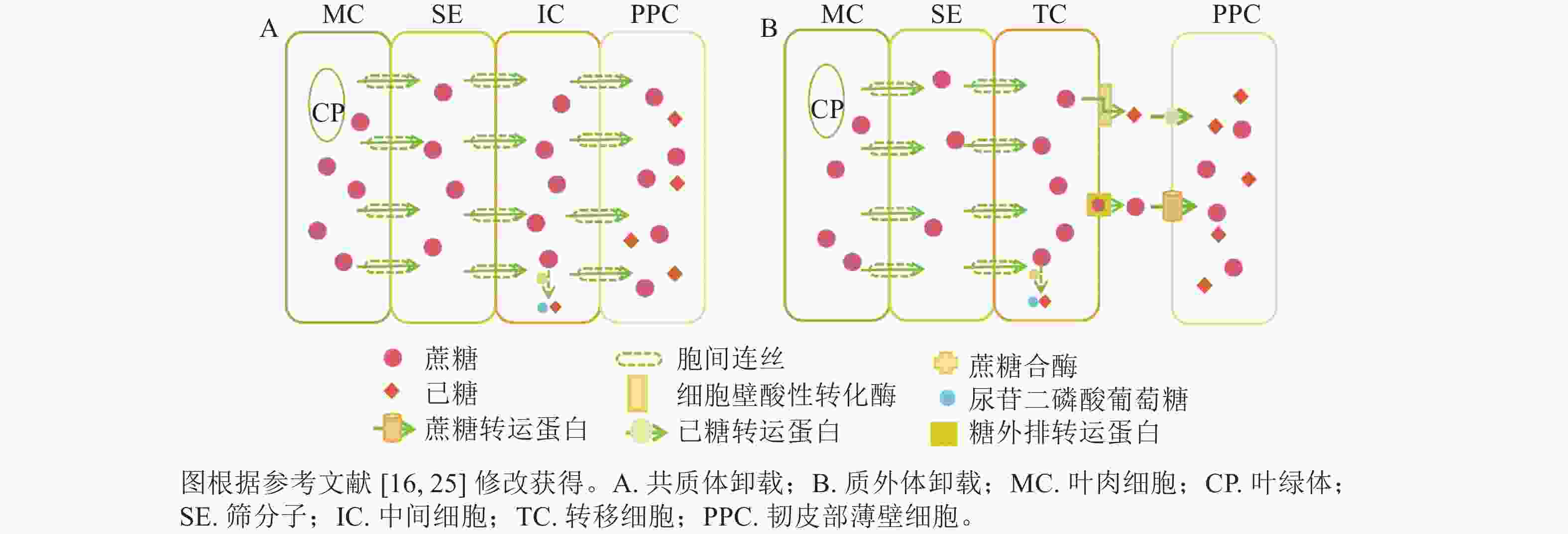
共质体卸载途径指光合同化物通过胞间连丝从筛分子伴胞复合体中将同化物运输到周围韧皮部薄壁细胞,并进一步运送到库器官的过程[14−16]。共质体卸载途径主要受胞间连丝与中间细胞影响,属于顺浓度梯度的被动运输过程。近期研究表明:胞间连丝的种类(如漏斗型)、分叉情况、是否处于闭合态等均会影响胞间连丝的功效,即意味着有时即便存在胞间连丝,但若胞间连丝是闭合态,也无法采用共质体运输[17−19]。在硬骨凌霄Tecoma capensis的研究中发现:中间细胞与周围韧皮部薄壁细胞存在大量的胞间连丝,这进一步证实了中间细胞是共质体卸载的又一重要形态标志[16, 20]。此外,糖类代谢酶在韧皮部卸载过程中有显著作用,如蔗糖合酶(SuSy)与共质体卸载途径密切相关(图1A),可见对关键代谢酶的研究尤为重要,是证明共质体卸载方式的重要证据。
-
质外体卸载途径是指光合同化物从筛分子伴胞复合体中跨膜进入质外体空间,再经过机体代谢和(或)跨膜蛋白转运,被周围韧皮部薄壁细胞吸收并运输到库器官的过程[14]。因此,质外体卸载途径与共质体卸载途径的主要区别:一是质外体卸载不通过胞间连丝,二是质外体卸载需借助各类糖转运蛋白逆浓度梯度的主动运输过程[16]。以质外体卸载为主的研究中,转移细胞是判断质外体卸载途径的主要形态标志[16, 21]。在拟南芥Arabidopsis thaliana韧皮部薄壁转移细胞的功能研究中发现,蔗糖通过影响韧皮部薄壁转移细胞中蔗糖输出活性来调节细胞壁向内生长[22],这与先前关于增加的质膜表面积从而提高物质跨膜运输效率的假设是一致的[15]。在代谢酶方面,细胞壁酸性转化酶是调控韧皮部质外体卸载的主要酶,可在胞外空间分解蔗糖(图1B),细胞壁酸性转化酶活性及转录本的表达变化常与共质体向质外体转化时间变化相一致[23−24]。
-
用于研究同化物卸载途径的传统方法主要有空中皮技术、新浆果杯法和组织圆片技术等[14−15]。植物体内物质运输细胞学路径的方法也得到较大程度的革新,目前植物组织及细胞学路径研究的主要方法包括透射电子显微镜技术、荧光染料示踪法、绿色荧光蛋白示踪法、胶体金免疫定位技术等。
-
韧皮部及其周围薄壁细胞的超微结构观察可为同化物韧皮部卸载提供细胞学证据。如以‘富有’甜柿Diospyros kaki ‘Fuyu’果实为研究对象,发现韧皮部伴胞与维管薄壁细胞上均分布一定数量的胞间连丝,说明韧皮部卸载路径为共质体路径[26]。此外,有研究表明栽培枣Ziziphus jujuba和野生酸枣Z. jujuba var. spinosa在果实成熟阶段胞间连丝密度以及可溶性糖含量差别较大,前者存在大量胞间连丝,加速了以蔗糖为代表的可溶性糖的显著积累,后者胞间连丝很少且可溶性糖积累不明显[27−28]。这表明超微结构可用于揭示韧皮部卸载强度,是完成卸载的结构基础。
-
目前最常用的共质体标记物为羧基荧光素 (carboxyfluorescein, CF),CF可长距离运输,属“膜不透性”探针[10, 29−30],但会受到质体外微环境pH和液泡区隔化的限制[31]。与CF不同的是,荧光黄染料 (lucifer yellow CH, LYCH)不受pH影响,在生理pH值下有较高的解离度,故不易透膜,因此,同样可以作为共质体标记物[32]。目前CF广泛应用于园艺植物的根、茎、叶、果实[11, 33−40]中,用以判断卸载路径的变化。由表1可见:在大部分已研究的园艺植物中都用该方法来研究韧皮部卸载路径。前期研究也采用CF表明:东方百合‘索邦’Lilium ‘Sorbonne’[24]和石蒜Lycoris radiata[41]的鳞茎形成后期以共质体运输为主。
分类 种名 研究内容 研究方法 卸载方式 参考文献 果树 ‘富有’甜柿Diospyros kaki ‘Fuyu’ 果实发育 半薄切片法 共质体 [26] 扁桃Prunus dulcis 种皮发育 半薄切片法 共质体 [52] 果实发育 质外体 [53] 核桃Juglans regia 种皮发育 半薄切片法、胶体金免疫
定位技术、CFDA示踪共质体 [32, 47] 果皮发育 质外体 桃Amygdalus persica 果实发育 半薄切片法 质外体 [55] 蓝莓Vaccinium uliginosum 果实发育 半薄切片法、CFDA示踪 质外体 [46] 苹果Malus domestica 果实发育 胶体免疫金定位技术、CFDA示踪 质外体 [39, 54] 草莓Fragaria ananassa 果实发育 CFDA示踪 质外体 [37] 鸭梨Pyru bretschneideri 果实发育 CFDA示踪 质外体 [38] 猕猴桃Actinidia chinensis 果实发育 半薄切片法、CFDA示踪 质外体 [36] 荔枝Litchi chinensis 果皮发育 CFDA示踪 质外体 [40] 葡萄Vitis vinifera 果实发育 绿色荧光蛋白、CFDA示踪 共质体-质外体 [44] 无花果Ficus carica 果实发育 CFDA示踪 共质体-质外体 [56] 文冠果Xanhoceras sorbifolium 果实发育 半薄切片法、CFDA示踪 共质体-质外体-共质体 [61] 蔓越橘Vaccinium macrocarponl 果实发育 半薄切片法、CFDA示踪 共质体-质外体-质外体 [60] 枣Ziziphus jujuba 果实发育 胶体免疫金定位技术、CFDA示踪 共质体-质外体-质外体 [57−59] 质外体-共质体-质外体 [28, 30, 49] 蔬菜 豌豆Pisum sativum 茎发育 14CO2标记 共质体 [63] 蚕豆Vicia faba 茎发育 14CO2标记 共质体 [64] 黄瓜Cucumis sativus 果实发育 绿色荧光蛋白、CFDA示踪 质外体 [10, 65] 西瓜Citrullus lanatus 果实发育 CFDA示踪 质外体 [11] 甜菜Beta vulgaris 叶片发育 14CO2标记 共质体 [67−69] 根发育 共质体-质外体 番茄Solanum lycopersicum 果皮发育 CFDA示踪 共质体-质外体 [66] 观赏植物 黄梁木Neolamarckia cadamba 叶柄发育 CFDA示踪 共质体 [35] 云南箭竹Fargesia yunnanensis 地上茎发育 CFDA示踪 共质体 [70−71] 硬骨凌霄Tecoma capensis 叶脉 半薄切片法 共质体 [20] 南林-95杨Populus × euramericana ‘Nanlin95’ 叶发育 CFDA示踪 共质体 [33] 茎发育 共质体 根发育 质外体 毛地黄Digitalis purpurea 蜜腺 半薄切片法 质外体 [72] 龟背竹Monstera deliciosa 气根发育 14CO2标记 质外体 [73] 牡丹Paeonia suffruticosa 叶柄发育 半薄切片法、CFDA示踪 生长期:共质体为主,质外体为辅 [34] 茎发育 休眠期:共质体 根发育 慈竹Bambusa emeiensis 地上茎发育 CFDA示踪 共质体-质外体 [35] 油茶Camellia oleifera 果实发育 CFDA示踪 共质体-质外体-共质体 [74−75] Table 1. Summary of studies on phloem unloading of horticultural plants
绿色荧光蛋白是直观性极强的遗传标记物,属共质体探针,与CF相比,可获得更准确的示踪结果[15];该方法在拟南芥[42]、木薯Manihot esculenta[43]卸载路径鉴定上得到了很好的应用,但在园艺植物中应用较少,仅在葡萄Vitis vinifera上有报道[44]。
-
Esculin可被蔗糖转运蛋白(SUT)及专一运输蔗糖的SWEET等运输,可指示是否为韧皮部质外体卸载途径[11, 45]。此PTS (trisodium, 3-hydroxy-5,8,10-pyreno trisulfonate)和SRG (sulphorhoda-mine G)等荧光染料只限制在质外体中,不能被细胞壁偶联,也是较为理想的质外体标记物[46]。综合来看,荧光染料可通过不同注射技术引入韧皮部,代谢多久可观察到明显荧光则由园艺植物种类决定,一般为12~72 h[23, 46];绿色荧光蛋白则较为稳定,但其应用受转化体系建立与否的制约,耗时相对长。以上荧光观察均可通过荧光显微镜或者激光共聚焦显微镜进行,从而辅助明确卸载路径。
-
在质外体卸载的研究中,常用胶体金免疫定位技术研究酸性转化酶在植物器官内的亚细胞定位,以此明确韧皮部卸载的机制[14]。该方法在园艺植物研究中仅涉及到核桃Juglans regia[32, 47]、枣[30]和苹果Malus domestica[39]等少数植物。
-
蔗糖是韧皮部运输的主要糖分之一,研究酸性转化酶和蔗糖合酶的活性与基因表达对韧皮部卸载有重要意义。在慈竹Bambusa emeiensis幼笋韧皮部卸载研究中发现:竹笋后期细胞壁酸性转化酶活性及表达量与前期相比明显升高,且同一时期韧皮部卸载方式由共质体向质外体转变,说明细胞壁酸性转化酶与韧皮部卸载方式变化保持一致[48],相似的结果在枣[49]、黄瓜[10]中也有体现。相反,蔗糖合酶活性在马铃薯Solanum tuberosum块茎形成过程中,由质外体途径向共质体途径转变时同步增高[50],蔗糖合酶表达量则在‘索邦’百合[24]和石蒜[41]鳞茎形成的后期阶段显著提高。
-
韧皮部通过质外体和(或)共质体途径对光合同化物等进行转运来调控果实发育,故韧皮部卸载在提高果树果实质量及产量方面有重要作用[51]。目前对果树韧皮部卸载的研究相对较多。大多数果树卸载路径为单一路径,即只有共质体或质外体卸载路径,还有一些果实韧皮部卸载路径存在转换。由表1可见:蓝莓Vaccinium uliginosum等10种果实的卸载方式为单一路径,且以质外体卸载路径为主;扁桃Prunus dulcis果实维管束结构中没有发现筛分子伴胞复合体与周围韧皮部薄壁细胞存在胞间连丝[52−53],而且其余9种果实在研究中发现荧光染料CF均未从维管束中卸出[26, 36−40, 46, 54−55]。表明其韧皮部卸载路径是质外体路径,现有报道中仅甜柿Diospyros kaki果实的卸载方式因胞间连丝的存在被判定为共质体卸载路径[20, 26]。
共质体卸载路径和质外体卸载路径并不相互排斥,而是可以互相转化的,在果实韧皮部卸载研究中,同化物在果实的不同发育期呈现不同的卸载路径。葡萄、灵武长枣Ziziphus jujuba ‘Lingwuchangzao’、中宁圆枣Ziziphus jujuba ‘Zhongningyuanzao’、无花果Ficus carica和蔓越橘Vaccinium macrocarponl等果实的韧皮部卸载都遵循共质体到质外体路径的转变过程,有相似也有差异。其中葡萄和无花果果实存在发育前期和发育后期2个生长期,在发育前期两者筛分子伴胞复合体和韧皮部薄壁细胞之间均存在大量的胞间连丝,但在发育后期不存在胞间连丝[44, 56]。枣果实的生长期包括膨大前期、快速膨大期、着色期和完熟期。灵武长枣和中宁圆枣的研究发现:荧光染料CF只在膨大前期从维管束中卸出,其他时期CF均未卸出[57−58],通过超微结构观察,进一步验证了灵武长枣果实的卸载路径[59]。而在另一些枣品种中却在果实发育前中后期存在由质外体—共质体—质外体卸载路径相互转换的过程[30, 49]。蔓越橘果实的生长期分为幼果期、膨果期、转色期与成熟期,胞间连丝只在幼果期与膨果期被发现,在转色期和成熟期均未被发现[60]。对核桃果肉韧皮部及其周围薄壁细胞组织定位研究[32]发现:核桃果皮韧皮部卸载主要采取质外体路径,而种皮内则采用共质体路径,说明在果实不同部位存在差异。文冠果Xanthoceras sorbifolium果实在发育前期筛分子伴胞复合体与韧皮部薄壁细胞存在胞间连丝,发育中期未发现胞间连丝,但发育后期胞间连丝重新出现,表明在果实发育过程中韧皮部卸载途径存在共质体—质外体—共质体的转化过程[61]。因此,韧皮部卸载路径可能随着果实的发育进程会出现一次甚至多次的转化,应结合发育阶段准确分析,且具有品种(种)特异性,不同部位也会呈现差异。
-
蔬菜类包括茎菜类、根菜类、果菜类等。蔬菜通过光合作用产生的化合物经韧皮部运输到库器官,而不同库器官之间的光合产物分配被认为是影响其产量的主要因素[62]。目前蔬菜韧皮部卸载研究主要在果菜类和根菜类,尤以果菜类的相关研究较多。针对果菜类不同器官及不同发育过程卸载方式均有研究(表1)。在豌豆Pisum sativum根尖发育过程中发现卸载方式主要以共质体卸载路径为主[63],在蚕豆Vicia faba中也有相似结果[64];在水苏糖运输型植物黄瓜[10]和西瓜[11]的研究中,均发现荧光染料CF并未从维管束中卸出,表明韧皮部卸载路径以质外体为主,此外,己糖转运蛋白CsSUC4在黄瓜果实发育过程中均被限制在韧皮部内,进一步证实黄瓜韧皮部卸载为质外体路径[65]。但在同样为果菜类的番茄Solanum lycopersicum中发现:前期CF可以在番茄果皮薄壁细胞间移动,但后期则不可。表明番茄幼果期以共质体路径为主,发育后期则转变成质外体路径[66]。
近年对根菜类甜菜Beta vulgaris韧皮部卸载的研究较为透彻,不仅包括肉质根还涉及到叶片。通过对甜菜肉质根中细胞壁酸性转化酶和蔗糖合酶表达模式研究,明确了在直根发育过程中韧皮部卸载存在从共质体向质外体转化[67];在叶片研究中,将PCMBS (parachloromercuribenzene sulfonic acid)引入发育中的甜菜叶片,发现同化物的输入未受到影响,且同化物从韧皮部的卸出也未经过跨膜运输,故其韧皮部卸载路径以共质体为主[68−69]。可见,各类蔬菜的韧皮部卸载路径会因器官和发育阶段的不同而有所差异,且在不同器官的发育过程中存在转化现象。后续相关研究应根据蔬菜品种具体分析。
-
观形植物多以树形优美的乔木为主,但在观形植物研究方面很少涉及韧皮部卸载途径。在‘南林95杨’Populus ×euramericana ‘Nanlin95’[33]研究中发现:CF在“库”叶、茎尖和根尖都可以卸出,表明韧皮部卸载方式为共质体路径,而在次生茎和次生根中无法卸出,则证明其主要采用质外体路径。同样在黄梁木Neolamarckia cadamba叶柄研究中也发现:CF可在韧皮部薄壁细胞内卸载,证实其卸载方式以共质体路径为主[35]。
-
观花植物的研究对象既有草本也有木本植物,木本植物的韧皮部卸载路径较草本植物多变。在硬骨凌霄叶脉中发现中间细胞与周围韧皮部薄壁细胞存在大量胞间连丝,证明其韧皮部卸载方式为共质体[20]。在毛地黄Digitalis purpurea蜜腺中发现:在花蜜分泌过程中质外体占主导地位[72]。而在油茶Camellia oleifera果实研究中发现:在果实发育的早、中、晚期蔗糖运输途径有差异,遵循从共质体—质外体—共质体的转换规律[74],该结论同样在油茶品种‘华硕’C. oleifera ‘Huashuo’中得到验证[75]。另外,在牡丹Paeonia suffruticosa的研究中发现:牡丹在生长期和休眠期同化物运输方式各不相同,生长期除根部外,各器官之间均存在胞间连丝,即光合同化物在韧皮部中以共质体卸载为主,质外体卸载为辅;但在休眠期光合同化物的运输则以共质体卸载为主[34]。
-
观叶类植物的研究目前只涉及竹Bambusoideae和龟背竹Monstera deliciosa,但是关于竹笋或竹秆中糖的韧皮部卸载和卸载后路径的信息较少。已有研究发现:慈竹幼笋CF能够扩散出韧皮部,但被限制在维管束内,表明蔗糖在维管束内的卸载以共质体路径为主,但在维管束与周围薄壁细胞间为质外体路径[35];在云南箭竹Fargesia yunnanensis韧皮部卸载过程中存在很多胞间连丝,确定其韧皮部卸载为共质体路径[70−71];龟背竹气根中的蔗糖被细胞壁酸性转化酶分解,经己糖转运至库细胞,这为质外体卸载提供了有力证据[73]。
-
韧皮部卸载是园艺植物生长发育过程中的关键,是植物体内多因素共同作用的结果,包括自身细胞结构、代谢酶和蔗糖转运蛋白等。通过了解韧皮部卸载的途径与内部机制有助于提高园艺植物的产量与质量。目前对园艺植物韧皮部卸载的研究正处于发展阶段,研究由初期的细胞层面过渡至生理生化层面,并开始关注到分子层面。单种方法可能带来错误的表征,后续研究可综合采用细胞学,糖含量、酶活性及基因表达等定量方法从生理化及分子等多层面明确卸载路径。有研究表明蔗糖代谢酶以及蔗糖转运蛋白与韧皮部卸载途径的变化有关,但在园艺植物韧皮部卸载方面的研究还很欠缺。因此,需重视园艺植物韧皮部卸载与代谢酶以及糖转运蛋白的联系。
现有的报道认为蔷薇科植物以山梨醇为主要运输糖,葫芦科植物以棉子糖类为主,但同科内物种研究仍较少,应扩大研究科内物种数量,寻找不同物种卸载路径中的共性与个性。决定园艺植物质量和产量的重要性状大多与糖的转运密切相关,集中在果实的糖含量、地下变态器官的碳储藏等方面,因此韧皮部卸载在相关生物学过程中可发挥极为重要的作用,但现有研究并未揭示韧皮部卸载路径的精确调控机制。
总体而言,园艺植物韧皮部卸载机制的研究已取得了一些进展,但仍有很多问题未解决。今后的研究重点可包括:①从细胞学、生理生化学及分子生物学等不同层面进行研究,明确卸载路径中的系统性、有效性及互证性;②不同运输糖类在韧皮部卸载中的异同;③糖信号是否介导了韧皮部卸载路径的根本性改变;④结合突变体,深入阐述糖韧皮部卸载的调控机制。应结合分子生物学、基因工程学等领域,进一步揭示韧皮部卸载在园艺植物生长发育中的作用机制,为园艺植物的质量与产量的提升提供更深入的理论支撑。
Research progress of phloem unloading in horticultural plants
doi: 10.11833/j.issn.2095-0756.20230427
- Received Date: 2023-08-09
- Rev Recd Date: 2023-12-25
- Available Online: 2024-03-21
- Publish Date: 2024-04-01
-
Key words:
- horticultural plants /
- phloem unloading /
- symplasmic unloading /
- apoplasmic unloading
Abstract: Phloem unloading contains a series of complex processes. Phloem unloading plays an important role in the transportation and distribution of assimilates in horticultural plants (fruit trees, vegetables, ornamental plants). This study summarizes the main ways of phloem unloading, and focuses on the main research results of phloem unloading in the growth and development of horticultural plants, including: (1) the main sugar substances transported by phloem; (2) phloem unloading mode; (3) research approaches for phloem unloading; (4) research on phloem unloading in horticultural plants. The key enzymes and sucrose transporters involved in phloem unloading process need further clarification with in-depth research combining the emergence of new technologies, which will help to further elucidate the underlying molecular regulation mechanism of phloem unloading during the key economic and ornamental traits formation in horticultural plants, as well as to provide new ideas for detail research on phloem unloading in other plants. [Ch, 1 fig. 1 tab. 75 ref.]
| Citation: | LIU Jie, HUANG Ziyang, KANG Jie, et al. Research progress of phloem unloading in horticultural plants[J]. Journal of Zhejiang A&F University, 2024, 41(2): 437-446. DOI: 10.11833/j.issn.2095-0756.20230427 |









 DownLoad:
DownLoad: