-
麸皮是小麦Triticum aestivum加工工业的副产物,约占小麦籽粒质量的26%。小麦麸皮中含有丰富的阿拉伯木聚糖,占小麦麸皮干质量的18.8%~21.4%[1]。虽然小麦麸皮在食品工业中有应用,但是大部分小麦麸皮被用作动物饲料或酿造原料。此外,小麦麸皮未被充分利用及产生的废物也会造成环境问题。
全球范围内,中国的核桃Juglans regia种植量稳居首位。随着核桃产业的迅猛发展,核桃榨油副产物——核桃饼粕数量急剧增加。这些副产品不仅对环境造成了负担,而且在一定程度上阻碍了核桃类产品的进一步开发和利用[2]。为了突破这一瓶颈,利用核桃饼粕生产核桃肽(WP),是对核桃饼粕的综合利用、实现核桃饼粕的价值增值、开辟核桃饼粕的应用途径,成为核桃加工新的利润增长点,但是制备核桃活性多肽在提高核桃粕工业价值的同时,也会使生产成本大幅提升[3]。
美拉德反应是还原糖的羰基与氨基酸、肽或蛋白质的氨基之间复杂的非酶褐变反应[4−5],具有绿色、高效、温和等优点,被认为是修饰天然蛋白质结构和功能特性的最有前途的方法之一[6],可以改善蛋白或肽的乳化性、发泡性;增加溶解度、抗菌活性、抗氧化性、热稳定性;改善食物质地、风味等[7]。目前大多研究集中在大豆肽、鱼肽等肽类与碳水化合物的美拉德反应方面,但有关核桃肽的研究较少。因此,本研究以核桃肽、阿拉伯木聚糖(AX)和低聚木糖(XOS)为原料,研究阿拉伯木聚糖、核桃肽及其美拉德反应产物理化性质的变化以及糖基化对核桃肽功能特性的影响,为核桃肽功能性食品的开发提供参考。
-
材料主要包括麸皮(徐州宏昌)、核桃粕(长春)、低聚木糖(上海源叶)。
-
基于前期研究结果[8],核桃肽通过酶促水解制备。按质量体积比为9∶100的料液比在核桃粉中加入去离子水,进行15 min超声后,在90 ℃条件下处理15 min,冷却至50 ℃,用1 mol·L−1的氢氧化钠调至pH 9.0,加入7 000 ×16.67 nkat·g−1的碱性蛋白酶,在50 ℃下搅拌2 h,沸水浴15 min以灭活酶,通过4 500 r·min−1离心15 min分离水解物的可溶性和不溶性部分,将核桃肽的可溶性部分冷冻干燥备用。
基于前期研究结果[9],阿拉伯木聚糖通过碱提醇沉法制备。按质量体积比为1∶20的料液比在麸皮中加入去离子水,加入耐高温的α-淀粉酶,在95 ℃、pH 6.0条件下持续搅拌3 h。冷却至室温后加入糖化酶,然后在37 ℃、pH 4.5条件下搅拌过夜,离心得到沉淀,烘干。沉淀按质量体积比为1∶20的料液比加入0.2 mol·L−1 的氢氧化钠溶液,常温搅拌提取12 h。离心并收集上清液,上清液pH调至4.0。上清液中加入无水乙醇,离心得到粗多糖沉淀,加入适量去离子水复溶沉淀,加入蛋白酶持续搅拌4 h,然后灭活10 min并再次离心去除蛋白。取上清液进行透析后,经离心除去不溶性多糖,收集上清液进行旋蒸浓缩后冻干,得到阿拉伯木聚糖样品,保存备用。
将1 g核桃肽、2 g阿拉伯木聚糖或低聚木糖溶于100 mL去离子水中,之后将样品进行冻干,收集冻干粉末,放置于预热到80 ℃的干燥器中,利用饱和溴化钾溶液控制体系内相对湿度为79%,反应5 h得到核桃肽的糖基化产物。将产物加蒸馏水溶解,进行透析和离心,以去除游离氨基酸和不溶性成分。
-
(1)蛋白质量分数测定参考GB
5009.5 —2016凯氏定氮法[10],根据标准曲线,采用BCA试剂盒法进行测定。(2)总糖质量分数测定使用苯酚-硫酸法[11],根据标准曲线,测定波长490 nm处的吸光度。
(3)溶解度测定:称取10 mg的阿拉伯木聚糖、核桃肽或糖基化产物于2 mL离心管中,加入1 mL去离子水,在室温下混合90 min(每30 min摇匀5 s),9 000 r·min−1离心10 min,去除上清液,将离心管及内部沉淀真空冷冻干燥后称量[12]。每份样品平行测定3次。
(4)根据标准曲线,相对分子量测定采用高效液相色谱仪(HPLC)。①多糖分子量测定[13]:以不同分子量的葡聚糖(分子量分别为10、40、70、500、2 000 kDa)作为标准品。色谱条件如下:Ultrahydrogel Column Linear凝胶色谱柱(10 μm,7.8 mm×300.0 mm),柱温为40 ℃,流速为0.6 mL·min−1,流动相为0.1 mol·L−1的硝酸钠溶液,进样量为20 μL,运行时间为25 min。②肽分子量测定[14]:以不同分子量的物质作为标准品。色谱条件如下:TSKgel 2000 SWXL色谱柱(5 μm,7.8 mm×300.0 mm),柱温为30 ℃,流速为0.5 mL·min−1,流动相为V(乙腈)∶V(水)∶V(三氟乙酸)=450∶550∶1,进样量为20 μL,运行时间为40 min。
(5)单糖组成成分的测定采用气相色谱法测定阿拉伯木聚糖的单糖组成,参考WANG等[15]的方法,并稍作修改。以标准单糖(鼠李糖、阿拉伯糖、木糖、甘露糖、半乳糖、葡萄糖)做混合标准单糖简称混标。称取5 mg阿拉伯木聚糖或标准品于三氟乙酸中,120 ℃水解4 h后用硼氢化钠还原,再经V(乙酸酐) ∶V(吡啶)=1∶1进行乙酰化,过0.22 μm有机滤膜待测。测定参数如下:HP-5MS色谱柱,进样口温度为250 ℃,进样量为2 μL,流速为1.0 mL·min−1,氮气载气分流体积比为1∶20。
-
参考文献[16]的方法测定接枝度。精准称取40 mg的邻苯二甲醛(OPA)溶于1 mL的甲醇中,与质量浓度为20%的十二烷基硫酸钠(SDS) 2.5 mL、0.1 mol·L−1的四硼酸钠25.0 mL以及100.0 μL的β-巯基乙醇混合,然后用蒸馏水定容至50.0 mL,得到OPA试剂,现配现用。测定时,在200 μL样品溶液加入4 mL的OPA试剂,室温避光保存5 min。然后测定波长340 nm处的吸光度。以OPA试剂中加入200 μL蒸馏水的混合物作为空白。同时,用L-亮氨酸标准溶液(0~0.5 g·L−1)校准曲线计算游离氨基质量浓度。
-
糖基化产物用蒸馏水分别稀释至0.05和0.50 g·L−1,以蒸馏水作为空白对照,分别在波长294和420 nm处测定吸光度,重复3次取平均值。波长294 nm处的吸光度表示美拉德反应的中间产物含量;波长420 nm处的吸光度表示反应过程中最终产物含量,例如类黑素[17]。
-
粒径是影响肽功能特性的重要因素之一,因此分析糖基化改性前后核桃肽的平均粒径。使用Malvern Mastersizer 2000型激光粒度仪和Hydro 2000S取样装置测定样品的平均粒径。使用乙醇作为分散剂材料来读取样品,每个样品平行测量3次,最后取平均值。Zeta电位是表征分散系稳定性的重要指标之一。使用Malvern Zetasizer Nano-Z设备测量样品的Zeta电位,以2 g·L−1的质量浓度制备样品溶液,在室温下测定溶液的Zeta电位,每个样品平行测量3次,最后取平均值[18]。
-
将待测样品涂抹于导电胶的表面,喷涂约10 nm的电镀层,在15 kV加速电压下进行电镜扫描,放大500倍[19],观察阿拉伯木聚糖、低聚木糖、核桃肽以及其糖基化产物的微观形态。
-
差示扫描量热法是评估蛋白质、肽等物质热稳定性的常用技术手段,可通过变性温度(Td)和焓值变化(ΔH)来表示稳定性的大小。Td用于描述蛋白的热稳定性,而ΔH则表示克服变性过程中非共价所需的能量。在通氮气条件下,利用差示扫描量热仪对样品的热稳定性进行测定。将5~10 mg样品装入DSC坩埚中,采用铝片进行密封处理。仪器参数设定如下[20]:升温速率为10 ℃·min−1,温度为25~110 ℃,气体流速为50 mL·min−1。以空铝坩埚作为空白对照。
-
在糖基化反应中,游离巯基和二硫键交换在蛋白质交联和聚集中起着重要作用。参考文献[21],准确称取40 mg 5,5-硫硝基苯甲酸溶于10 mL的Tris-甘氨酸缓冲液(0.086 mol·L−1 Tris,0.090 mol·L−1甘氨酸,4.000 mmol·L−1乙二胺四乙酸,pH 8.0)中,配制成Ellman试剂。用Tris-HCl缓冲液将样品配制成质量浓度为6 g·L−1的溶液,在10 000 r·min−1条件下离心10 min。吸取2 mL离心后的样品,加入 80 μL Ellman试剂混均,反应5 min,测定波长412 nm处的吸光度,即为游离巯基质量摩尔浓度。吸取2 mL样品上清液,加入β-巯基乙醇溶液(体积分数为0.2%)混匀,反应2 h后加入4 mL三氯乙酸溶液(质量浓度为12%),反应1 h,10 000 r·min−1条件下离心10 min,再用三氯乙酸溶液重复洗涤沉淀,将沉淀用3 mL Tris-HCl缓冲液(pH 8.0)溶解,测定波长412 nm处的吸光度,即为总巯基质量摩尔浓度。
-
2, 2′-联氮-双-3-乙基苯并噻唑啉-6-磺酸(ABTS)自由基清除能力测定参考LIU等[22]的方法,2, 2-二苯基-1-苦味肼(DPPH)自由基清除能力测定参考SUN等[23]的方法,羟自由基清除能力测定参考ZHANG等[24]的方法,氧自由基吸收能力(ORAC)测定参考ZHANG等[25]的方法。
-
采用SPSS 26.0进行t检验和方差分析,结果以平均值±标准差表示,显著水平为0.05。利用Origin 2022作图。
-
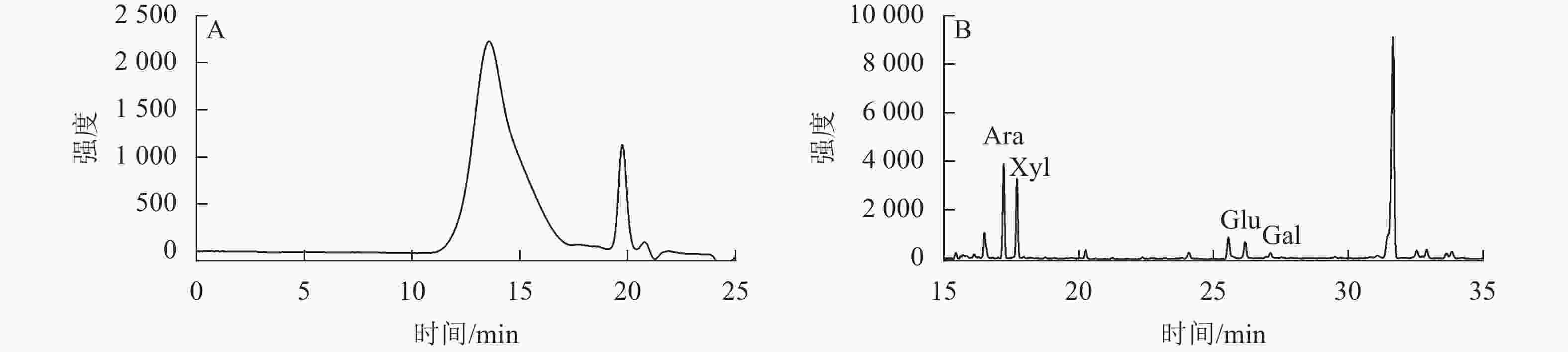
通过高效液相色谱图(图1A)可知:信号峰出现在13.56 min时,主要为高相对分子量组分。通过气相色谱图(图1B)可知:混标从左至右出峰顺序为阿拉伯糖(Ara)、木糖(Xyl)、葡萄糖(Glu)、半乳糖(Gal),发现阿拉伯木聚糖主要由阿拉伯糖、木糖、葡萄糖和半乳糖组成。从表1可见:阿拉伯木聚糖的蛋白和总糖质量分数分别为0.73%和81.39%,相对分子量为413.45 kDa,阿拉伯糖和木糖的质量分数之和约占整体的90%。
样品 相对分子量/kDa 总糖质量分数/% 蛋白质量分数/% 溶解度/% 各单糖质量分数/% 阿拉伯糖 木糖 葡萄糖 半乳糖 阿拉伯木聚糖 413.45±1.98 81.39±2.05 0.73±0.11 72.00±2.65 61.52±2.10 27.27±2.48 8.48±0.45 2.73±0.07 Table 1. Physical and chemical properties of arabinoxylan
-
为确定核桃肽-阿拉伯木聚糖的反应产物(WP-AX)和核桃肽-低聚木糖的反应产物(WP-XOS)的美拉德反应程度,对接枝度和褐变程度进行了分析。如图2A所示:WP-AX在美拉德反应前后的游离氨基质量浓度分别为0.10和0.09 g·L−1,WP-XOS在美拉德反应前后的游离氨基质量浓度分别为0.09和0.04 g·L−1,表明游离氨基质量浓度在美拉德反应后有所降低。经计算,WP-AX、WP-XOS的接枝度分别为12.31%±3.52%和54.46%±3.26%,表明核桃肽中分别有12.31%和54.46%的游离氨基在美拉德反应后分别与阿拉伯木聚糖和低聚木糖结合。

Figure 2. Effect of Maillard reaction on free amino groups (A), intermediate product formation (B) and browning degree (C)
D(294)和D(420)能够分别反映美拉德反应中无色中间体化合物生成量和糖基化产物褐变程度[26]。吸光度越低,褐变程度越小,说明糖基化反应程度越小,反之越大。如图2B所示:WP-AX和WP-XOS的中间产物显著上升(P<0.05),而且WP-XOS比WP-AX产生了更多的中间产物——还原酮和高级产物类黑素。如图2C示:WP-XOS的褐变程度具有显著差异(P<0.05),且WP-XOS的褐变程度明显高于WP-AX。
-
核桃肽在美拉德反应后的分子量分布如图3所示。图3A为核桃肽分子量的HPLC洗脱,核桃肽中存在多种大小不一的多肽片段,其分子量主要分布在3 000 Da以下,其中小于2 000 Da的占64.35%,小于1 000 Da的占37.28%,表明核桃肽富含由2~10个氨基酸残基组成的寡肽。图3B为WP-AX分子量的HPLC洗脱,WP-AX小于1 000 Da的占43.59%,相比于核桃肽,WP-AX寡肽部分的含量有一定程度的提高。图3C为WP-XOS分子量的HPLC洗脱,而WP-XOS小于1 000 Da的只占26.30%,但分子量大于3 000 Da部分占40.20%,相对于核桃肽有明显的提高。这2种不同的情况可能是因为肽在美拉德反应过程中存在2种转化方式:①肽直接参与美拉德反应生成产物;②通过热降解形成小分子肽,这些小分子肽再参与美拉德反应生成产物,说明美拉德反应的最佳肽段可能存在原料差异性。
-
如表2所示:与核桃肽相比,引入碳水化合物进行糖基化后,WP-AX和WP-XOS的游离巯基和总巯基显著增加(P<0.05)。与WP-AX相比,WP-XOS的二硫键质量摩尔浓度更高(P<0.05)。因此,可推断出WP-XOS的结构更稳定。
样品 总糖质量分数/% 蛋白质量分数/% 溶解度/% 总巯基/(μmol·g−1) 游离巯基/(μmol·g−1) 二硫键/(μmol·g−1) WP 9.96±0.24 c 77.28±0.10 a 99.68±0.55 a 7.49±0.01 c 5.66±0.25 c 0.91±0.12 b WP-AX 36.27±0.77 a 23.82±0.30 c 94.75±1.19 b 24.79±0.02 a 23.01±0.28 a 0.89±0.13 b WP-XOS 19.18±0.75 b 50.15±1.11 b 96.35±1.56 b 10.97±0.03 b 7.18±0.23 b 1.90±0.10 a 说明:WP为核桃肽;WP-AX为核桃肽和阿拉伯木聚糖的美拉德反应产物;WP-XOS为核桃肽和低聚木糖的美拉德反应产物。同列不同小写字母表示不同样品间差异显著(P<0.05)。 Table 2. Analysis of thiol and disulfide bond content in WP, WP-AX, and WP-XOS
-
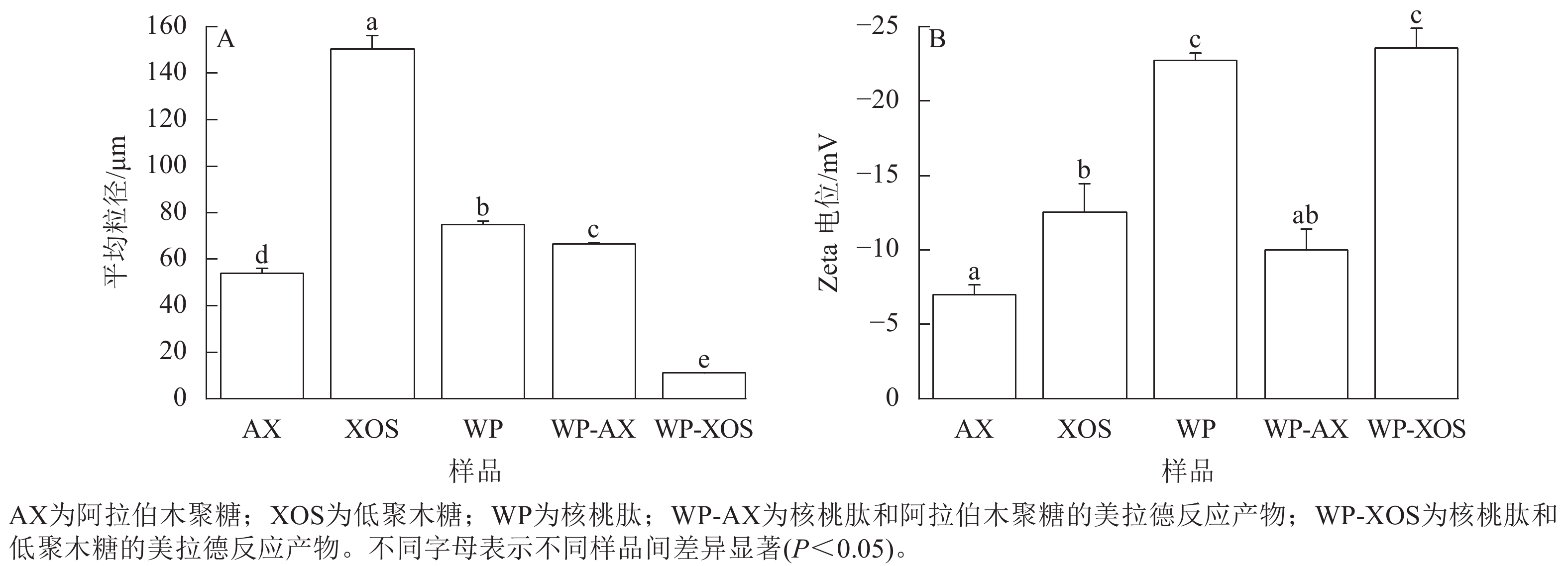
由图4A可知:未处理的核桃肽颗粒相对较大,经过糖基化反应后,WP-AX和WP-XOS的粒径显著降低(P<0.05),分别为66.45和10.93 μm,这可能是核桃肽与碳水化合物共价结合后,其结构发生了变化,从而使原有的片状结构被破坏,形成粒径更小的体系。

Figure 4. Particle size plots (A) and Zeta potential plots (B) before and after the Maillard reaction of different samples
由图4B可知:WP-AX的Zeta电位绝对值显著低于WP-XOS (P<0.05),这可能是引入的阿拉伯木聚糖的Zeta电位绝对值显著低于低聚木糖(P<0.05),说明WP-XOS溶液的稳定性比WP-AX好。
-
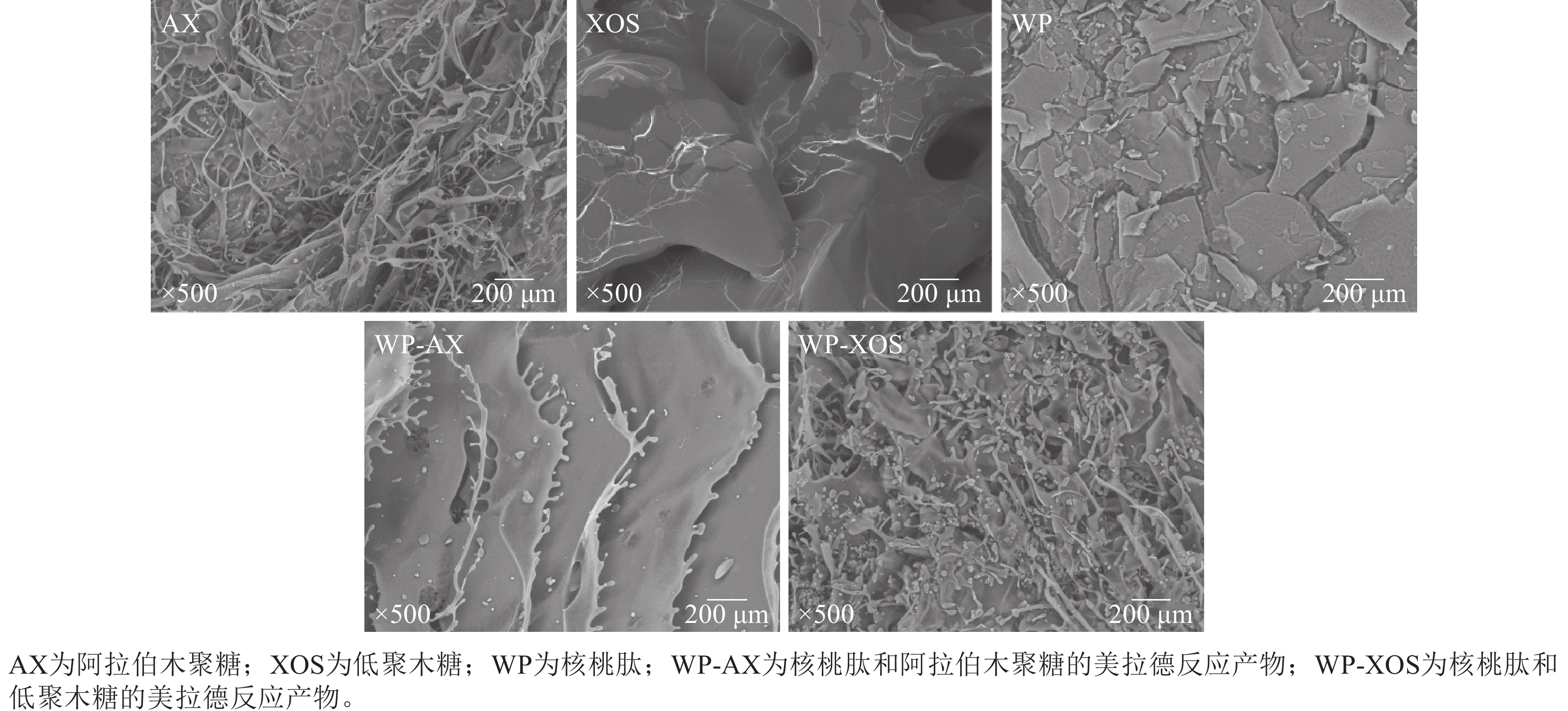
由图5所示:阿拉伯木聚糖呈现丝状的纤维结构,没有规律性;低聚木糖表面光滑,呈相互连接的状态;核桃肽呈现不同大小的片状结构。经过糖基化反应后,WP-AX和WP-XOS呈丝状分支结构,这可能是因为核桃肽分别与阿拉伯木聚糖和低聚木糖发生了共价结合,导致核桃肽的结构遭到破坏,分子充分展开,从而使核桃肽从片状结构变为片状带有丝状分支的结构。因为WP-XOS的接枝程度比WP-AX高,所以表现出更多的丝状无序结构。
-
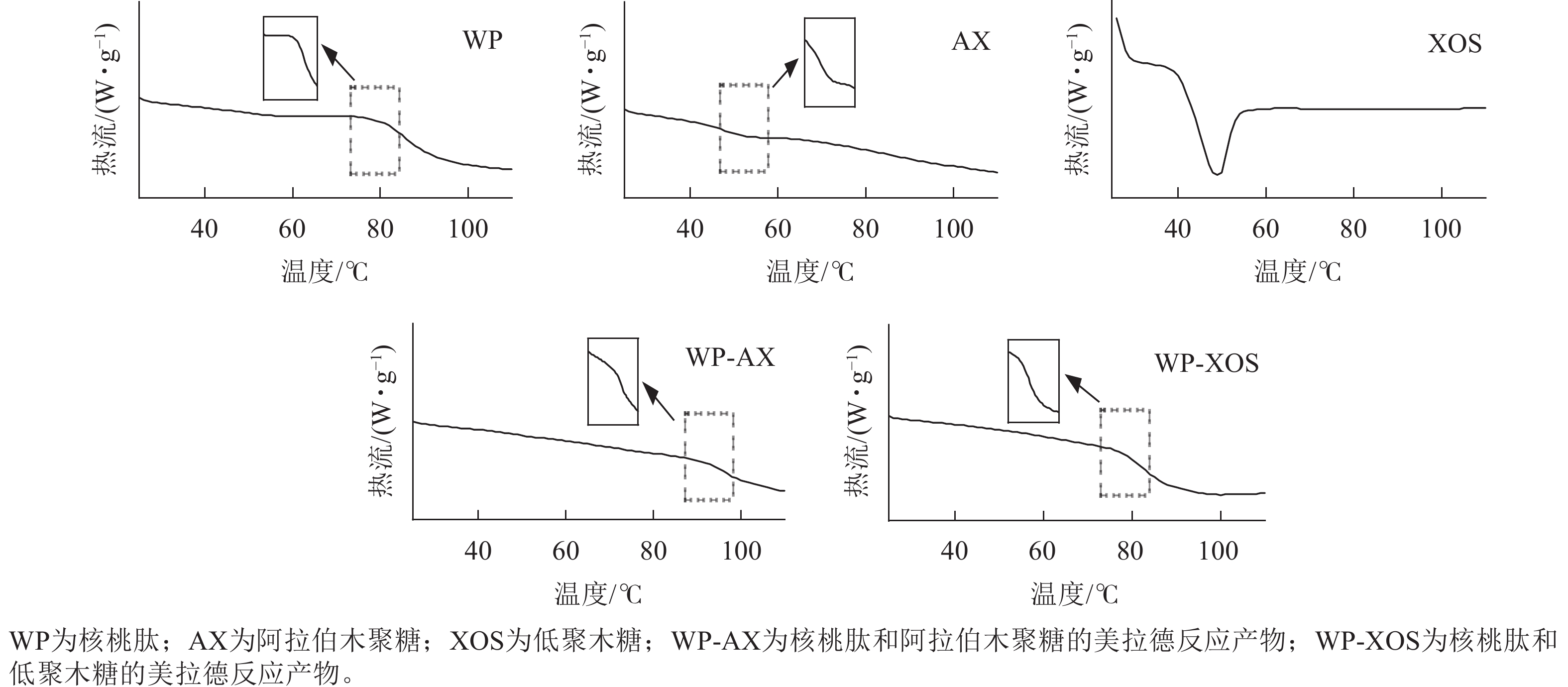
如图6所示:WP、AX和XOS的变性温度(Td)分别为82.18、53.23、48.52 ℃,焓值变化(ΔH)分别为3.11、0.60、9.66 J·g−1,WP-AX和WP-XOS的玻璃化转变温度(Tg)为95.78、83.53 ℃。WP经过糖基化改性后,WP-AX和WP-XOS并未出现热变形峰,而是出现了玻璃化转变峰,热变形峰可能是样品不稳定或者样品结构破坏所导致的,这是不可逆的,但是玻璃化转变是可逆的,说明经糖基化改性后热稳定性增加。
-
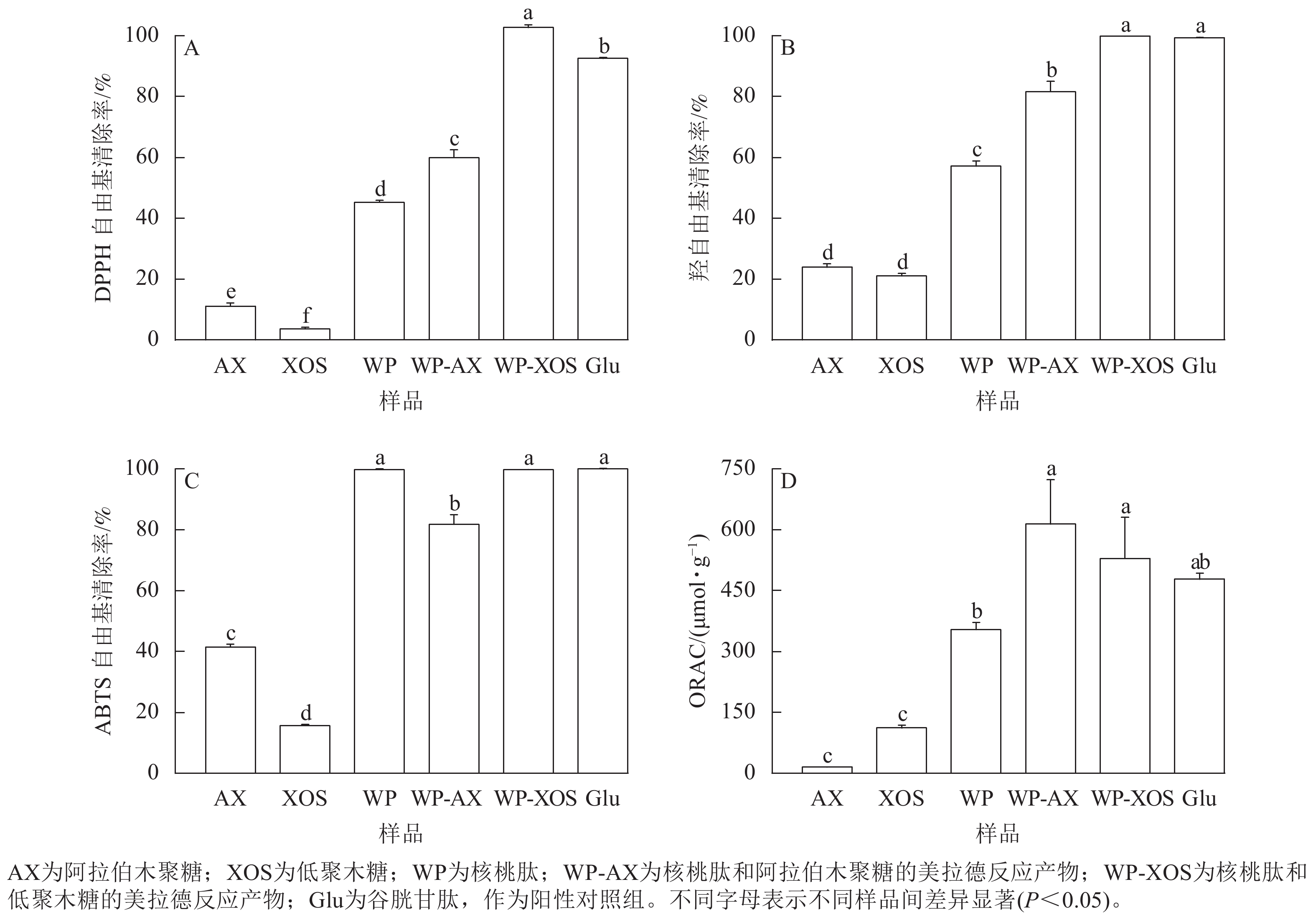
如图7A所示:与核桃肽相比,当阿拉伯木聚糖和低聚木糖分别与核桃肽共价结合时,产物的清除能力显著提升(P<0.05)。与WP-AX相比,WP-XOS表现出了更强的自由基清除能力(P<0.05)。

Figure 7. Comparison of antioxidant activity before and after Maillard reaction of different samples
如图7B所示:当阿拉伯木聚糖和低聚木糖分别与核桃肽进行糖基化反应后,其羟自由基清除能力得到了显著提高(P<0.05),同时,WP-XOS表现出了更强的自由基清除能力(P<0.05)。
如图7C所示:由于核桃肽本身的ABTS自由基清除能力已经很高,当阿拉伯木聚糖和低聚木糖分别与核桃肽进行糖基化反应后,WP-AX和WP-XOS并未表现出更强的自由基清除能力。这是因为WP-AX的接枝度较低,引入了清除能力较低的阿拉伯木聚糖,从而导致了WP-AX比核桃肽表现出了更弱的自由基清除能力(P<0.05)。
如图7D所示:与核桃肽相比,经过糖基化后,WP-AX和WP-XOS的氧自由基吸收能力(ORAC)显著提升(P<0.05),其中WP-AX表现出了更强的氧自由基吸收能力(P<0.05)。
-
现阶段大量研究发现:阿拉伯木聚糖具有抗氧化、抗炎和改善记忆等多种生物活性,是蛋白质和多肽等生物活性物质的理想载体[27−29]。低聚木糖生物活性范围较宽,具有抗氧化活性、抗菌、抗感染、抗过敏以及抗炎等特性,能选择性增加细胞活性,进行免疫调节等[30],是一种理想安全的食品原料[31]。此外核桃肽的生物活性潜力巨大,具有抗氧化、降血压、提高免疫活性、抗疲劳和改善记忆等功效[32]。美拉德反应可以提高蛋白质和肽的抗氧化、乳化等性能[33]。
本研究表明:核桃肽2种不同底物的美拉德反应导致了终产物中肽分子量分布的不同变化,说明小分子肽和碳水化合物的交联作用跟大分子肽的热降解作用有关[34]。核桃肽糖基化后,游离巯基和总巯基质量摩尔浓度都显著增加,一方面,可能是由于核桃肽在糖基化反应过程中,空间结构改变,使得嵌入内部的游离巯基暴露,从而质量摩尔浓度增加[35];另一方面,可能是糖基化反应导致二硫键断裂,使二硫键转化成新的巯基[36],游离巯基质量摩尔浓度随着反应的加深而增加,这与现有的研究结果一致[37]。美拉德反应后样品粒径均减小,这是由于核桃肽与碳水化合物的结合改善了肽的热变性,破坏了表面水合层,从而使得粒径下降[38];也有可能在糖基化反应过程中,肽的表面电荷减少,肽和碳水化合物之间的静电相互作用减少,导致粒径减小[39]。有研究表明:糖基化显著降低了肌源性纤维连接蛋白的粒径,表明共价接枝可以有效抑制加热过程中的蛋白质聚集[40]。通过扫描电镜发现糖基化反应后,出现了纤维结构,缀合物的结构变得更加无序,这与已有的研究结果一致[41]。通过差示扫描热分析发现:美拉德反应能提高肽的热稳定性,缀合物在110 ℃内未出现热变形,这可能得益于疏水的相互作用和氢键的增加[42]。有研究表明:蛋白质与碳水化合物经过糖基化后,变性温度都有不同程度的提高,可见,可通过提供碳水化合物来增加蛋白质的热稳定性或三级构象的稳定性[43]。将核桃肽分别与阿拉伯木聚糖和低聚木糖进行美拉德反应,成功提高了核桃肽的抗氧化活性,这可能与非挥发性美拉德中间产物还原酮和高级阶段产物蛋白黑素具有较强的抗氧化活性有关,同时,也与挥发性含氮、硫的杂环化合物等低分子量物质较强的抗氧化性能有关[44−45]。本研究通过美拉德反应制备的糖肽,在降低肽成本的同时,可提高肽的功能特性,可为以糖肽为原料的保健品或功能食品的应用提供数据支持。
-
本研究表明:WP-XOS的接枝度和褐变程度都比WP-AX高。核桃肽美拉德产物的平均粒径降低,负电荷降低,活性巯基质量摩尔浓度增大。扫描电镜结果表明:碳水化合物的加入使肽的片状结构更加松散,呈现丝状分支结构。热分析图谱显示:核桃肽的变性温度为82.18 ℃,其美拉德反应产物并未出现热变形的现象。此外,WP-AX、WP-XOS的DPPH自由基清除率、羟自由基清除率和ORAC都比核桃肽显著提高了;而ABTS自由基清除率由于核桃肽本身就拥有较高的清除能力,所以并未得到提高,反而引入了阿拉伯木聚糖且接枝度低,导致WP-AX的ABTS自由基清除率比核桃肽低。综上所述,糖基化改性是提升核桃肽热稳定性和抗氧化活性的有效途径,可为核桃肽在食品工业生产中的应用提供参考依据。
Effect of glycosylation modification on structure and functional properties of walnut peptides
doi: 10.11833/j.issn.2095-0756.20240621
- Received Date: 2024-11-25
- Accepted Date: 2025-02-17
- Rev Recd Date: 2025-01-23
- Available Online: 2025-04-01
- Publish Date: 2025-04-01
-
Key words:
- Maillard /
- glycosylation /
- walnut peptides /
- functional properties
Abstract:
| Citation: | LIAO Yanchao, LIU Yan, ZHANG Juncheng, et al. Effect of glycosylation modification on structure and functional properties of walnut peptides[J]. Journal of Zhejiang A&F University, 2025, 42(2): 219−229 doi: 10.11833/j.issn.2095-0756.20240621 |













 DownLoad:
DownLoad: