-
微胶囊是由微小的颗粒或液滴被涂层包围而产生的具有许多特性的胶囊[1],被广泛用于载运对光照、温度等外界环境敏感的生物活性物质。微胶囊通常形成核壳结构包埋活性物质,壁材具有阻挡光线、氧气、水等的物理屏障作用,因此可以保护核心材料免受不利外部环境条件的影响[2]。近几年,壁材材料引起了人们的关注,包括蛋白质、海藻酸盐、多糖、抗性淀粉和美拉德产物等。海藻酸钠可被高碘酸盐氧化形成多个功能醛基团,通常被称为海藻酸二醛(ADA)。海藻酸二醛的醛基官能团非常活跃,可以与氨基发生反应形成席夫碱,促进与含氨基聚合物的共价交联[3]。蛋白-多糖二元复合物在包埋生物活性物质方面显示出巨大的潜力,不仅可以将蛋白的优异乳化特性和多糖的稳定作用相结合,还可以提升复合物在不同温度、离子强度和pH下的稳定性[4]。COPADO等[5]制备了酪蛋白酸钠-乳糖共聚物微胶囊,微胶囊表现出良好的分散性,经过美拉德反应的酪蛋白酸钠-乳糖共聚物提高了奇亚籽油的氧化稳定性。JIA等[6]通过美拉德反应制备了乳清蛋白(WPI)分离物-低聚糖共聚物,并用于包埋番茄红素,包埋率和包封产率分别达94%和86%。共聚物微胶囊不仅提高了番茄红素的储藏稳定性和氧化稳定性,还将番茄红素的生物利用度从16%提高到60%。
本研究主要探讨WPI和ADA共聚物间的共价交联对微胶囊中姜黄素(CUR)稳定性和释放特性的影响。通过测定褐变强度、接枝度、粒径、Zeta电位和流变性能探究不同WPI和ADA质量比下共聚物乳液的理化性质;利用傅里叶红外光谱(FTIR)和X射线衍射光谱(XRD)分析WPI-ADA共聚物的结构变化特征,通过热重分析(TGA)研究WPI-ADA共聚物微胶囊的热稳定性;最后测定姜黄素在胃肠道模拟消化中的释放特性,以期提高姜黄素的生物利用度并为新型运载体系提供理论基础。
-
WPI和CUR购自上海麦克林生化科技有限公司;海藻酸钠购自南京化学试剂有限公司;四硼酸钠、2-巯基乙醇购自国药集团化学试剂有限公司;人工胃液、人工小肠液、中链甘油三酯(MCT)、邻苯二甲醛(OPA)购自上海源叶生物科技有限公司。
-
将WPI与ADA以1∶0、3∶1、2∶1、1∶1、1∶2和1∶3的质量比分散在去离子水中(分别记为ck、W3A1、W2A1、W1A1、W1A2和W1A3),得到固形物质量分数为100 mg·g−1的溶液。将溶液在人工气候箱(温度为65 ℃,相对湿度为90%)中保持24 h,在−80 ℃预冷后进行冷冻干燥,得到WPI-ADA共聚物。
-
WPI-ADA共聚物分散在去离子水中。测定304 nm处吸光度用于证明Amadori化合物的形成[7],测定294 nm处吸光度证明美拉德反应早期产物和中间产物的存在,测定420 nm处吸光度证明美拉德反应终产物的存在[8]。
-
接枝度是评估美拉德反应幅度的关键指标。美拉德反应的发生可以通过游离氨基的含量进一步确定,因此反应体系中的接枝程度可以反应WPI游离氨基的变化[9]。根据ZHANG等 [10]的方法测定接枝度。将80.0 mg OPA加入2.0 mL甲醇中,然后与50.0 mL四硼酸钠缓冲溶液(0.1 moL·L−1,pH=9.7)、5.0 mL十二烷基硫酸钠(0.2 kg·L−1)和200.0 μL β-巯基乙醇混合。用去离子水定容到100.0 mL获得OPA试剂。将200.0 μL样品溶液(2 g·L−1)和4.0 mL OPA试剂混合并在35 ℃下保持5 min。测定340 nm处的吸光度获得游离氨基的含量。空白对照为4.0 mL OPA试剂和200.0 μL去离子水的混合物。
-
通过红外光谱仪(Nicolet iS20)测定红外光谱图。干燥后的ADA、WPI和WPI-ADA共聚物在400~
4000 cm−1内测定收集红外光谱信号,分辨率为4 cm−1。 -
将1 mg·g−1 CUR溶于MCT中,避光过夜搅拌。同时,将不同比例的WPI-ADA共聚物分散于去离子水中,室温下搅拌24 h,4 ℃过夜水合。将水相和油相以19∶1的质量比混合,通过高速性高剪切分散乳化机(FA25)将溶液以10 000 r·min−1剪切3 min得到粗乳液,使用超声波细胞粉碎机(JY-99-IIDN)超声10 min (开3 s,关3 s)制备乳液,制备过程中使用冰浴。形成的乳液于−80 ℃预冷后进行冷冻干燥,得到WPI-ADA微胶囊。
-
测试前用去离子水将乳液稀释100倍,利用粒径仪(Nano ZS)在室温下测定乳液粒径、PDI和电位。
-
使用旋转流变仪(HR-1)测定乳液的流变特性。设置应变为0.5%,在角频率1.0~100.0 rad·s−1下记录储能模量(G′)和损耗模量(G″)。选定直径40 mm的平行板,调整间距为500 μm,在剪切速率为0.01~100.00 s−1内记录样品的黏度值。通过Herschel-Bulkley模型分析剪切速率与剪切应力之间的关系:
式(1)中:σ为剪切应力(Pa);σ0为屈服应力(Pa);K为稠度系数(Pa·sn);γ是剪切速率(s−1),n为流动行为指数。
-
使用数码显微镜(BA310)观察水包油(O/W)乳液的微观结构。
-
为了量化微胶囊中CUR的总量,将制备好的微胶囊溶解在蒸馏水中,用乙醇提取CUR。根据CUR溶液的标准曲线,在425 nm处测定总CUR质量浓度(M0)。将微胶囊分散在无水乙醇中,在425 nm处测定游离CUR质量浓度(M1)。包埋率(E)通过以下公式得到:
式(2)中:M0为总CUR质量浓度(mg·L−1),M1为游离CUR质量浓度(mg·L−1)。
-
使用X射线衍射仪(Ultima Ⅳ)得到WPI-ADA微胶囊的XRD图谱,扫描角度范围为5~80°,扫描速度为2°·min−1。
-
将WPI-ADA微胶囊样品加入Tarsus热重分析仪(TG 209 F3)的铝盘中,测试温度为30~600 ℃,升温速率为10 ℃·min−1。
-
根据欧盟委员会(EU)10/2011号法规,以体积分数为50%的乙醇为食品模拟剂[11],根据已报道的方法,使用透析袋进行微胶囊的体外释放实验 [12−14]。将微胶囊样品与模拟胃液(SGF,2 g·L−1 氯化钠,3.2 g·L−1胃蛋白酶)混合并将pH调至1.2,将该溶液装入透析袋置于乙醇释放介质中。模拟胃消化结束后,将溶液pH调整至7.0以抑制胃蛋白酶活性。随后,收集模拟胃消化产物与模拟肠液(SIF,10 g·L−1胰蛋白酶,6.8 g·L−1磷酸二氢钾)混合,并将pH调至7.4,将溶液装入透析袋置于乙醇释放介质中。体外释放均在37 ℃,100 r·min−1下进行,在预定的时间间隔内取样,并更换相同量的释放介质以保持相同体积的释放介质。释放介质中CUR的质量浓度通过在425 nm处吸光度得到。
式(3)中:mt为在时间t时释放的CUR质量浓度(mg·L−1),m∞为微胶囊中CUR的质量浓度(mg·L−1)。
-
所有数据以平均值±标准差表示,采用单因素方差进行显著性分析,使用DPS数据处理系统进行Tukey事后检验显著性分析,使用Origin Pro 2022 b绘图。
-
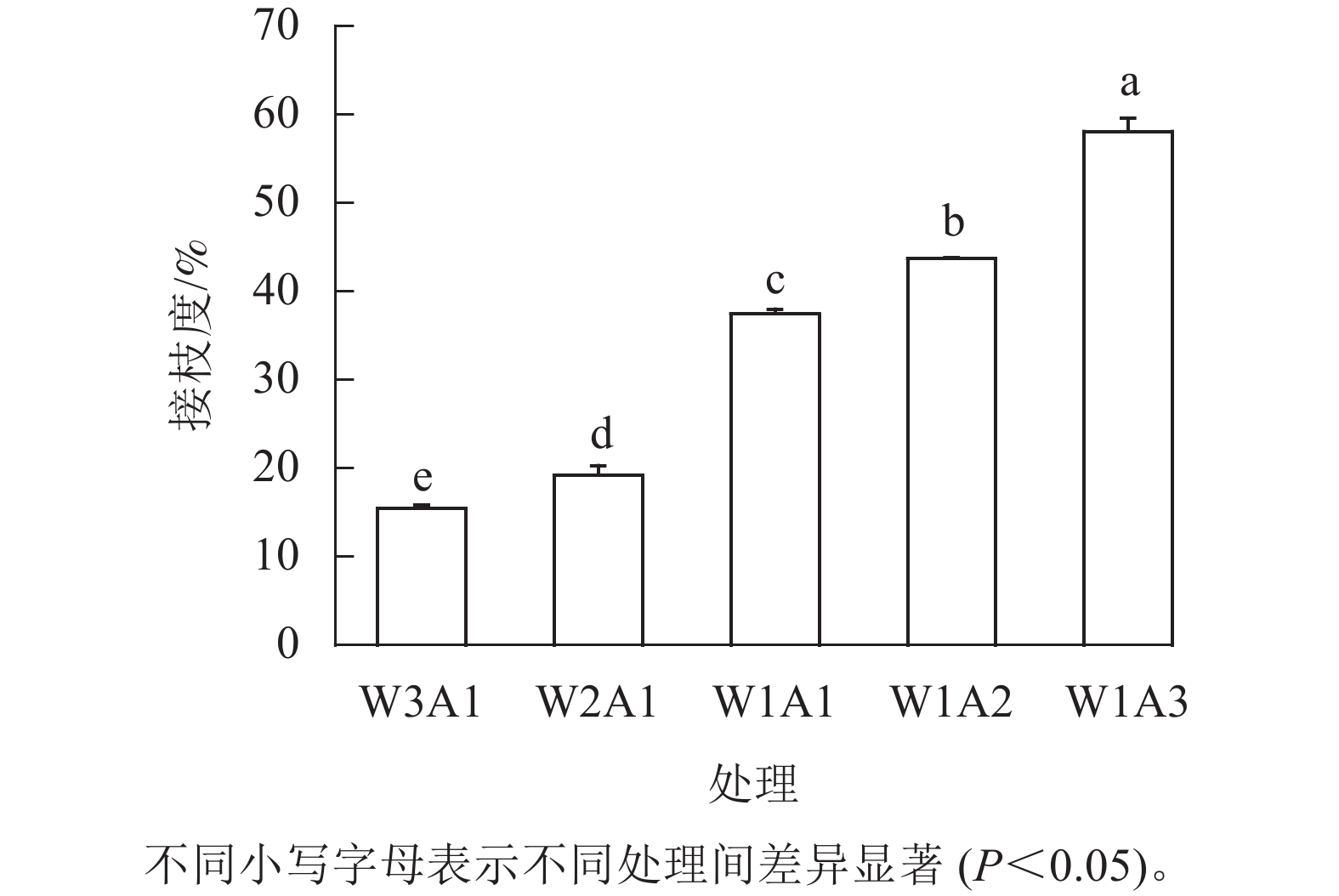
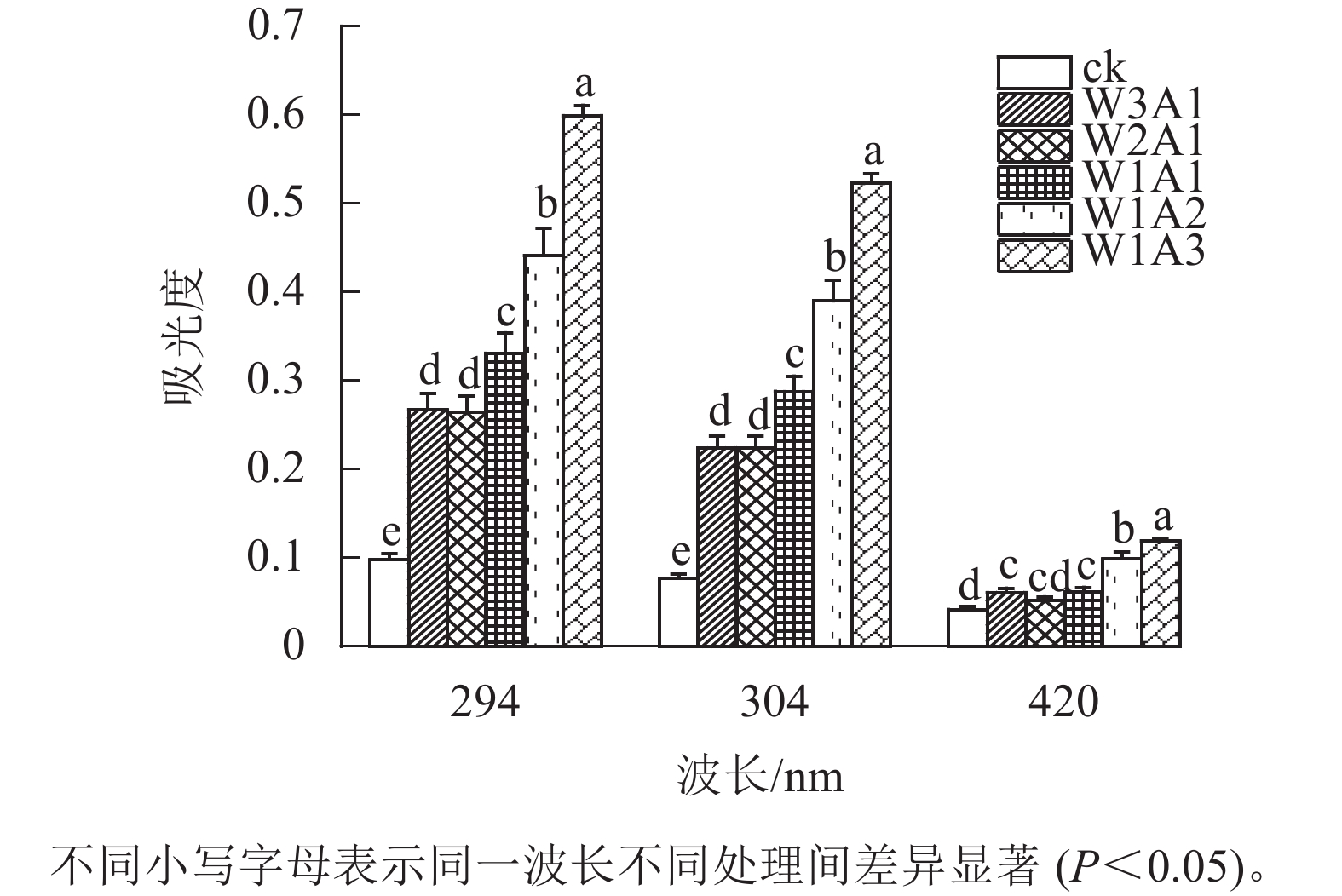
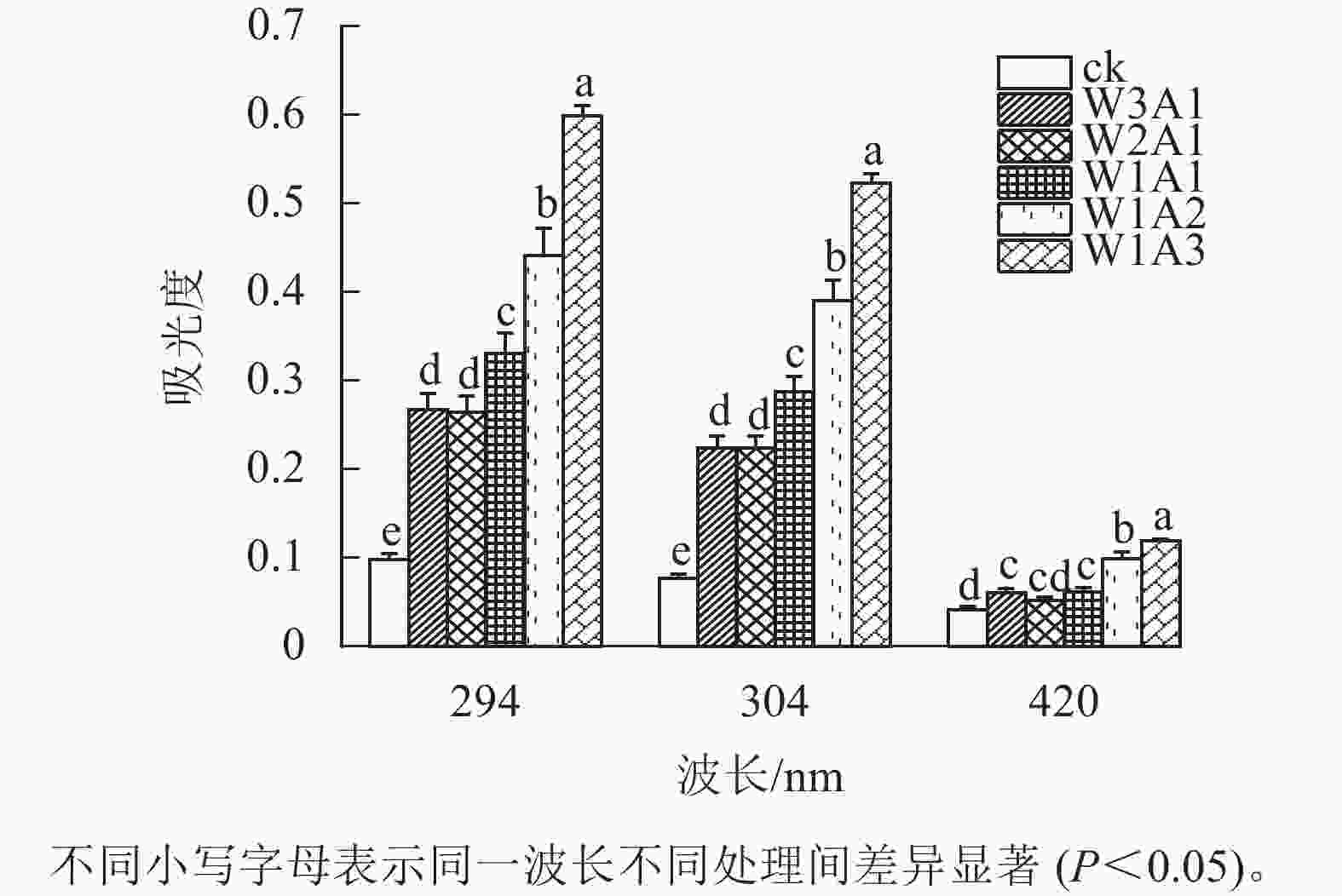
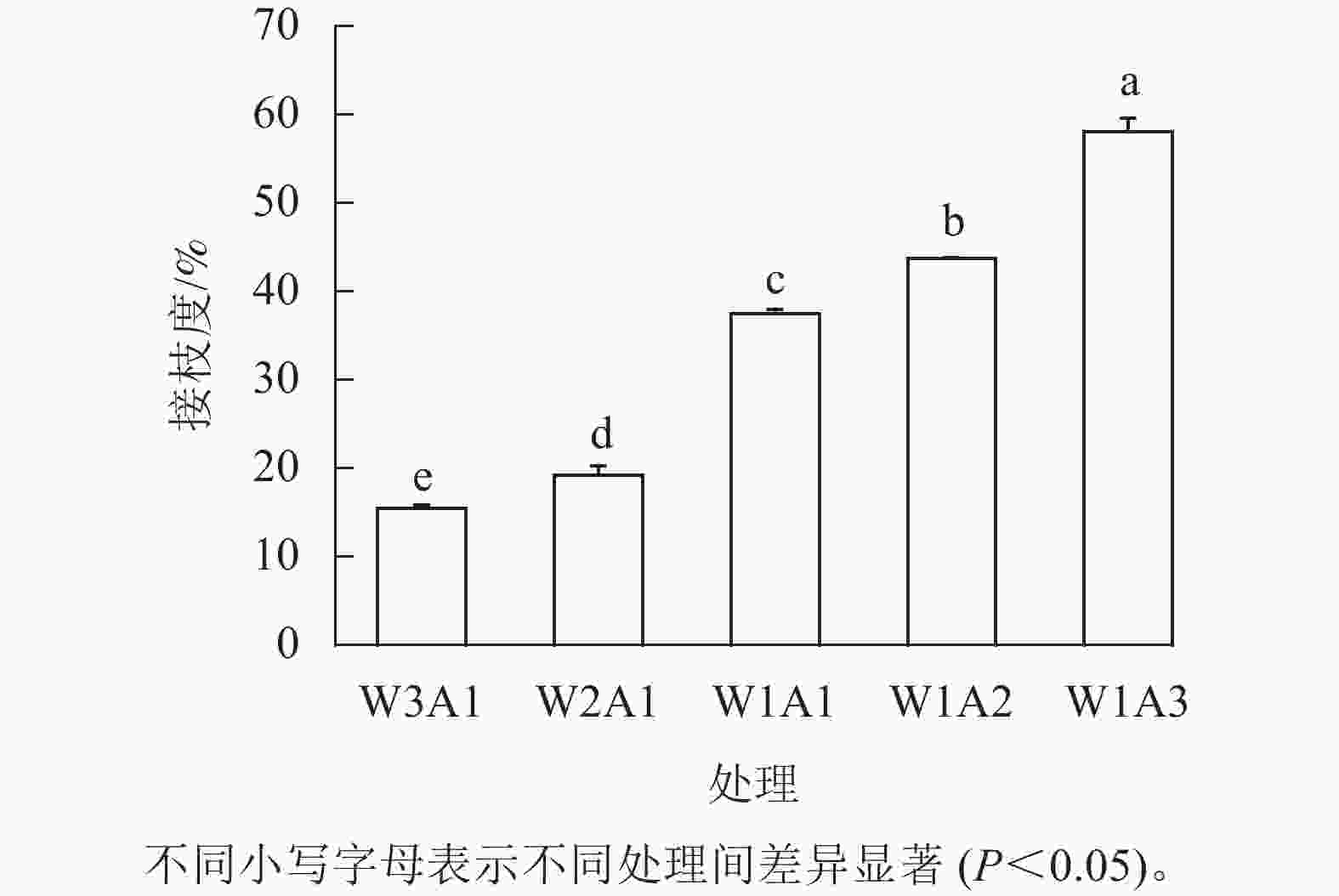
如图1所示:除了W2A1处理组在420 nm处的吸光度外,其他处理组在294、304和420 nm处的吸光度均比ck显著增加(P<0.05)。吸光度随着WPI和ADA质量比的降低而增加,这与观察到的接枝度变化一致。同时,所有处理组在294 nm处的吸光度均高于304 和420 nm处。由图2可知:随着WPI∶ADA从3∶1变化到1∶3,WPI-ADA共聚物的接枝度从15.5%增加到56.1%。这表明在一定的范围内,高ADA能够促进与WPI的美拉德反应,使接枝度增加。
-
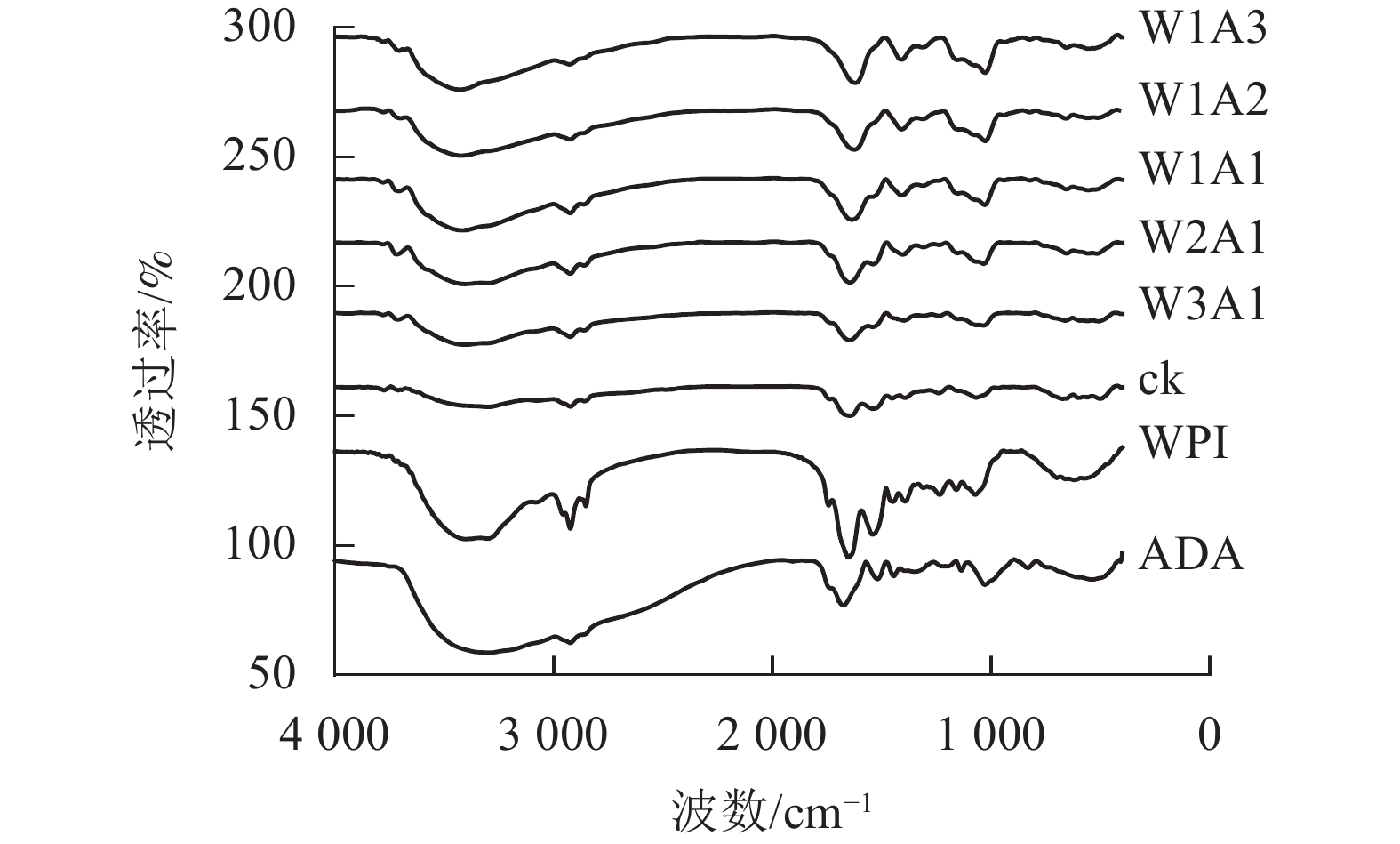
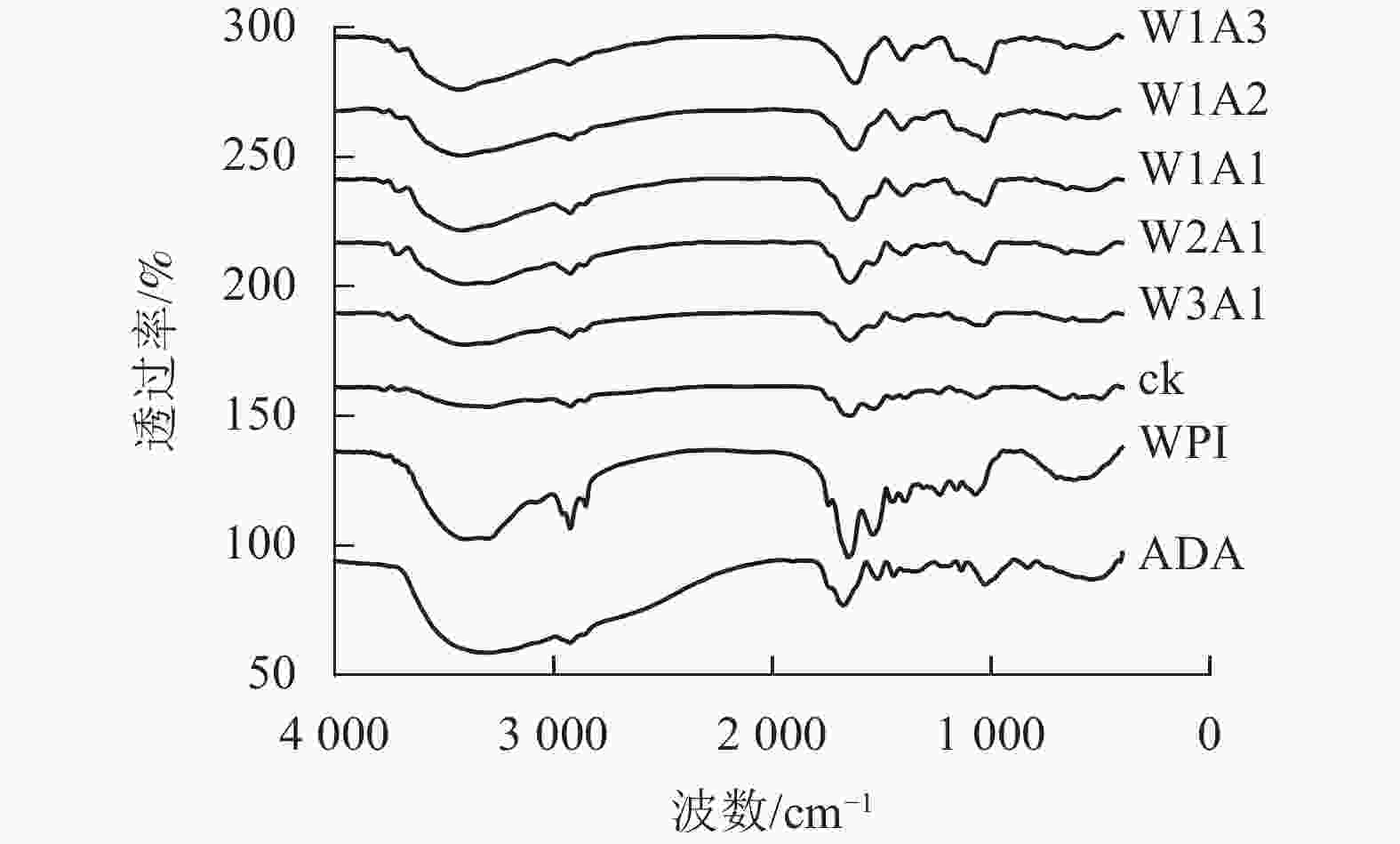
如图3所示:美拉德反应导致处理组的FTIR图谱发生条带的位置和强度的变化。WPI的特征峰在1 649、1 541和1 392 cm−1出现,分别对应的是C=O拉伸振动,C—N拉伸振动和N—H弯曲振动(酰胺Ⅰ),N—H弯曲振动和C—N拉伸振动(酰胺Ⅱ)以及C—N拉伸振动和N—H弯曲振动(酰胺Ⅲ)。随着WPI与ADA的比例从0∶1到1∶3,处理组的FTIR图谱表现出不同程度的蓝移或者峰强的改变。WPI的特征峰从1 649 cm−1移至1 621 cm−1,从1 541 cm−1移至1 534 cm−1(W1A1),该峰的强度逐渐变弱直至消失,位于酰胺Ⅲ带的特征峰从1 392 cm−1移至1 409 cm−1,并且该峰强度逐渐增强。这是美拉德反应诱导的蛋白结构变化所导致的结果。
-
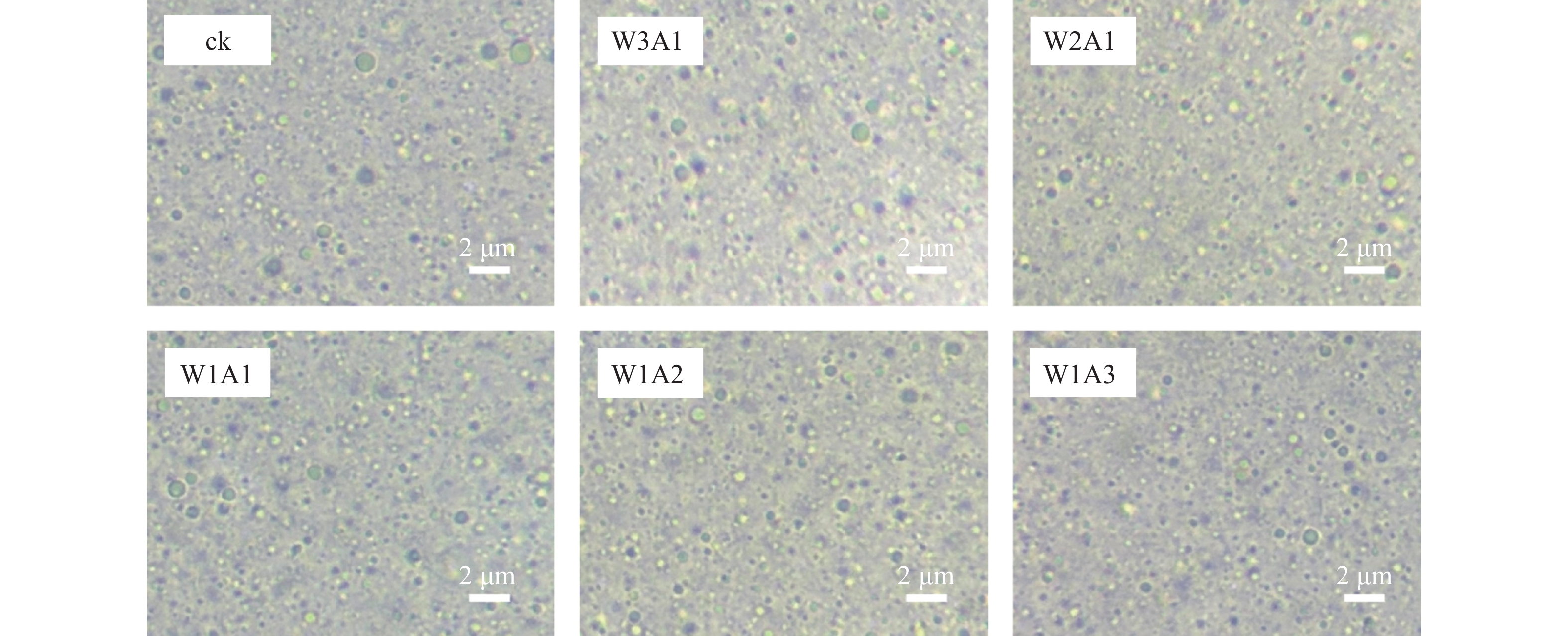
如表1和图4所示:与ck相比,处理组的粒径从415.4 nm降低到325.9 nm,PDI从0.356降低到0.215,这表明共聚物能够形成更小更均匀的液滴。乳液Zeta电位的绝对值反映了其静电相互作用的强度,这对保持乳液稳定性至关重要。处理组比ck有着更高的电位绝对值,ck的电位为−25.0 mV,处理组的Zeta电位绝对值为39.7~43.9 mV,说明处理组的稳定性较好。
处理 粒径/nm PDI值 Zeta电位/mV ck 415.4 ± 3.0 d 0.356 ± 0.001 d −25.0 ± 0.4 d W3A1 369.4 ± 2.5 c 0.354 ± 0.042 d −39.7 ± 1.4 c W2A1 370.5 ± 4.9 c 0.323 ± 0.004 cd −41.4 ± 1.0 bc W1A1 366.7 ± 4.3 c 0.286 ± 0.022 bc −42.1 ± 0.0 abc W1A2 335.1 ± 0.8 b 0.258 ± 0.014 ab −43.9 ± 1.2 ab W1A3 325.9 ± 2.0 a 0.215 ± 0.009 a −44.2 ± 0.9 a 说明:不同小写字母表示同一指标不同处理间差异显著(P<0.05)。 Table 1. Particle size, PDI and Zeta Potential of WPI-ADA emulsions
-
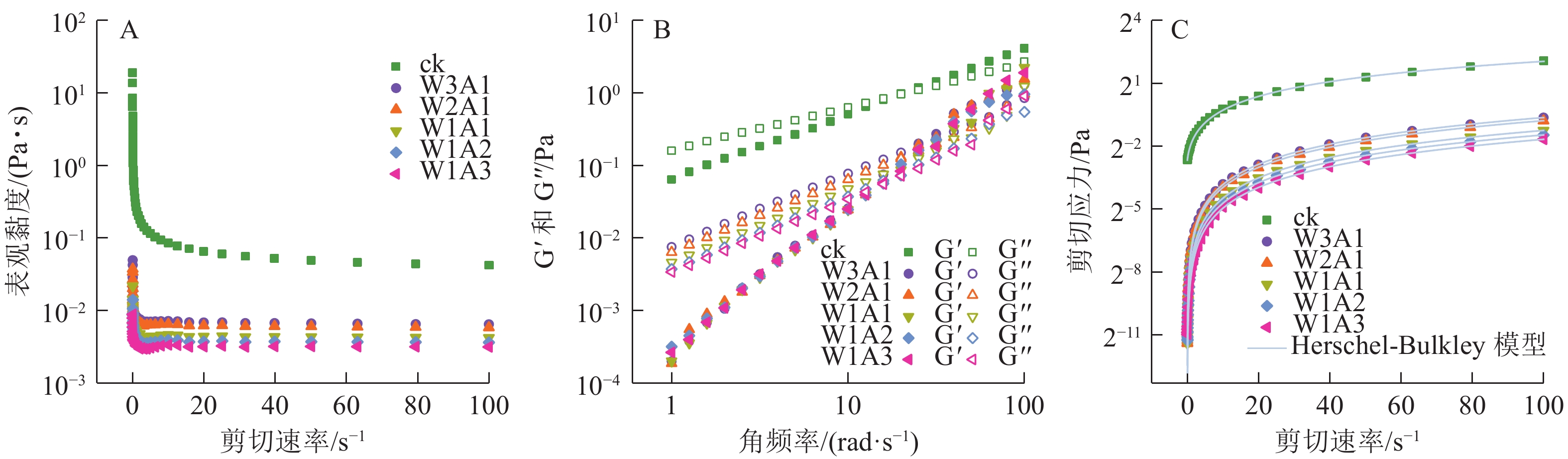
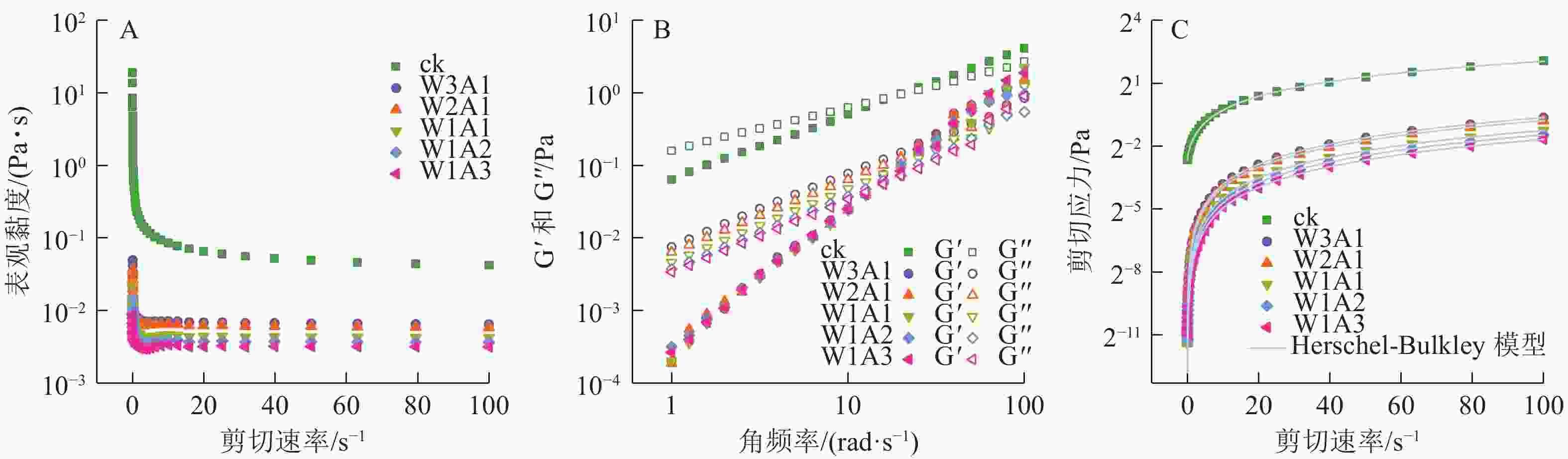
如图5A所示:在测试的剪切速率范围内,所有乳液都表现出非牛顿假塑性流体的剪切稀化行为。这种行为可以通过剪切过程中缠绕的聚合物网络的断裂来解释,其中断裂的分子间纠缠速率大于重组的分子间纠缠速率,导致分子间流动阻力更小,使乳液的表观黏度降低。储能模量G′反映样品的固体状行为,损耗模量G″反映样品的液体状行为。如图5B所示:在所有样品中观察到溶液-凝胶过渡点,证实了乳液到凝胶的转变。图5C显示了测量的剪切应力与剪切速率数据与Herschel-Bulkley模型具有良好的相关性 (R2>0.99)。剪切速率与剪切应力显示出非线性关系,并且所有乳液的n<1,证实了乳液为非牛顿流体,且n值随着WPI的增加从0.988 1降低到了0.774 8,这表明与牛顿流体的行为偏差逐渐增加。ADA比例的增加导致σ0值从0.154 8 Pa (ck)降低到1.713 2×10−5 Pa (W1A3)。
-
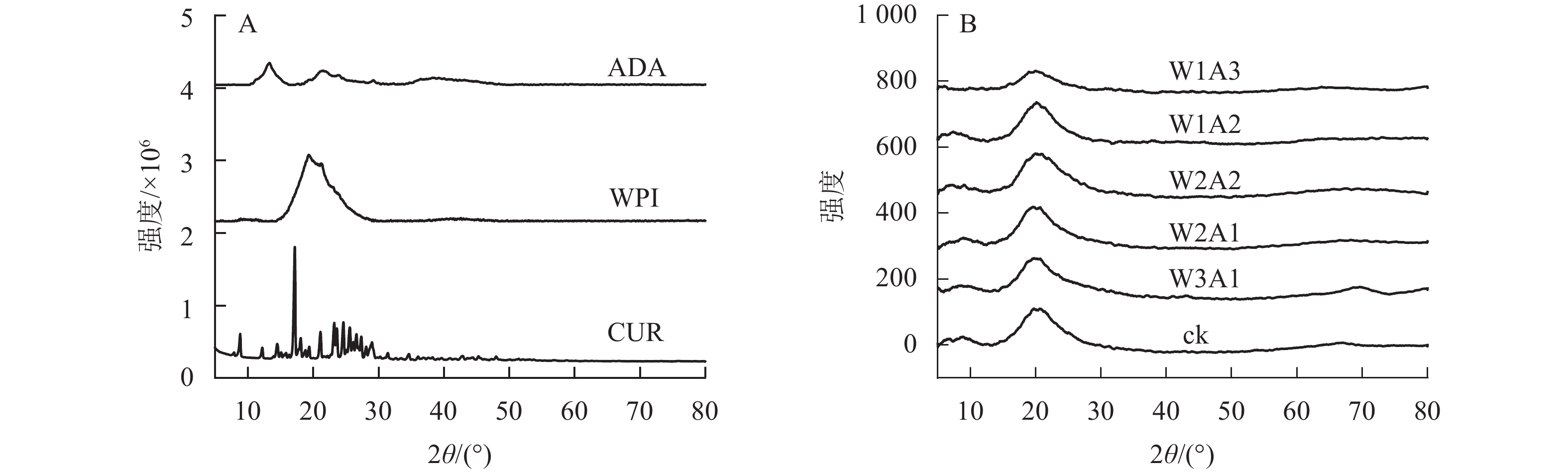
由图6可知:WPI在20°出现衍射峰,ADA在14°和24°出现宽峰,峰形宽阔而柔和,这意味着WPI和ADA具有无定形结构。CUR的衍射图出现尖锐而强烈的结晶峰(8.8°、12.2°、17.2°、21.1°和24.6°),但是在微胶囊中CUR的特征峰(图6B)消失。
-
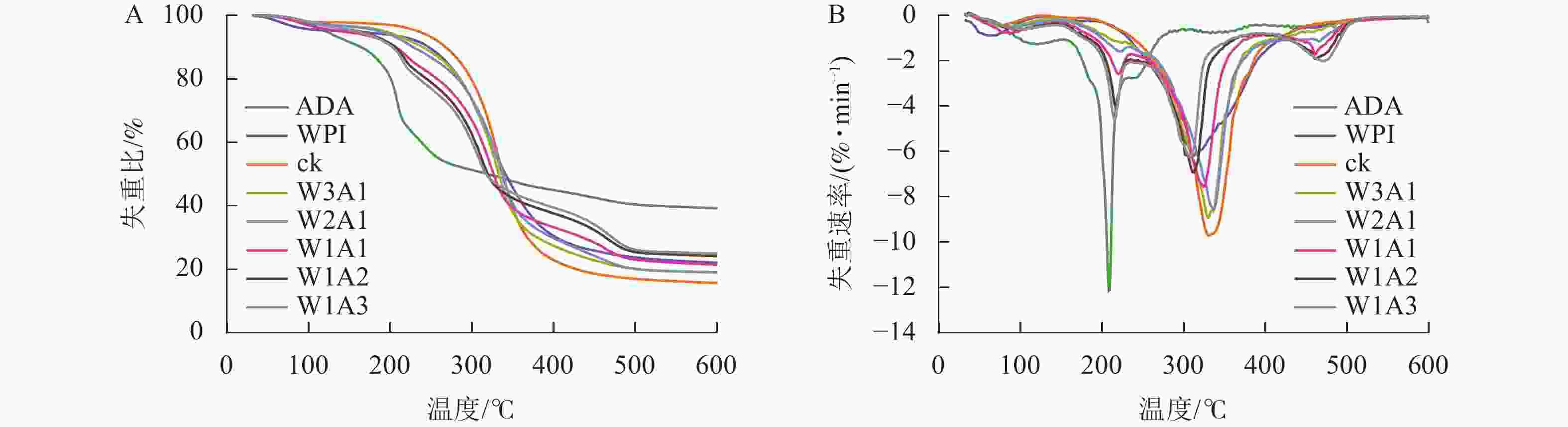
微胶囊的失重曲线由3个阶段组成(图7):第1阶段(0~200 ℃)对应游离水和结合水的损失,这一阶段的质量损失为2.13%~5.15%;微胶囊在第2阶段(200~400 ℃)显示出较快的质量损失,可归因于蛋白的分解和多糖的解聚;第3阶段的质量损失是多糖和蛋白的碳化所导致的。所有样品在第2阶段损失了大部分的质量,其中,ck的质量损失最大,达82.13%,处理组的失重比为55.62%~77.71%。ck在600 ℃时的残渣残余量是15.71%,处理组的残渣残余量是19.05%~24.99%。
-
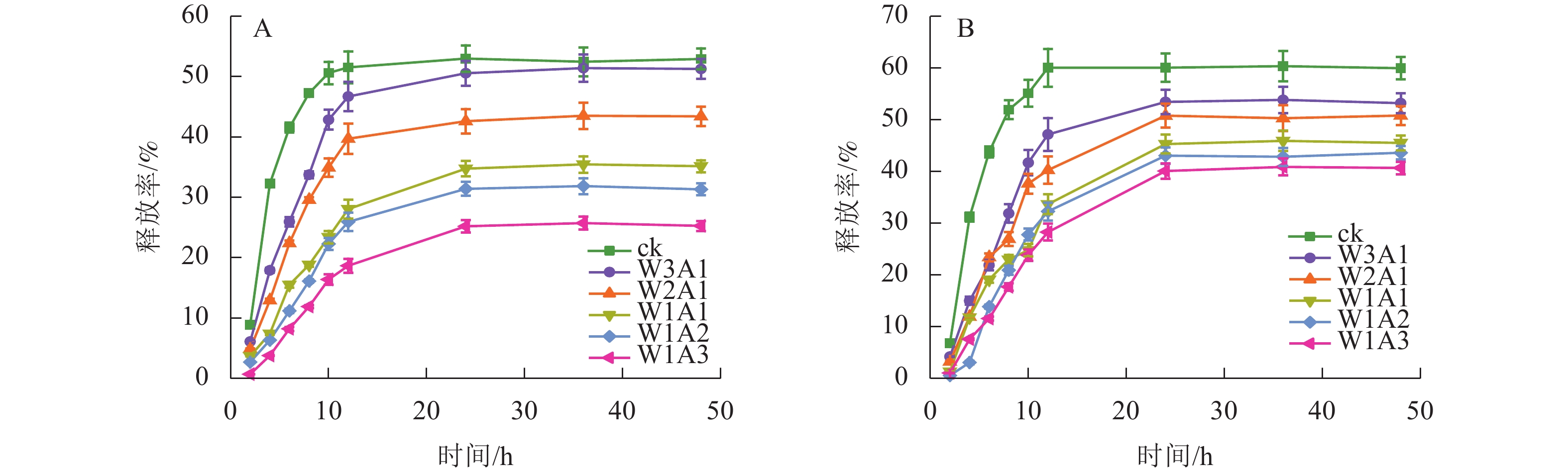
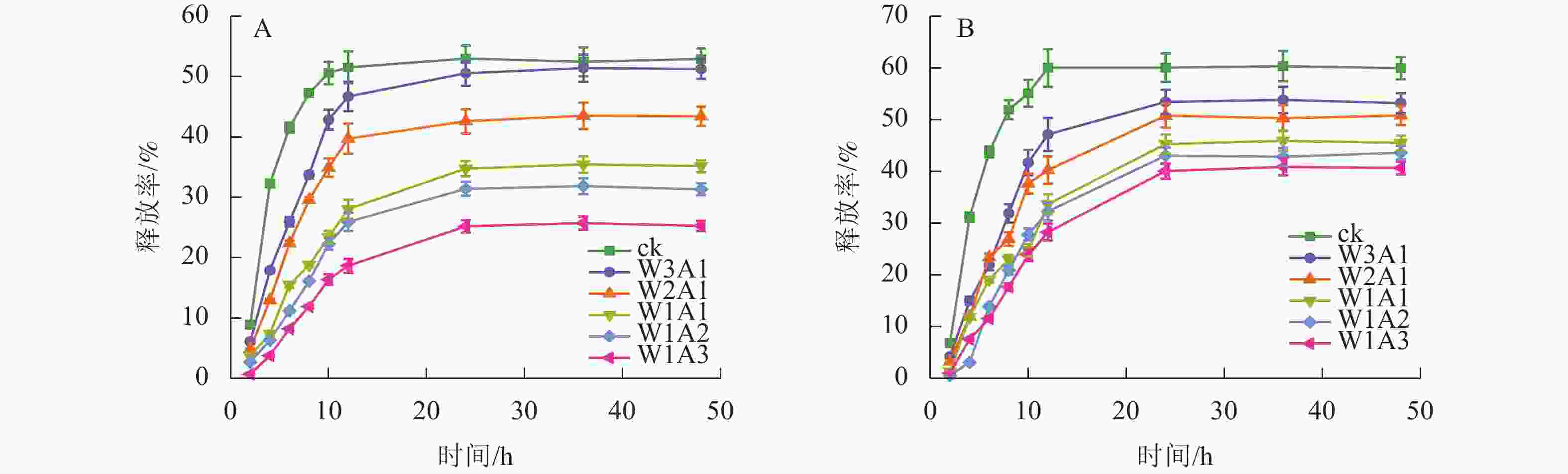
ck、W3A1、W2A1、W2A2、W1A2和W1A3微胶囊的包埋率分别为83.9%、87.8%、91.0%、91.5%、93.4%和95.4%。由图8可知:所有微胶囊表现出相似的释放曲线,首先表现出较高的释放速率,然后缓慢释放至平衡阶段。与ck相比,在模拟胃液(图8A)和模拟肠液(图8B)中,处理组中的CUR释放更缓慢,且释放时间从12 h延长至24 h。CUR在模拟肠液中的释放速率大于模拟胃液,表明处理组能够保护CUR免受模拟胃液环境降解,并在模拟肠液条件下释放,有望靶向肠道,促进肠道细胞摄取,从而提高CUR的生物利用度。
-
本研究结果表明:除了W2A1处理组在420 nm处的吸光度外,其他处理组在294、304和420 nm处的吸光度均比ck显著增加(P<0.05),表明中间物质的形成,其中一部分进一步聚合产生棕色物质[15]。同时,处理组在294 nm处的吸光度均高于在304和420 nm处的吸光度。这表明处理组早期-中期美拉德产物的含量很高,这与DELGADO-ANDRADE等[16]的研究一致。FTIR图谱显示:WPI酰胺Ⅰ带特征峰发生蓝移,该峰的强度逐渐变弱直至消失;位于酰胺Ⅲ带的特征峰发生红移,并且该峰强度逐渐增强,这是美拉德反应诱导的蛋白结构变化所导致的结果。此外,处理组的1 000~1 160 cm−1(C—O拉伸)和3 200~3 600 cm−1(O—H拉伸)的吸收强度增加,这是糖化蛋白形成的典型特征[9]。
乳液粒径、PDI和Zeta电位显示:与ck相比,处理组形成了更小更均匀的液滴。这可能是由于美拉德反应使蛋白变性,导致表面疏水性和柔韧性增加,共聚物到界面的迁移率增加,界面张力降低,快速吸附在液滴表面形成黏弹性厚层,从而获得更小、分布更均匀的油滴[17]。处理组的电位绝对值高于ck,这与JIANG等[18]研究一致。由WPI-羧甲基纤维素共聚物稳定的乳液的Zeta电位显著高于由WPI稳定的乳液,这可能是美拉德反应使NH3+水平降低,而共聚物提供的阴离子羧基浓度增加所导致的。流变特性显示:糖基化减少了水相中ADA聚集体的形成,降低了流动阻力和乳液水相中的黏度[19]。与ck相比,处理组的G′值较低,这可能与偶联物难以形成凝胶网络的二硫键有关[20]。同样,CONSOLI等[21]发现酪蛋白酸钠-玉米淀粉水解物偶联物乳液的黏度低于对照乳液(酪蛋白酸钠-玉米淀粉水解物加热时间为0 h)的黏度。也有研究表明糖基化产生的空间位阻通过二硫键和疏水键的子间缔合来抑制凝胶状结构的形成[22]。
微胶囊射线衍射图中CUR的特征峰消失,表明CUR以无序的结晶相或无定形的分子分散在微胶囊中[23]。热重分析数据显示:处理组的减重更慢,残余质量保持更高,这证明了WPI与ADA通过美拉德反应增强了样品的热稳定性,这可能与接枝子区域抗断裂的化学键强度增加有关[24]。包埋率与接枝度结果表现出相似的趋势,表明较高的接枝度可能对微胶囊包埋率产生积极影响,这与JIA等[6]的研究结果一致。体外模拟消化实验显示:微胶囊初始的较高释放速率可归因于微胶囊表面或微胶囊网络中弱结合的CUR的释放,随后的缓慢释放阶段可归因于壁材降解导致的CUR释放[25]。在模拟胃液和模拟肠液中,处理组中CUR缓慢持续释放,这一现象是由于美拉德反应制备的共聚物增强了WPI的稳定性并降低了胃蛋白酶对CUR的敏感性,导致CUR释放速率降低和释放量减少[26]。此外,WPI和ADA之间的共价连接导致静电斥力不能充分解离WPI和ADA,但能够使复合结构松散,使胰酶接触并逐渐水解WPI,因此WPI和ADA的糖基化能够延缓模拟肠液中CUR的释放,但不能完全阻止WPI被胰酶消化,从而最终释放CUR[27]。
-
在共价相互作用下,WPI-ADA 共聚物能够改善微胶囊的理化性质和姜黄素的释放特性。褐变强度、接枝度、傅里叶红外光谱分析证实了美拉德反应的发生,随着WPI∶ADA质量比从1∶3到3∶1,共聚物的接枝度从15.5%增加到56.1%,并且在美拉德反应过程中有高分子量物质的形成。随着共聚物中ADA比例的增加,乳液粒径从415.4 nm减小到325.9 nm。共聚物制备的乳液表现出假塑性流体的特征,与 Herschel-Bulkley 模型有良好的相关性。WPI-ADA 共聚物微胶囊具有较高的包封率(87.8%~95.4%),WPI-ADA 共聚物的应用也增强了微胶囊的热稳定性。将微胶囊暴露于模拟胃肠道条件后,姜黄素在模拟胃液和模拟肠液中的释放时间都由12 h延长到24 h。本研究为开发基于蛋白-多糖美拉德产物修饰微胶囊体系和疏水性活性物质的递送提供了参考。
Fabrication and controlled release characteristics of whey protein-alginate dialdehyde microcapsule
doi: 10.11833/j.issn.2095-0756.20240243
- Received Date: 2024-03-19
- Accepted Date: 2024-07-12
- Rev Recd Date: 2024-07-05
- Available Online: 2024-09-06
- Publish Date: 2024-11-20
-
Key words:
- curcumin /
- protein-polysaccharide complex /
- intermolecular interaction /
- controlled release properties
Abstract:
| Citation: | YANG Ye, WU Shaping, WANG Kaijun, et al. Fabrication and controlled release characteristics of whey protein-alginate dialdehyde microcapsule[J]. Journal of Zhejiang A&F University, 2024, 41(6): 1274-1282. DOI: 10.11833/j.issn.2095-0756.20240243 |










 DownLoad:
DownLoad: