-
香果树Emmenopterys henryi为茜草科Rubiaceae香果树属Emmenopterys高大落叶乔木,是中国特有的第四纪孑遗植物之一,零星分布在中国西北、华中、华东、华南和西南等15个省(自治区)[1−2]。目前,香果树的野外生存受到一定的威胁,已经被列为国家Ⅱ级重点保护植物[3]。过去几十年,国内学者对香果树进行了广泛的研究[4],但对于香果树生境土壤微生物的研究几乎空白。土壤微生物作为陆地生物多样性的重要组分,不仅在陆地生态系统物质循环和能量流动过程中起着重要作用[5],还通过植物-微生物反馈影响着植物的代谢、功能性状及其对环境胁迫的适应能力,从而影响植物的健康和多样性[6−7]。细菌和真菌是土壤中最重要的两大微生物类群,一般来说真菌对环境胁迫的抗性更强,而细菌对资源水平波动的适应性更强[8]。因此,学者普遍认为资源质量低的环境中微生物群落往往以真菌为主,资源不稳定或资源水平高的环境中微生物群落往往以细菌为主[9]。香果树喜生于海拔430~1 630 m处岩石裸露度高、土层浅薄的山谷林中[10]。在这种严苛的环境中,微生物常对珍稀植物的生长和繁育过程起着更为重要的促生作用[11]。有学者已经从香果树营养器官中筛选到了多种具溶磷、解钾、分泌吲哚乙酸或具有2种以上功能的真菌[12]。除提高土壤养分可利用率和刺激植物释放激素外,微生物还可以有效抑制植物病害的发生[11, 13]。活体植物根系周围既是病原微生物的聚集地,也是共生抗病微生物的聚集地,因此植物体周围的微生物多样性和物种组成对于该植物的生存和繁衍具有重要影响[14]。然而,目前香果树生境微生物多样性和物种组成时空变化格局及机制仍未得到深入了解。
微生物多样性和物种组成的时空变化格局受多种生态因素的影响,这些因素相互作用,但主控因素仍然不明[15]。海拔是影响土壤微生物多样性的重要因素[16]。不同森林生态系统中表层土壤微生物普遍呈现海拔变化格局,但对于土壤微生物组成和多样性沿海拔变化的趋势至今没有统一定论[10, 14]。其主要原因在于各种生态因子,如土壤温度、湿度、土壤理化性质和植被类型,都会随海拔的升高而改变,进而对土壤微生物群落造成不同影响[16−17]。虽然土壤特性对微生物多样性的影响一直受到关注[15],但研究多围绕土壤养分含量,很少分析土壤物理性质等对微生物多样性的影响。此外,植物可以通过凋落物和根系分泌物对土壤微生物产生直接影响,也可以通过改变土壤理化性质对土壤微生物产生间接影响[18−19]。植物群落的物种组成或多样性与群落中土壤微生物物种组或多样性呈显著相关[20−21]。同时,空间距离对土壤微生物多样性的影响也不容忽视。除了微生物多样性本身,样本间土壤理化性质和植被特征的相似性也都与空间距离有关。因此,需要尽可能同时检验这些生物和非生物因素(包括空间因素)对珍稀植物生境土壤微生物多样性的影响。
为探究香果树生境土壤微生物多样性和物种组成时空变化信息以及造成这些变化的主控因素,本研究利用高通量测序技术检测浙江九龙山国家级自然保护区香果树生境土壤细菌和真菌的多样性格局,并分析香果树生物因素和环境因素对这些格局的影响,以期为香果树的保护和繁育提供科学依据。
-
浙江九龙山国家级自然保护区位于浙、闽、赣交界处的丽水遂昌西南部(28°19′10″~28°24′43″N,118°49′38″~118°55′03″E)。该区气候属中亚热带湿润季风气候,四季分明,雨量充足,光照适宜,气候具有垂直地带性,年内雨季和干季交替明显,南北坡气候存在差异[20]。2021年平均气温为16.3 ℃,年降水量为2 171.5 mm;2022年平均气温为16.0 ℃,年降水量为2 064.2 mm。土壤属富铝土纲,红壤土类,包括老红壤、红壤、黄红壤和红黄壤等亚类,随着海拔的变化,各亚类按序替代。土壤中有机质、全氮及钾素丰富,有机质转化迅速,腐殖质组成以富里酸占优势[22]。植被以亚热带常绿阔叶林为主,该区也是香果树在浙江的主要分布地之一[22−23]。
-
2021年7—10月,在九龙山国家级自然保护区内的大岩前、岩背坑、陈坑、源大坑、内阴坑、内北坪等6处香果树分布地共设置34个样地。每个样地都是以1株香果树为圆心,15 m为半径的圆。定位每个样地的海拔和地理坐标,测量样地所在山坡的坡向,测量样地内所有胸径大于2.5 cm的乔、灌木的胸径及与样地中心香果树的距离,并鉴定物种。
-
分别于2021年11月中旬(24个样地)和2022年10月底(10个样地)分2次采集了34个样地的土壤样品。采样时在中心香果树冠幅范围内去除表层凋落物后任意采集5份土壤(深度0~15 cm)进行混合,剔除碎石等杂物。将采集土样放入提前标记好的无菌密封袋密封,置于冰盒内带回实验室。在实验室去除可见根系和动植物残体,一部分用于微生物高通量测序,另一部分自然风干后过2 mm土筛,用于土壤理化性质测定。
-
土壤理化性质分析参考《土壤分析技术规范》[24]。采用电位法测定土壤pH,外加热重铬酸钾容量法测定有机质,凯氏蒸馏法测定全氮,碱解扩散法测定碱解氮,盐酸-氯化铵浸提-钼锑抗比色法测定速效磷,醋酸铵浸提-火焰光度法测定速效钾;采用指测法确定土壤质地;采用比重瓶法结合环刀法测定和计算土壤总孔隙度。
-
土壤样品保存在干冰中送至实验室后,使用土壤DNA提取试剂盒(Omega Bio-tek,Norcross,GA)进行土壤微生物总DNA抽提,并利用质量分数为1%的琼脂糖凝胶电泳对抽提的总DNA纯度和浓度进行检测。本研究采用通用引物338F和806R扩增细菌16S rDNA基因的V3~V4区域;采用引物ITS1F和ITS2R扩增真菌ITS1区域。每个样本3个重复,重复的PCR扩增产物混合后经检测、回收、纯化和荧光定量后构建Miseq文库,并进行双向测序。原始序列数据经拼接、过滤质控和去除嵌合体等步骤后,得到高质量的有效序列数。在97%相似性水平下,划分所有序列的操作分类单元(OTU)[18]。
-
Shannon-Wiener指数和Simpson指数是衡量群落α多样性最常用的指数[25],但这2个指数在比较不同群落α多样性的差异时,群落间的多样性差异程度容易失真[26]。为了更好地揭示不同样地间α多样性变化的影响因素,本研究使用Shannon-Wiener指数当量(H')和Simpson指数当量(D')(即保持多样性指数值不变,群落内物种都具有相同多度时,群落中预期的物种数量)来表征香果树生境土壤细菌和真菌的α多样性。本研究分别统计所有土样细菌、真菌的OTU数量和门类,并计算每个土样细菌和真菌的Shannon-Wiener指数、Simpson指数及其当量。用t检验比较2个年份土壤细菌和真菌的H'和D'。
为分析不同样地土壤微生物α多样性差异与地形、土壤理化性质以及香果树大小的关系,本研究以各样地土壤细菌和真菌的H'或D'作为应变量,以样地的海拔、坡向、土壤理化性质、样地中心香果树的胸高断面积、树木α多样性作为自变量进行回归分析。取坡向的正弦和余弦值作为自变量来提取坡向的信息。同时,考虑到样地间的空间自相关,以及不同取样年份造成的影响,本研究将香果树分布地和取样年份作为随机效应加入回归方程,建立线性混合效应模型。利用F检验对各自变量固定效应的显著性进行检验。
-
采用基于丰度的Sorenson相异性指数作为配对样本之间的β多样性(即样本之间的微生物组成的差异,Btotal)的度量,并将β多样性分解为物种周转组分(Repl)和物种丰富度差异组分(Rich)[23]。当Rich/Btotal大于50%时,表明β多样性主要由物种丰富度差异组分主导,Repl/Btotal大于50%时,表明β多样性主要由物种周转组分主导[25]。
利用偏Mantel检验,在控制样地空间距离条件下,分析取样年份与香果树生境土壤微生物β多样性及其组分的Spearman相关;或控制取样年份条件下,分析样地间空间距离与香果树生境土壤微生物β多样性及其组分的相关性。同时控制样地空间距离和取样年份的情况下,分析其他因子与土壤微生物β多样性及其组分的关系。
最后,通过方差分解,探究对香果树生境土壤微生物β多样性及其组分有显著影响因素的相对重要性[25]。该分析以土壤样本细菌或真菌β多样性及其2个组分为响应变量,以地形、土壤理化性质、样地树木物种组成、中心香果树胸高断面积、取样年份或样地空间距离等因素作为解释变量。本研究依据最低残差原则选择“最好”的因素组合,去除共线性因子。空间距离因子由邻体矩阵主坐标分析(PCNM)引入,选择正相关的PCNM特征值作为空间因子指标[25]。以树种组成矩阵主坐标排序(PCoA)的前10个排序轴(含总变异的89.3%)作为代表各样地树种组成的变量。
-
所有数据分析都在R软件中完成。α多样性指数计算、偏Mantel检验、PCNM分析和方差分解采用vegan程序包,线性混合效应模型分析通过lme4程序包,β多样性分解通过adespatial程序包,PCoA排序通过ade程序包实现。
-
本研究从香果树生境土壤中共检测到8 750个细菌OTUs和10 968个真菌OTUs,其中8 612个可注释的细菌OTUs归属于38个门类,7 742个可注释的真菌OTUs归属于17个门类。2021年采集的24个土壤样品共检测到6 935个细菌OTUs和9 198个真菌OTUs,其中6 838个可注释的细菌OTUs归属于37个门类,6 554个可注释的真菌OTUs归属于16个门类;2022年采集的10个土壤样品共检测到4 168个细菌OTUs和4 592个真菌OTUs,其中4 096个可注释的细菌OTUs归属于37个门类,3 432个可注释的真菌OTUs归属于15个门类。
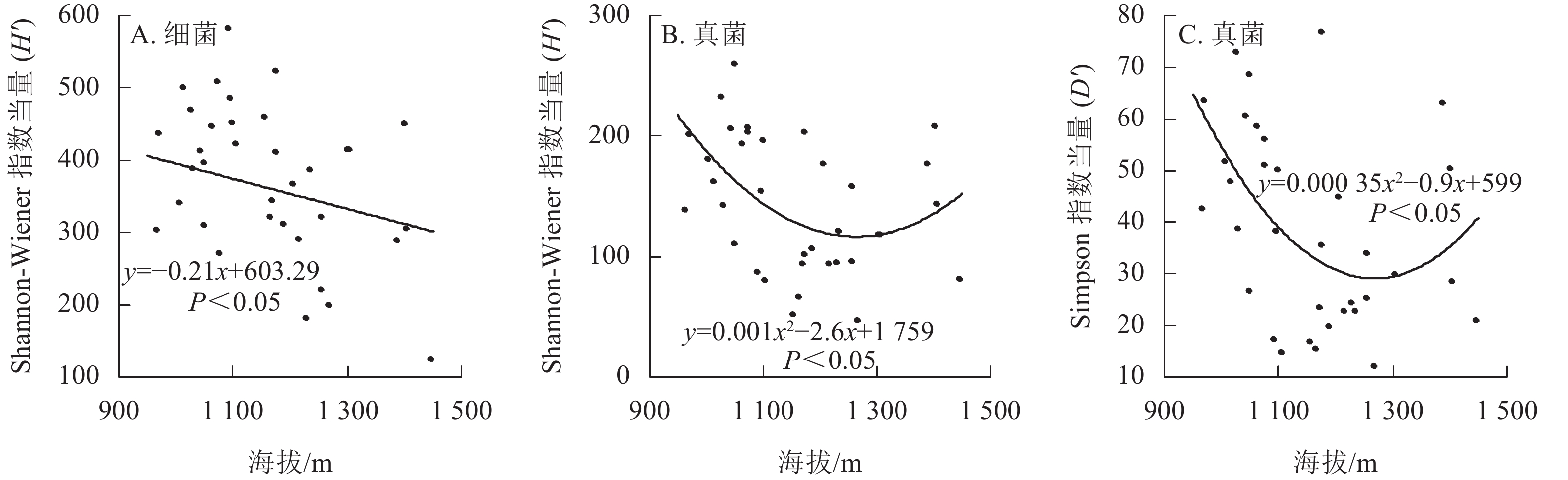
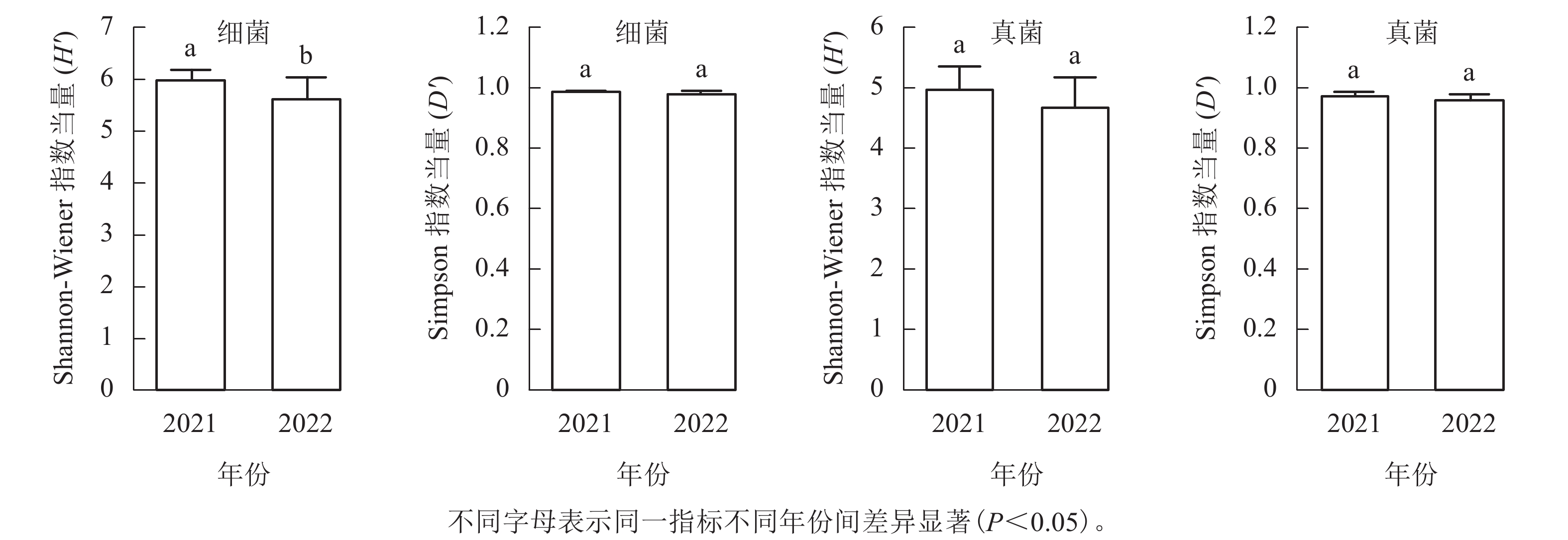
从图1可见:每个土壤样品细菌的Shannon-Wiener指数为4.82~6.37,均值为5.87,Simpson指数为0.95~0.99,均值为0.98;真菌Shannon-Wiener指数为3.84~5.56,均值为4.88,Simpson指数为0.92~0.99,均值为0.97。2021年采集的土壤细菌H'显著高于2022年(P<0.05),但D'无显著差异;真菌的H'和D'在2个取样年份间差异不显著。
线性混合效应模型分析表明:香果树生境土壤细菌H'与海拔呈显著负相关(P<0.05),即随着海拔上升,土壤细菌H'下降(图2A),而土壤细菌D'与海拔无关。海拔及其二次方对土壤真菌H'和D'都有显著影响(P<0.05),随着海拔的上升,香果树生境土壤真菌α多样性呈现先下降后上升的趋势(图2B和图2C)。模型分析表明:样地坡向与土壤细菌和真菌α多样性都无显著关系。土壤理化性质中,速效钾与细菌D'呈显著负相关(P<0.05);pH与细菌H'呈显著正相关(P<0.05);碱解氮与真菌H'、D'都呈显著负相关(P<0.05);其余性质都与真菌和细菌的α多样性无显著关系。植物因子中,仅中心香果树胸高面积与土壤真菌D'呈显著正相关(P<0.05),土壤细菌、真菌α多样性都与样地树种多样性无关(表1)。
影响因素 细菌H' 细菌D' 真菌H' 真菌D' 回归系数 P 回归系数 P 回归系数 P 回归系数 P 坡向正弦 0.00 1.00 0.01 0.95 0.18 0.38 0.12 0.10 坡向余弦 0.01 0.97 0.18 0.33 −0.25 0.29 −0.11 0.99 土壤质地 −0.06 0.88 −0.10 0.65 −0.05 0.86 −0.07 0.90 总孔隙度 0.11 0.42 0.10 0.46 0.12 0.36 0.14 0.38 有机质 −0.43 0.56 0.10 0.90 −0.78 0.40 −0.14 0.86 全氮 1.21 0.17 0.61 0.54 1.41 0.20 0.40 0.71 速效钾 0.88 0.11 −0.65 0.05 0.93 0.17 1.00 0.11 速效磷 1.28 0.19 1.36 0.18 −0.60 0.61 −0.55 0.60 碱解氮 −0.87 0.12 −1.00 0.09 −0.37 0.03 −0.73 0.02 pH 0.82 0.00 0.09 0.67 −0.06 0.83 −0.10 0.69 中心香果树胸高断面积 0.09 0.64 −0.11 0.60 0.15 0.56 0.42 0.05 树种多样性 0.25 0.24 0.26 0.18 0.14 0.60 0.19 0.37 Table 1. Influence of biotic and abiotic factors on α diversity of soil microbes in habitat of E. henryi
-
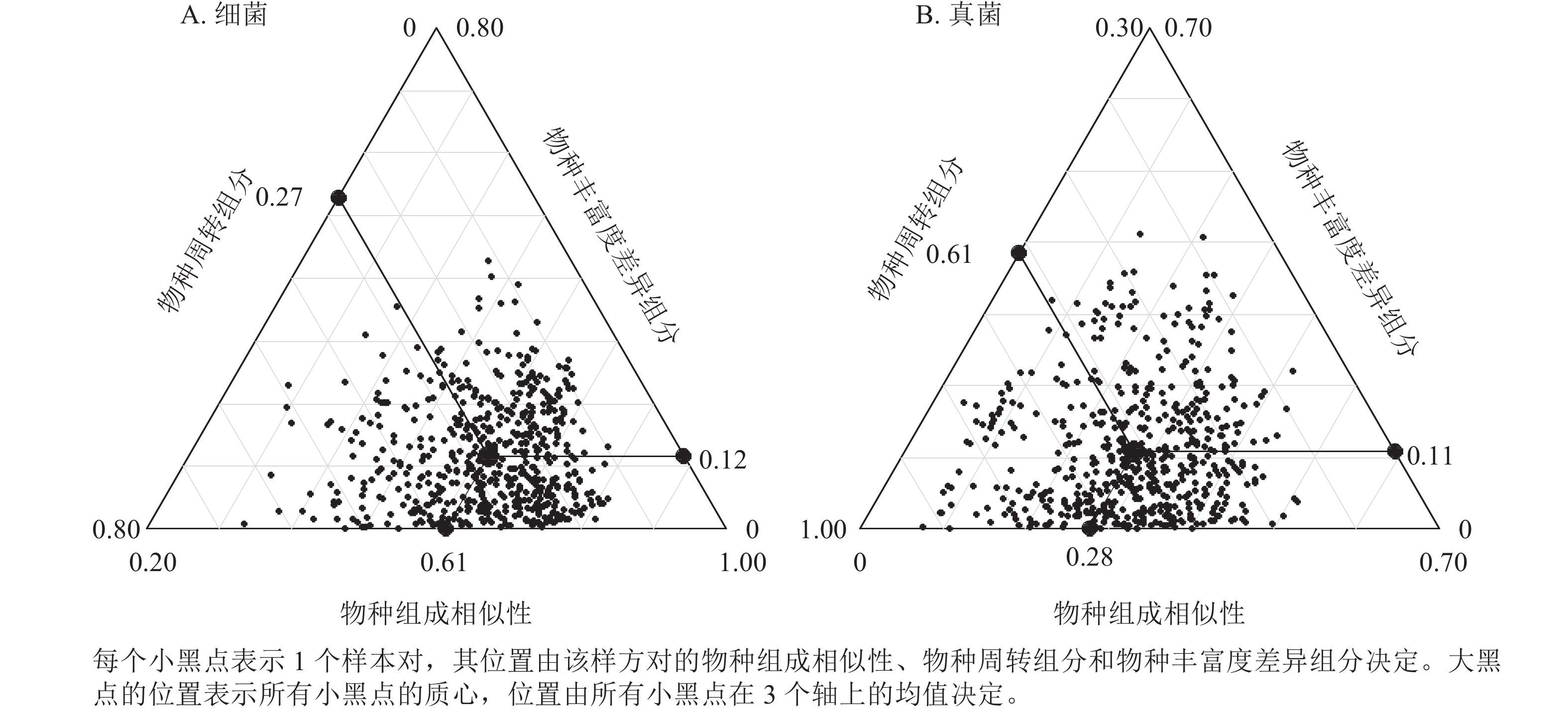
九龙山香果树生境土壤细菌β多样性为0.19~0.72,均值为0.39,其中物种周转组分为0.08~0.66,均值0.27,物种丰富度差异组分为0~0.43,均值为0.12 (图3A)。物种周转组分对土壤细菌β多样性的贡献达70.2%,而物种丰富度差异组分对土壤细菌β多样性的贡献占29.8%。香果树生境土壤真菌β多样性为0.49~0.96,均值为0.72,其中物种周转组分为0.37~0.92,均值为0.61,物种丰富度差异组分为0~0.41,均值为0.11 (图3B)。物种周转组分对土壤真菌β多样性的贡献高达85.1%,而物种丰富度差异组分对土壤真菌β多样性的贡献仅占14.9%。
-
偏Mantel检验表明:控制取样年份后,样地空间距离与土壤微生物β多样性及其物种周转组分都显著相关(P<0.05,表2);控制样地间空间距离后,仅土壤微生物物种丰富度与取样年份有关(表2)。取样年份解释了土壤细菌物种丰富度的42% (图4A),但仅解释了土壤真菌的8% (图4B)。
影响因素 细菌 真菌 β多样性 物种周转 物种丰富度差异 β多样性 物种周转 物种丰富度差异 相关系数 P 相关系数 P 相关系数 P 相关系数 P 相关系数 P 相关系数 P 空间距离 0.32 0.00 0.27 0.01 0.01 0.42 0.30 0.01 0.29 0.00 −0.04 0.64 年份 0.06 0.21 −0.38 1.00 0.65 0.00 0.02 0.42 −0.37 1.00 0.50 0.00 海拔 −0.06 0.73 −0.20 0.99 0.10 0.08 0.09 0.20 −0.27 1.00 0.33 0.00 坡向 0.02 0.28 −0.00 0.50 0.02 0.24 0.02 0.23 0.05 0.08 −0.02 0.65 土壤理化性质 0.46 0.00 0.39 0.00 0.01 0.25 0.43 0.00 0.26 0.02 0.12 0.13 质地 0.05 0.53 −0.06 0.77 0.05 0.43 0.02 0.44 0.06 0.29 0.06 0.38 总孔隙度 0.34 0.01 0.34 0.01 0.02 0.57 0.25 0.05 0.22 0.03 −0.04 0.87 有机质 0.45 0.00 0.41 0.00 −0.03 0.62 0.29 0.01 0.27 0.01 0.01 0.40 全氮 0.34 0.00 0.28 0.01 0.06 0.24 0.26 0.02 0.21 0.03 0.04 0.27 速效钾 0.31 0.01 0.31 0.00 −0.03 0.63 0.24 0.03 0.21 0.02 −0.03 0.61 速效磷 0.33 0.01 0.27 0.01 0.06 0.25 0.25 0.04 0.22 0.02 −0.01 0.49 碱解氮 0.24 0.01 0.18 0.04 0.01 0.43 0.35 0.00 0.14 0.09 0.15 0.06 pH 0.47 0.00 0.42 0.00 0.01 0.43 0.33 0.00 0.27 0.00 −0.02 0.53 中心香果树胸高断面积 0.08 0.21 −0.03 0.58 0.08 0.13 0.01 0.44 −0.21 0.99 0.18 0.03 树种组成 0.20 0.02 0.19 0.03 −0.06 0.76 0.23 0.02 0.13 0.09 0.01 0.45 Table 2. Partial spearman correlations between soil microbes β diversity and various environmental factors in E. henryi stands in Jiulongshan

Figure 4. Variance partitioning results of β diversity and its components of soil bacteria or fungi in E. henryi stands in Jiulongshan interpreted by biotic and abiotic factors
偏Mantel检验发现:控制采样年份和样地空间距离后,土壤细菌β多样性与土壤理化性质差异(总孔隙度、有机质、全氮、速效钾、速效磷、碱解氮和pH)以及树种组成显著相关(P<0.05,表2)。土壤理化性质、树种组成和空间结构共解释九龙山香果树生境土壤细菌β多样性52%的变异,其中土壤理化性质差异解释了26%,树种组成解释了12%,空间结构仅解释10%,土壤理化性质和树种组成共同解释额外10%的细菌β多样性(图4C)。土壤细菌物种周转与土壤理化性质(尤其是有机质、全氮、速效钾、速效磷和pH)及树种组成显著相关(P<0.05,表2)。样地的空间结构并不能单独解释土壤细菌的物种周转。土壤理化性质和树种组成共解释了44%的土壤细菌物种周转,其中土壤理化性质解释了36%,树种组成解释了18% (图4D)。土壤细菌物种丰富度差异与海拔、坡向、土壤理化性质、香果树种组成的相关性不显著(表2)。
控制取样年份和样地空间距离后,土壤真菌β多样性与土壤理化性质(总孔隙度、有机质、全氮、速效钾、速效磷、碱解氮和pH)及树种组成显著相关(P<0.05,表2)。土壤理化性质、树种组成和空间结构共解释了九龙山香果树生境土壤34%的真菌β多样性,其中树种组成解释了13%,土壤理化性质和空间结构各自解释了11%和10%的真菌β多样性,土壤理化性质和树种组成共同解释额外7% (图4E)。土壤真菌物种周转与土壤理化性质(总孔隙度、有机质、全氮、速效钾、速效磷和pH值)显著相关(P<0.05,表2)。土壤理化性质和空间结构共同解释了15%的土壤真菌物种周转,其中土壤理化性质和空间结构分别解释了5%和11% (图4F)。土壤真菌物种丰富度仅与海拔和中心香果树胸高断面积显著相关(P<0.05,表2)。海拔和取样年份交互作用解释了51%的土壤真菌物种丰富度差异,海拔和取样年份分别独立解释9%和8%的土壤真菌物种丰富度差异。中心香果树胸高断面积不能单独解释土壤真菌物种丰富度差异。
-
植物与微生物之间存在复杂的相互作用[11]。近年来,对森林土壤微生物多样性的研究较深入[27],但对珍稀濒危植物体内或生境中的微生物研究较少[11−12]。本研究分析表明:九龙山香果树生境土壤细菌α多样性高于真菌α多样性,且土壤微生物多样性显著高于该地树木的多样性[25]。这一特征符合森林土壤微生物多样性的基本规律[28]。极高的细菌和真菌多样性是出现宿主专一的病原微生物和共生菌的基础。专一的植物-微生物相互作用有助于缓解珍稀植物受到的环境胁迫[11]。从不同气候带森林土壤微生物多样性比较来看,九龙山国家级自然保护区香果树生境土壤细菌α多样性较其他地方低,而真菌α多样性较其他地方高[28]。这可能与香果树生境岩石裸露度高且土层浅薄的特性有关。在这种资源质量低的环境中,真菌种类丰富的微生物群落常体现出优势,丰富的真菌物种有利于维持环境中矿质资源的供给,改善植物的生存环境[8−9]。同时,本研究也发现:随着生境条件的变化,香果树生境中细菌群落的物种组成最为稳定,而真菌群落的物种组成变化较大[25]。植物、真菌和细菌群落在环境梯度上都是以发生物种周转为主,物种周转对于真菌群落的β多样性尤为重要。
-
本研究发现:2021年土壤细菌的H'显著高于2022年,而其余类群和多样性指数在不同年份间差异不显著。2022年土壤细菌群落H'显著降低,说明群落中不少稀有物种丧失或者丰度发生变化。已有研究表明:降水能显著影响森林土壤微生物群落的结构,且对细菌的影响尤为明显[29]。2022年6—8月,九龙山地区经历了明显的干旱过程,全年降水量比2021年下降了107.3 mm (5%)。降水的改变应该是九龙山香果树生境土壤细菌多样性降低的重要因素。
除了气候因素外,海拔和坡向是影响土壤微生物α多样性的重要因素[30−31]。有研究表明:土壤微生物α多样性在南坡和北坡对海拔变化的响应不同[31]。然而,本研究结果显示:九龙山香果树生境土壤微生物α多样性与坡向无关。海拔因子极其复杂,许多生态因子,如温度、湿度、辐射、植被等,都会随海拔的变化而改变,生态因子在海拔梯度上的变化比在纬度梯度上的变化快1 000倍[30]。目前,土壤微生物α多样性沿海拔梯度的变化模式主要包括单调递减、单调递增、单峰、下凹和无趋势等5种[31]。本研究表明:九龙山香果树生境细菌和真菌α多样性随海拔上升呈不同的变化趋势。
土壤理化性质是影响土壤微生物生存和繁殖的最直接因素,而且微生物的活动也影响着土壤理化性质,因此土壤理化性质与微生物多样性之间存在紧密联系[15]。九龙山香果树生境土壤pH全部小于6,属于酸性至强酸性土壤。细菌D'与土壤pH呈正相关,说明土壤酸性越低,细菌多样性越高,部分细菌类群不能在强酸性环境中生存。本研究发现:细菌D'与土壤速效钾呈负相关关系,可能有以下2种解释:首先,九龙山香果树生境中部分细菌类群可能不喜速效钾较高的生境;其次,生境中可能存在大量消耗速效钾的细菌类群,导致生境中速效钾降低。具体原因有待从细菌功能生态的角度进行深入研究。此外,本研究土壤碱解氮与真菌多样性之间呈显著负相关。主要原因在于真菌以利用分解程度低的凋落物为主,而在凋落物层较厚的环境中氮素多以有机态大分子形式存在,碱解氮质量分数常较低[11]。
在较大的地理尺度上或不同植被类型(或演替阶段)中,土壤微生物与植物多样性之间常存在紧密的正相关[32]。本研究的地理尺度较小,且限于单一的香果树群落,群落类型单一,因此并未检测到树木多样性与土壤微生物多样性之间的关系。然而,本研究依然发现样地中心香果树胸高断面积与其周围土壤真菌的D'之间呈显著正相关。有研究表明:不同树种的大树周围,土壤能以不同的速率积累病原真菌或菌根真菌[14]。如果积累的真菌种类大多为病原真菌,将导致香果树强烈的负密度制约效应,而积累多种菌根真菌,将有利于香果树幼苗从母树周围得到正反馈。珍稀植物如何招募并组装根内微生物,直接关系到其种群复壮前景[11]。张红芳等[12]已从香果树体内分离到了82株具溶磷效果和36株具解钾活性的内生真菌,说明有益真菌促进香果树种群维持和复壮是有可能的。
-
本研究发现:香果树生境土壤细菌和真菌群落的物种组成存在显著的空间正相关,即距离越近微生物群落的物种组成越相似。然而,微生物群落的空间结构并不完全由土壤理化性质和植物群落的空间自相关来解释。样地空间结构仅各独立解释了10%的细菌和真菌β多样性。其他具有空间自相关结构的生境因素,如土壤温度、湿度等,对于九龙山香果树生境土壤微生物物种组成具有重要影响。另外,取样年份对细菌和真菌群落的物种丰富度差异具有显著影响,说明气候波动对细菌和真菌群落物种丰富度的年际变化具有重要影响。树种是影响土壤微生物群落物种组成的重要因素[19]。在九龙山,土壤真菌、细菌β多样性以及细菌的物种周转组分与香果树群落的树种组成呈显著相关,且这一关系不能完全由香果树群落的树种组成的空间自相关因素解释。植物群落的树种组成差异促进了不同香果树生境土壤细菌和真菌群落间的物种替代。土壤是微生物生存的直接环境,土壤理化性质对土壤微生物β多样性具有重要影响[19]。本研究表明:土壤理化性质显著影响细菌和真菌群落β多样性及其物种周转组分,具有较高的独立和总和解释度。说明群落间土壤异质性是导致九龙山香果树生境土壤微生物(尤其是细菌) β多样性和物种周转的主要因素。除了重要的养分因子外,本研究还表明:影响土壤通气性、养分释放和移动以及热特性的土壤孔隙度对细菌和真菌群落的β多样性及其物种周转具有重要影响。虽然海拔梯度显著影响九龙山香果树生境土壤微生物α多样性,但是其对β多样性的影响很小。地形因素中,海拔差异仅与真菌群落物种丰富度差异显著相关,而坡向差异与细菌和真菌的β多样性及其2个组分都无显著关系。今后有必要进一步深入研究地形因子对土壤微生物α和β多样性影响的差异及其机制。
-
取样年份、海拔、土壤理化性质、中心香果树胸高断面积以及树种组成对浙江九龙山香果树生境土壤微生物群落的α和β多样性具有重要影响。细菌和真菌群落α多样性沿海拔梯度呈现不同的变化规律。此外,细菌和真菌群落β多样性体现出明显的空间结构。
-
感谢遂昌县气象局提供九龙山区气象数据。
Soil microbial diversity and its influencing factors in the habitat of rare plant Emmenopterys henryi in Jiulongshan, Zhejiang Province
doi: 10.11833/j.issn.2095-0756.20230532
- Received Date: 2023-11-01
- Accepted Date: 2024-04-24
- Rev Recd Date: 2024-04-11
- Available Online: 2024-09-25
- Publish Date: 2024-09-25
-
Key words:
- rare species /
- Emmenopterys henryi /
- soil /
- bacteria /
- fungi /
- altitude
Abstract:
| Citation: | LUO Zhengrong, ZHENG Weicheng, TANG Zhansheng, et al. Soil microbial diversity and its influencing factors in the habitat of rare plant Emmenopterys henryi in Jiulongshan, Zhejiang Province[J]. Journal of Zhejiang A&F University, 2024, 41(5): 1013-1023. DOI: 10.11833/j.issn.2095-0756.20230532 |











 DownLoad:
DownLoad: