-
菊花Chrysanthemum × morifolium又称女华、鞠华等,是菊科Asteraceae菊属Chrysanthemum多年生宿根花卉,起源于中国,栽培历史悠久,经长期人工选择培育为著名观赏花卉[1]。缨翅目Thysanoptera蓟马Thrips vulgatissimus是危害菊花的优势害虫类群[2],其中西花蓟马Frankliniella occidentalis是危害菊花的主要种类[3]。该虫起源于美国西部,后扩散至欧亚非多个国家和地区[4],属于缨翅目锯尾亚目Terebrantia蓟马科Thripidae花蓟马属Frankliniella,其对蔬菜、果树、花卉等植物造成严重危害[5−6],不仅造成直接取食危害[7],还在菊花生长过程持续传播病毒[8−9],影响菊花的观赏价值[10]。
植物抗虫性通常与其形态特征和化学防御机制密切相关[11−12]。腺毛作为关键表面结构,分为分泌型腺毛和T型腺毛[13]。鉴于西花蓟马的发生危害特点及寄主偏好性[14],其寄主选择研究成为热点[15]。蓟马在花卉上的种群数量从大到小依次为玫瑰Rosa rugosa、非洲菊Gerbera jamesonii、栀子花Gardenia jasminoides[16],对黄花美人蕉Canna indica var. flava和黄花槐Sophora xanthantha的偏好性强于凤尾兰Yucca gloriosa和夹竹桃Nerium oleander[6]。利用品种自身的抗性已成为蓟马综合防治的基本手段[17]。本研究以南京农业大学31种菊属及近缘属野生资源为研究对象,通过离体接种实验,系统分析其叶片腺毛密度与西花蓟马取食偏好性的关系,为培育抗西花蓟马菊花新品种、减少农药使用、促进菊花产业的健康可持续发展提供科学依据。
-
供试寄主材料:菊属及近缘属材料取自南京农业大学基地种质资源圃。供试的31份菊属及近缘属野生资源,均由依托南京农业大学建设的国家南方草本花卉种质资源圃(南京)提供。供试材料见表1。
植物 采集地 植物 采集地 濑户野路菊Chrysanthemum japonense var. debile 日本万叶 菊花脑C. nankingense 中国江苏南京 野菊C. indicum 中国江苏南京 矶菊Ajania pacificum 日本 菱叶菊C. rhombifolium 中国重庆巫山 花矶菊Ajania × marginatum 日本 野菊C. indicum 中国江苏溧水 毛华菊C. vestitum 中国河南伏牛山 野菊C. indicum 中国江苏汤山 大岛野路菊C. crassum 日本沿海 野菊C. indicum 中国陕西太平山 楔叶菊C. naktongense 中国黑龙江伊春 野菊C. indicum 中国陕西化羊御 萨摩野菊C. ornatum 日本广岛 细裂亚菊Ajania przewalskii 中国青海玉树 毛华菊C. vestitum 中国安徽天柱山 野菊C. indicum 中国湖南邵阳 野路菊C. japonense (RG2) 日本 若狭滨菊C. makinoi 日本筑波 野路菊C. japonense (RG8) 日本 岩菊C. zawadskii 日本广岛 花矶菊Ajania × marginatum 日本万叶 盐菊Ajania shiwogiku 日本 野菊C. indicum 日本 川甘亚菊Ajania potaninii 中国四川金川 野菊C. indicum 中国南京紫金山 达摩菊Aster spathulifolius 日本 银背菊C. argyrophyllum 中国陕西天竺山 神农香菊C. lavandulifolium var. aromaticum 中国湖北神农架 芙蓉菊Crossostephium chinense 中国福建 足摺野路菊C. japonense var. ashizuriense 日本筑波 Table 1. List of wild resources from Chrysanthemum and its closely related genera for testing
-
西花蓟马由南京农业大学植物保护学院朱敏老师提供。西花蓟马放置在人工气候室中,温度设定为(25±1) ℃,湿度设定为(50±5)%,光周期为16 h(光照)/8 h (黑暗),提前14 d进行统一继代处理[16],以室内连续饲养3代以上的成虫作为供试虫源。试验前将芸豆Phaseolus vulgari提前取出进行12 h饥饿处理[18]。
-
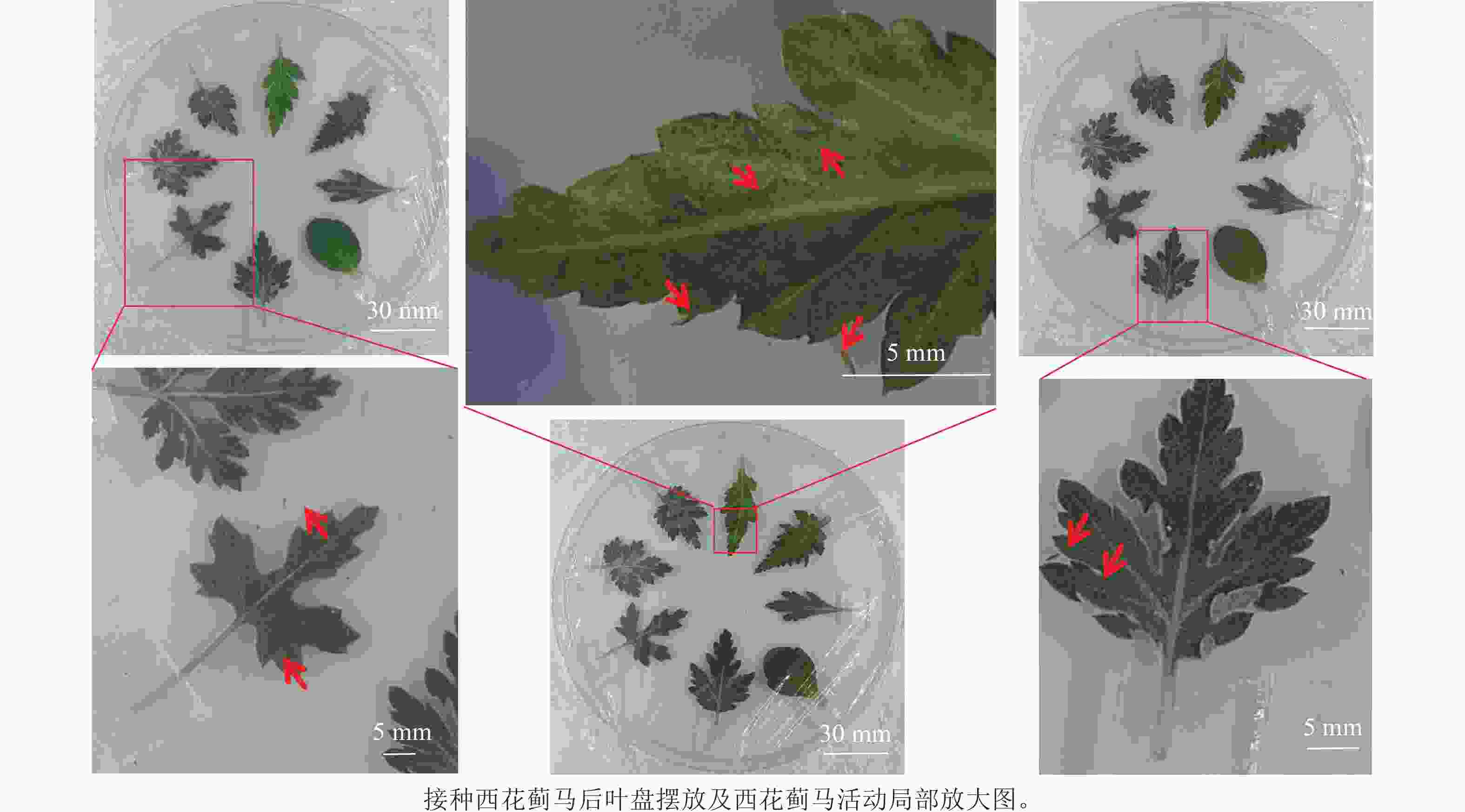
供试西花蓟马经12 h饥饿处理后,用软毛笔将成虫移至冰上培养皿(直径150 mm)中进行低温[(4±1) ℃]镇静。随后将西花蓟马精准转移至皿中心,设置每皿50头成虫的接种密度,5个生物学重复。接种后立即用透气聚乙烯膜封口,并用无菌注射针头(1 mL)在膜上制备均匀微孔(孔径0.45 mm)保障通气。数据采集于接种后12和24 h进行,通过目视计数统计叶片上西花蓟马数量,并按预设分级标准评定危害等级(图1)。
-
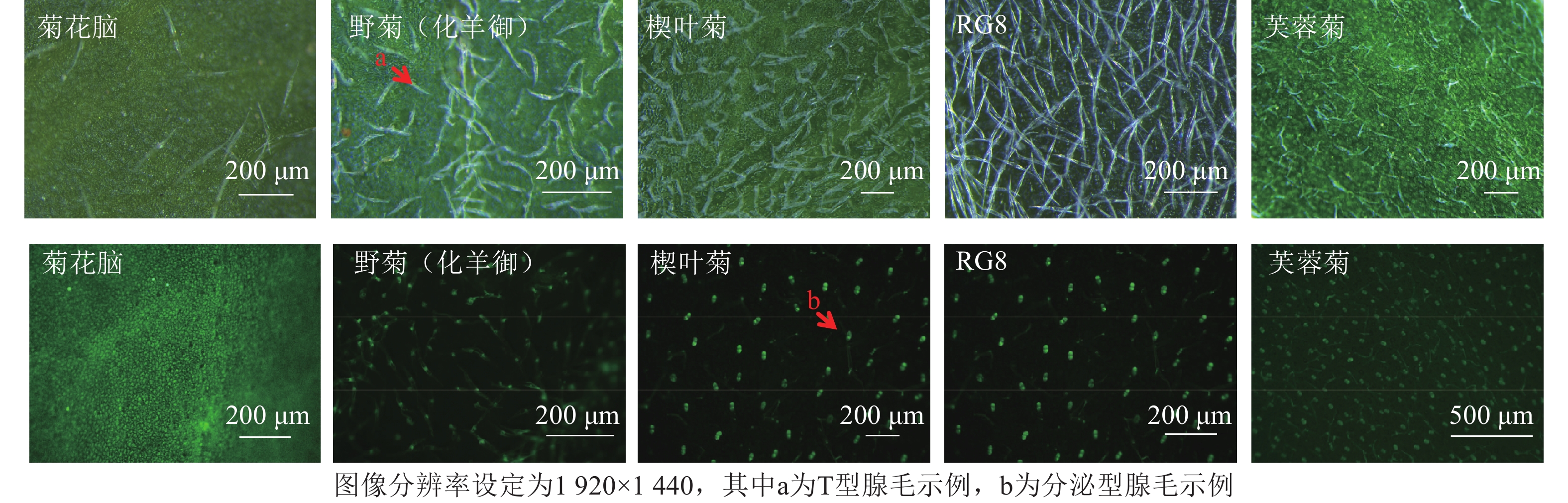
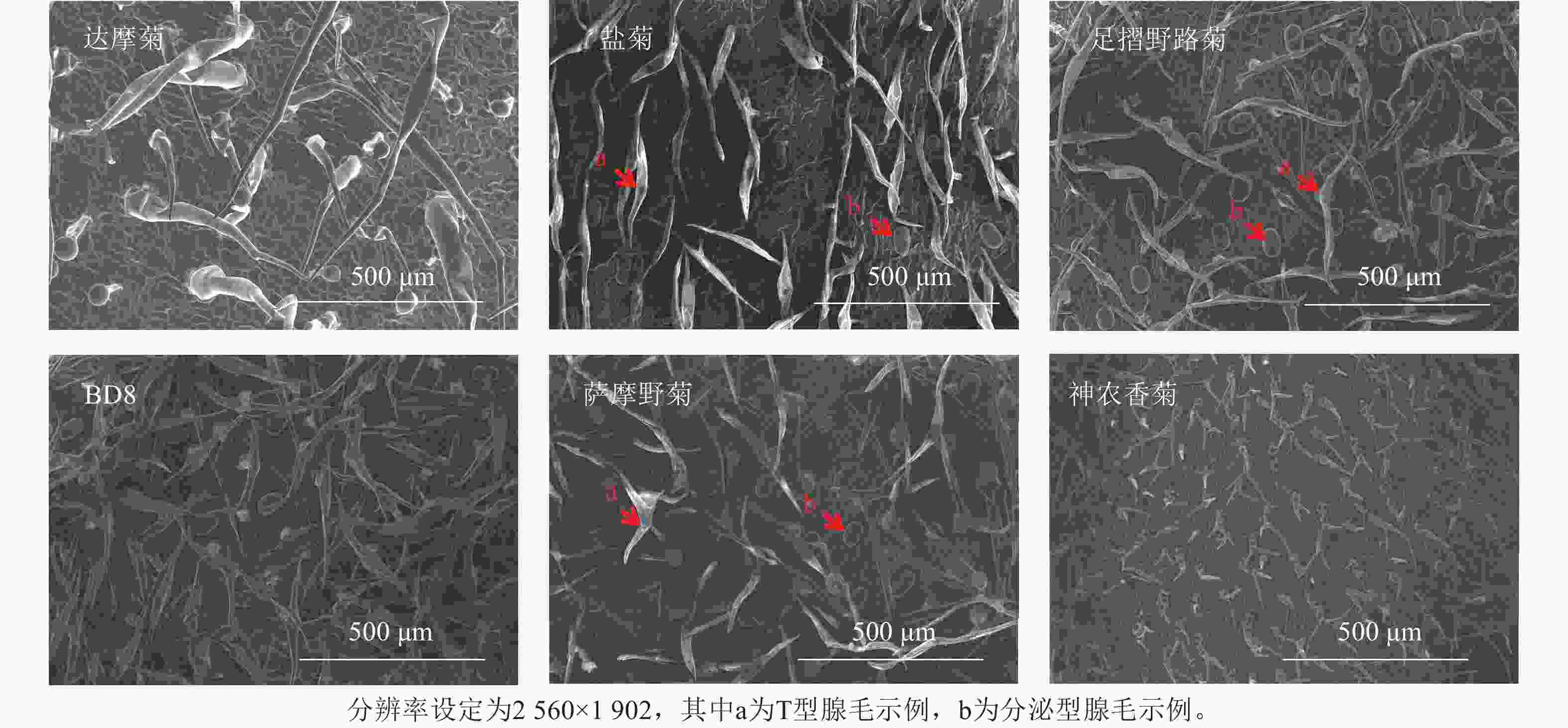
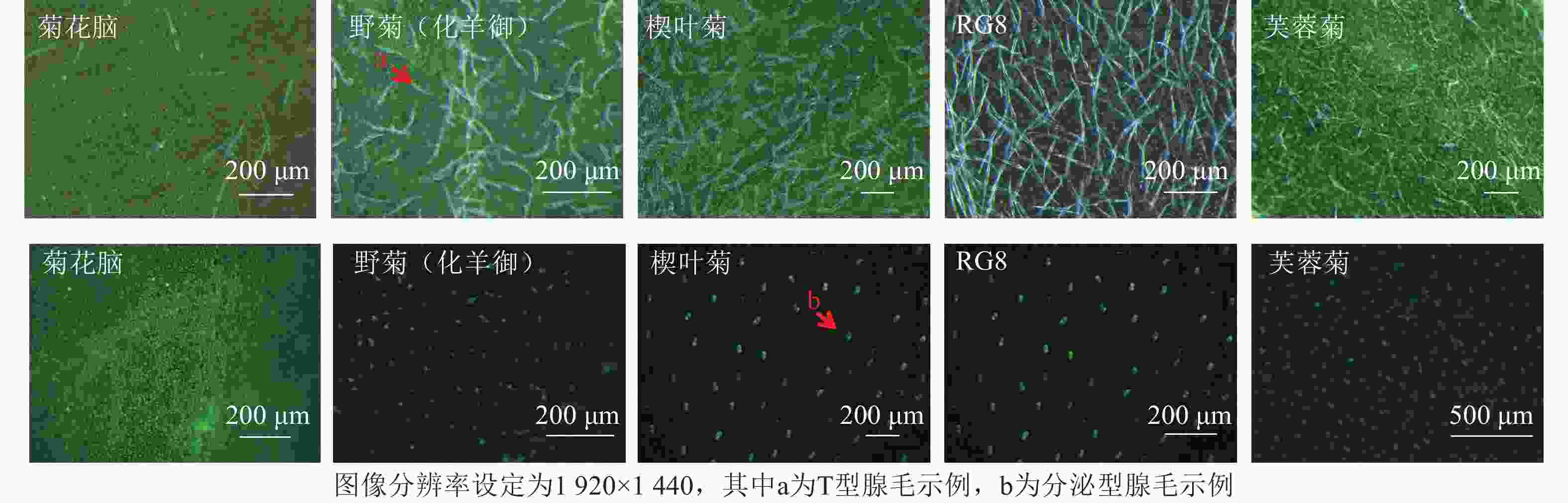
采集新鲜植物从顶芽开始第3片成熟健康叶片[19],用湿润滤纸保湿,一部分用于扫描电子显微镜(FlexSEM 1000Ⅱ)观察。另一部分叶片样本未经染色处理,直接置于体视荧光显微镜(M165FC)下,采用绿色荧光蛋白(GFP)、红色荧光蛋白(RFP)荧光通道,通过自发荧光特性区分分泌型腺毛(绿色或红色荧光信号)与T型腺毛(无特异性荧光)。
-
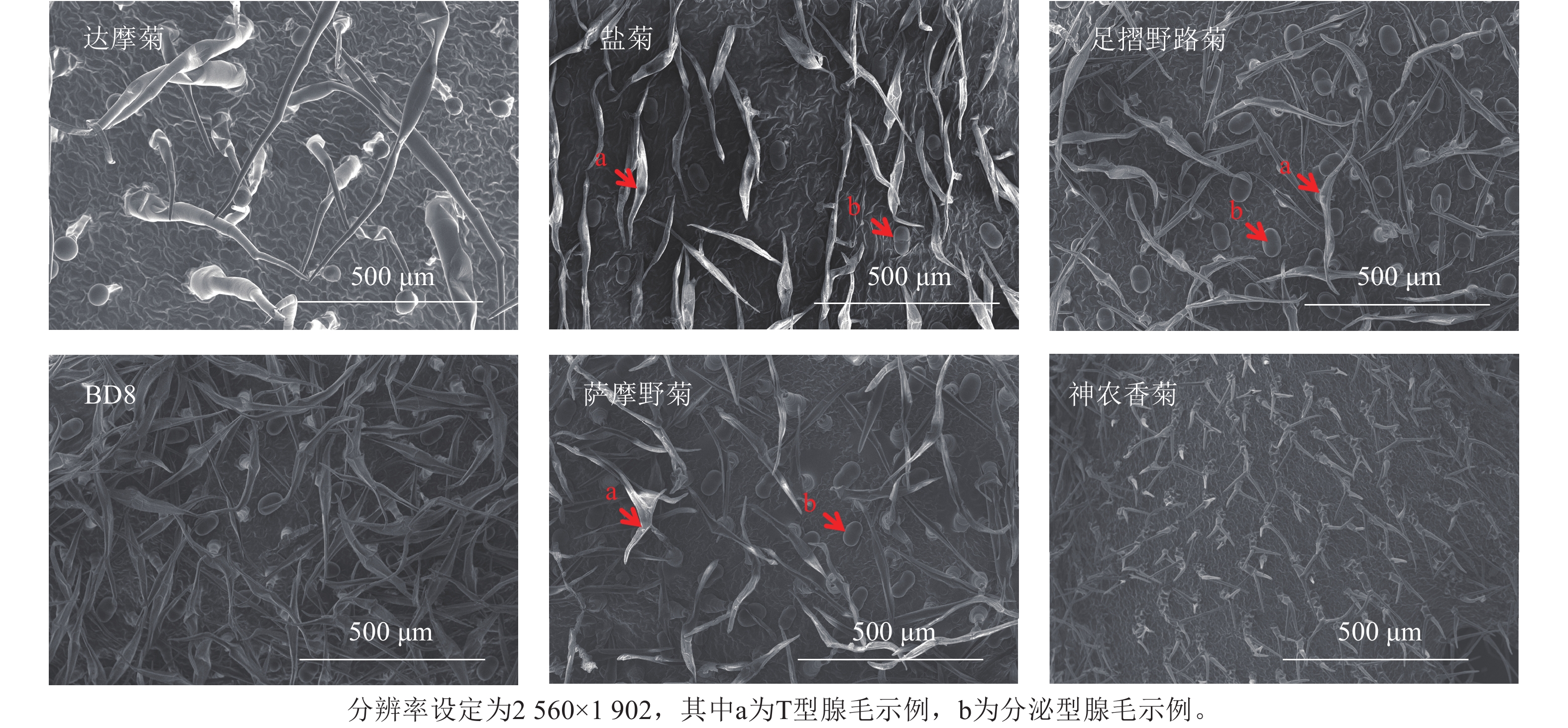
叶片正反面均分为近叶尖、中部、近叶基3个区域,每个区域随机选3个非重叠视野,共18视野。每生物学重复含3株植株叶片样本[20],总计54视野。扫描电子显微镜图像分辨率为2 560×1 902 (TIFF格式),荧光显微镜图像分辨率1 920×1 440 (PNG格式)(图2~3)。
-
基于显微图像内置标尺(扫描电子显微镜:50 μm标尺;荧光显微镜:100 μm标尺),设定图像空间分辨率(像素·μm−1)。分泌型腺毛依据绿色或红色荧光信号及球形形态特征人工标记;T型腺毛通过扫描电子显微镜图像中的非分泌型结构(直立或倒伏毛发状)进行区分。密度计算:统计单视野内2类腺毛数量,根据图像标尺计算实际视野面积(mm2),腺毛密度=腺毛数量/视野面积,根·mm−2。
-
本研究利用接种后西花蓟马种群数量进行抗性评价[6, 21],封闭空间接种50头西花蓟马,将种质划分为5个抗性等级:高抗西花蓟马(西花蓟马数量≤1头·叶−1)、中抗西花蓟马(2~4头·叶−1)、低抗西花蓟马(5~6头·叶−1)、易感西花蓟马(7~9头·叶−1)和高感西花蓟马(≥10头·叶−1)。
-
离体接种西花蓟马设置5个生物学重复,叶片腺毛密度统计设置3个生物学重复,每个重复取6个视野[22],以平均值±标准误表示。本研究使用GraphPad Prism 8.0.2软件绘制接种后西花蓟马数量箱线图,使用R 4.3.2软件绘制腺毛密度与西花蓟马偏好性相关性散点图。
-
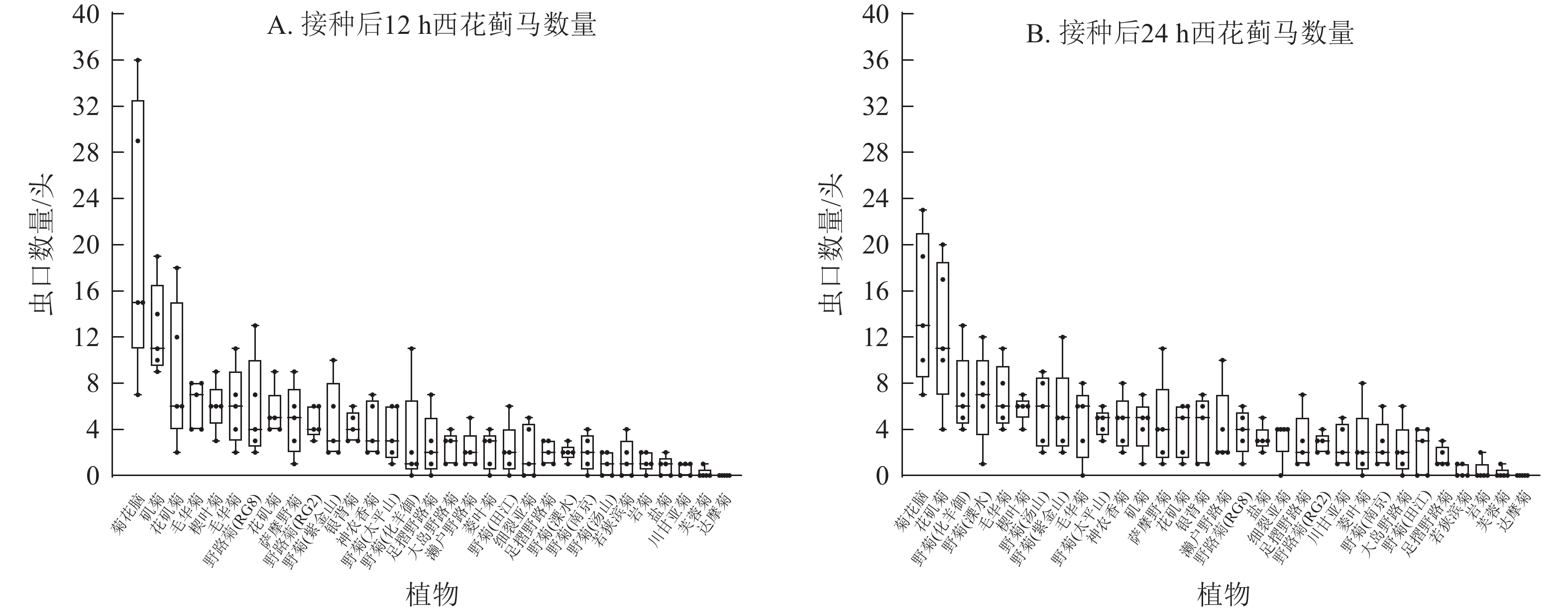
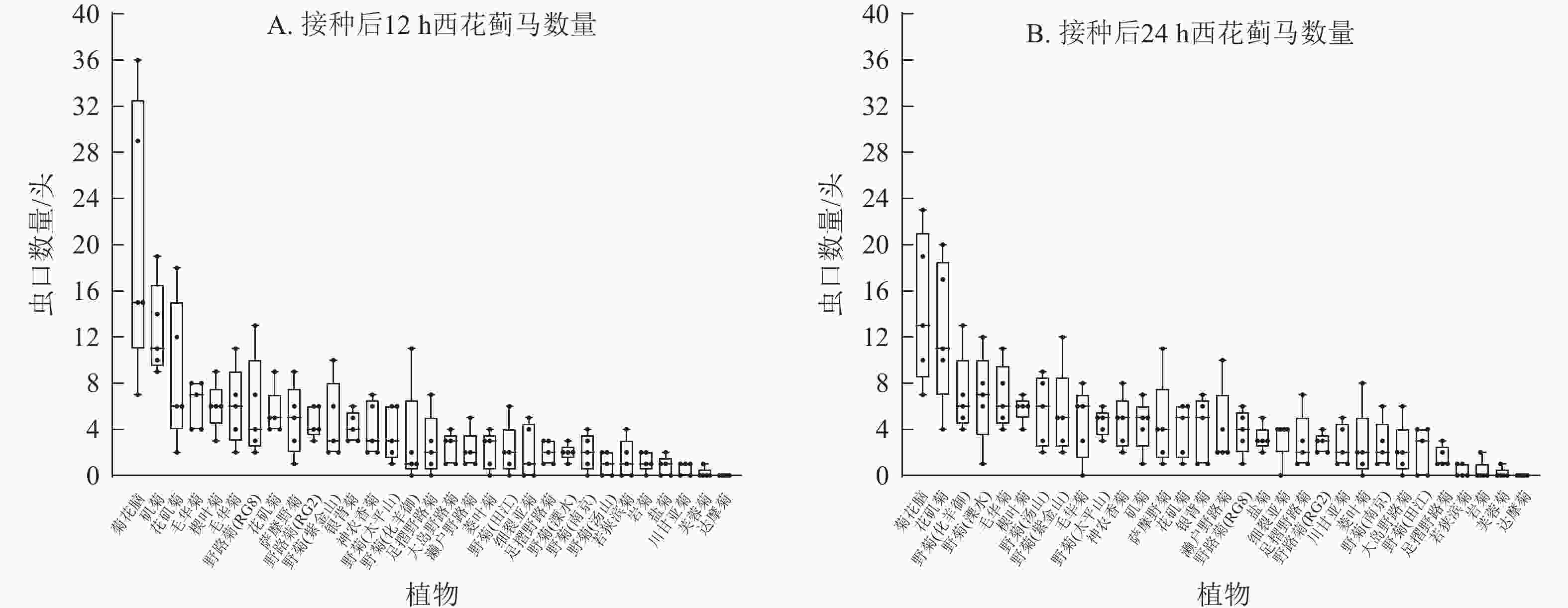
本研究通过离体接种实验发现:西花蓟马对不同菊属及近缘属种质的选择倾向不同,表现为虫口数量的差别。接种后12 h,菊花脑上西花蓟马虫口数量最高(36头),矶菊(19头)和花矶菊(18头)次之,芙蓉菊(2头)和达摩菊(0头)最低。至24 h,菊花脑仍维持最高密度(23头),花矶菊次之(20头),芙蓉菊与达摩菊持续低水平(0~2头)。值得注意的是,野菊(溧水)的虫口数量从12 h的3头升至24 h的12头,表明其取食偏好具有一定时间依赖性(图4)。菊属内不同种间西花蓟马虫口数量也有差别,接种后12及24 h,菊花脑西花蓟马种群密度最高(23~36头),岩菊最低(0~2头)。上述结果表明:不同菊属及近缘属以及属间不同种质都对西花蓟马的抗性存在差别,且西花蓟马的取食行为具有明显的时序性动态特征。
-
基于生物学意义、数据稳定性、动态变化特征及研究一致性等方面的综合考虑,本研究采用接种后24 h的西花蓟马数量进行抗性分级,能够更全面地反映菊属及近缘属种质资源对西花蓟马的抗性特征。
根据1.4节中菊属及近缘属种质抗性划分标准,从31份菊属及近缘属种质中筛选出4份高抗种质和2份高感种质(表2)。
植物 虫口数量/头 抗性等级 植物 虫口数量/头 抗性等级 植物 虫口数量/头 抗性等级 菊花脑 14.40±5.85 高感 矶菊 4.40±1.96 低抗 菱叶菊 2.60±2.80 中抗 花矶菊 12.40±5.61 高感 萨摩野菊 4.40±3.50 低抗 野菊(南京) 2.60±1.85 中抗 野菊(化羊御) 7.00±3.16 易感 花矶菊 4.00±2.10 低抗 大岛野路菊 2.20±2.04 中抗 野菊(溧水) 6.80±3.54 易感 银背菊 4.00±2.53 低抗 野菊(田江) 2.20±1.83 中抗 毛华菊 6.80±2.48 易感 濑户野路菊 4.00±3.10 低抗 野菊(日本) 1.60±0.80 中抗 楔叶菊 5.80±0.98 低抗 野路菊(RG8) 3.80±1.72 中抗 若狭滨菊 0.40±0.49 高抗 野菊(汤山) 5.60±2.73 低抗 盐菊 3.20±0.98 中抗 岩菊 0.40±0.80 高抗 野菊(紫金山) 5.40±3.50 低抗 细裂亚菊 3.20±1.60 中抗 芙蓉菊 0.20±0.40 高抗 毛华菊(伏牛山) 4.60±2.80 低抗 足摺野路菊 2.80±2.23 中抗 达摩菊 0.00±0.00 高抗 野菊(太平山) 4.60±1.02 低抗 野路菊(RG2) 2.80±0.75 中抗 神农香菊 4.60±2.06 低抗 川甘亚菊 2.60±1.62 中抗 Table 2. Grading of insect population on leaves of Chrysanthemum and related genera
菊属内不同种质抗性差异明显,既有高感的菊花脑,也有高抗的岩菊等;菊属近缘属(如亚菊属、紫菀属等)中的川甘亚菊表现为抗西花蓟马,达摩菊表现为高抗西花蓟马,可见近缘属中也存在优异的抗西花蓟马种质。
-
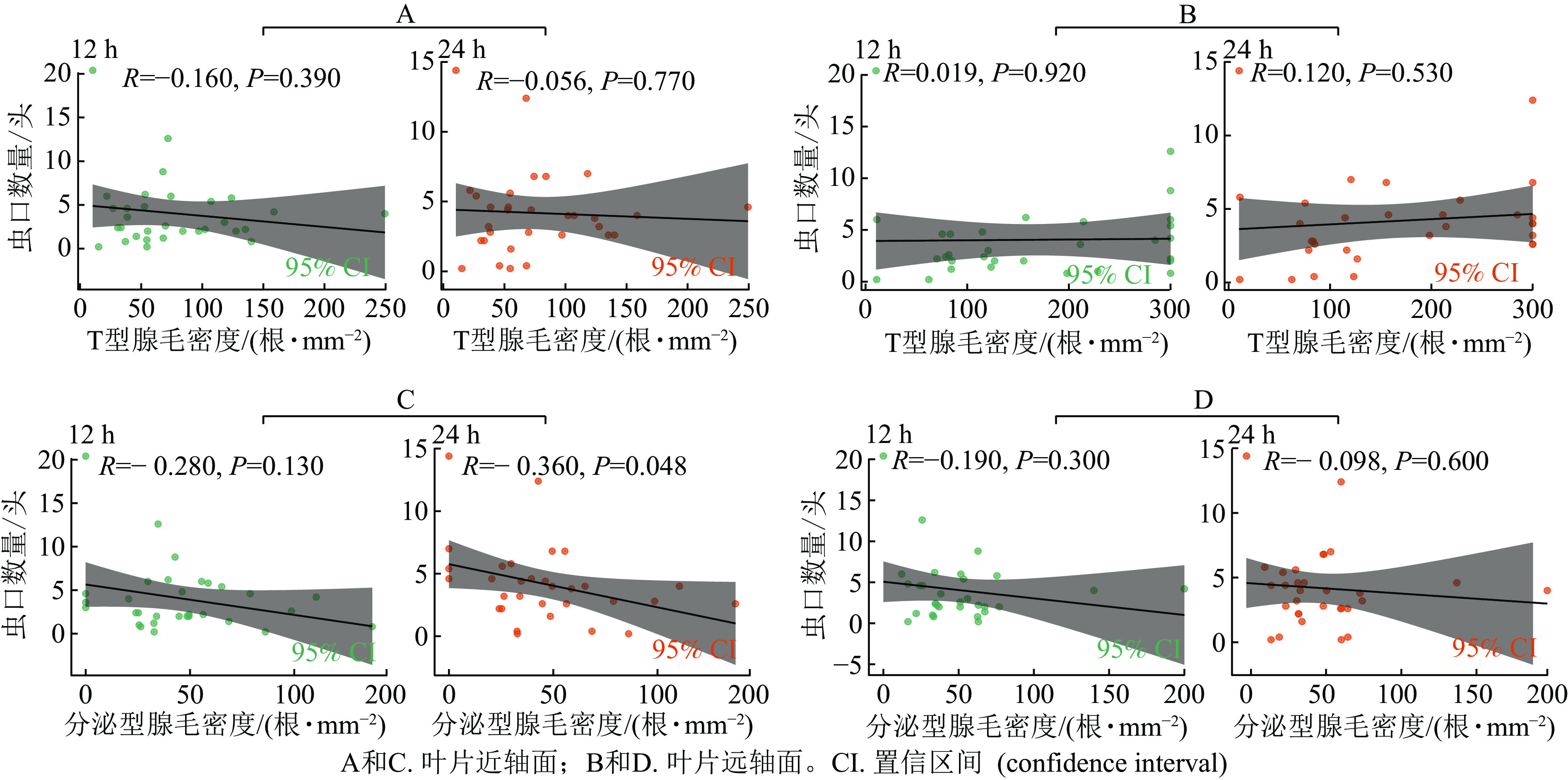
分析了西花蓟马取食行为与叶片腺毛密度的关系:叶片T型腺毛密度与接种后12和24 h的西花蓟马数量均无显著相关性 (图5 A和B)。这表明T型腺毛可能对西花蓟马的取食行为没有显著影响。
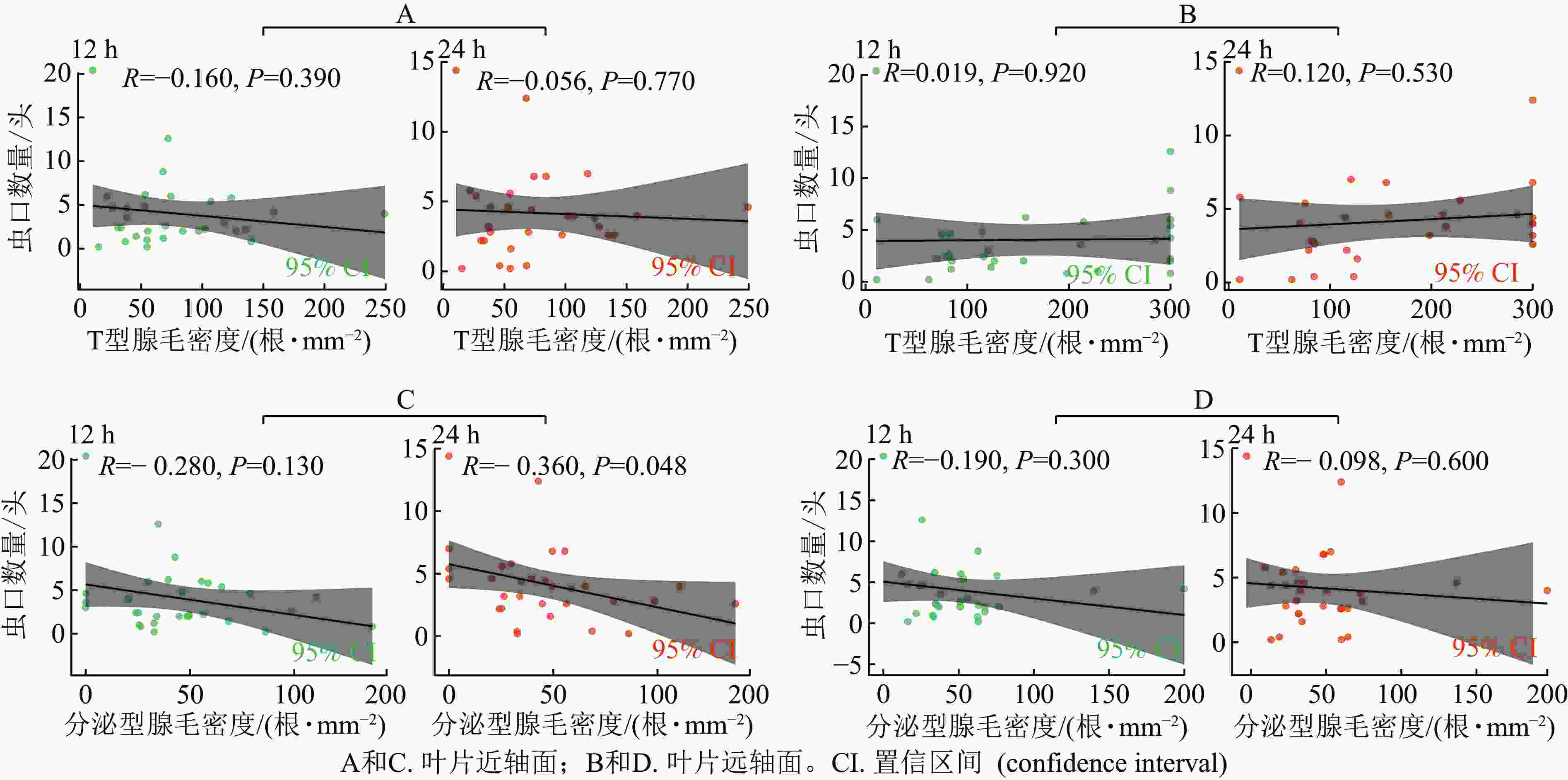
Figure 5. Correlation between leaf glandular hair density and the number of thrips inoculated in 31 species of Chrysanthemum and related genera
在离体接种后12 h,无论是叶片近轴面还是远轴面,分泌型腺毛密度与西花蓟马数量均无显著相关性 (图5 C和D)。接种后24 h,菊属及近缘属叶片近轴面的分泌型腺毛密度与西花蓟马数量呈显著负相关(R= −0.36,P=0.048,图5 C),即分泌型腺毛密度越高,西花蓟马数量越少。这可能是因为分泌型腺毛分泌的化学物质对西花蓟马有驱避或抑制作用。然而,叶片远轴面的分泌型腺毛密度与西花蓟马数量无显著相关性 (图5 D),这可能是因为远轴面腺毛的分泌物对西花蓟马的影响较小。
综上可见,T型腺毛对西花蓟马的取食行为没有显著影响。分泌型腺毛(尤其是叶片近轴面的)可能在较长时间内(24 h)对蓟马的取食行为产生抑制作用,表现为西花蓟马数量与腺毛密度呈负相关。短期内(12 h),分泌型腺毛的影响不显著。
-
本研究发现:不同菊属及近缘属野生资源对西花蓟马的抗性存在显著差异,其中菊花脑上的西花蓟马数量最多,达摩菊上最少。这种差异可能与植物的物理、化学及营养特性相关[13]。此外,西花蓟马数量在不同寄主叶片上随时间呈现动态变化,其时间依赖性可能与西花蓟马取食行为的时间动态性[17]、植物防御机制(如分泌型腺毛化学物质)的时效性[23]以及环境因素(光照、温度)变化有关[19]。
-
本研究发现:T型腺毛作为非分泌型物理毛状体,可能对西花蓟马的取食行为没有显著影响。T型腺毛防御功能主要依赖机械阻隔作用,然而,西花蓟马体长仅1~2 mm且口器(锉吸式)具有高度灵活性[24],可穿透腺毛间隙或通过调整取食位点规避物理屏障[23, 25]。研究证实:刺吸式昆虫可通过行为可塑性[23]快速适应单纯物理防御。例如,烟粉虱Bemisia tabaci可通过调整取食角度规避番茄Solanum lycopersicum非腺毛[11]。类似地,本研究中西花蓟马可能在接种后数小时内通过触角感器识别T型腺毛分布模式[26],并选择低阻力路径完成取食。这种适应性行为导致物理屏障的防御效率随时间推移进一步降低。
-
接种西花蓟马后24 h的种群数量与菊花叶片近轴面分泌型腺毛密度呈显著负相关,而接种后12 h时两者无显著相关。这表明分泌型腺毛的抗虫效应具有滞后性,其防御功能可能依赖于腺毛分泌物的持续释放或积累[27]。接种后12 h种群数量和腺毛密度相关性缺失可能与西花蓟马适应行为相关[23]。例如,通过触角嗅觉感器[23]快速调节对植物挥发物的响应阈值[12],或通过唾液鞘包裹腺毛尖端以规避物理损伤[11]。随着暴露时间延长(24 h),腺毛分泌物的化学防御物质可能协同干扰西花蓟马取食行为或消化生理[28],产生显著趋避效应。植物防御化合物通常在损伤或昆虫取食后数小时内逐步合成并释放[8],这与本研究中接种后24 h抗性表型的显现时间窗口相吻合。
-
本研究从31份菊属及近缘属野生种质中筛选出4份高抗种质(菊属2份,芙蓉菊属1份,紫菀属1份)和2份高感种质(菊属1份,亚菊属1份)。这表明野生种质资源作为遗传多样性载体,提供了庞大的基因库,是维持生态平衡和种质创新的核心资源,可进一步推动采集与保护。
-
本研究通过离体接种西花蓟马,分析西花蓟马对不同菊属及近缘属种质的取食偏好性。结果表明:离体接种后24 h,叶片近轴面分泌型腺毛密度与西花蓟马取食偏好性呈显著负相关。基于西花蓟马选择数量建立了5级抗性评价体系,筛选出4份抗西花蓟马种质。该结果为菊花抗西花蓟马育种提供了优异抗性资源,为更多品种的抗性评价鉴定奠定了基础,并为基于植物次生代谢产物的绿色防控技术研究提供了良好材料。
Screening of germplasm resources for resistance to Frankliniella occidentalis in Chrysanthemum and related genera
doi: 10.11833/j.issn.2095-0756.20250493
- Received Date: 2025-08-30
- Accepted Date: 2025-10-08
- Rev Recd Date: 2025-10-07
- Publish Date: 2025-10-20
-
Key words:
- Chrysanthemum /
- Frankliniella occidentalis /
- glandular trichomes /
- insect resistance /
- evaluation and identification of germplasm resources
Abstract:
| Citation: | SONG Zhaoli, LIAO Yuan, LIU Zhiyong, et al. Screening of germplasm resources for resistance to Frankliniella occidentalis in Chrysanthemum and related genera[J]. Journal of Zhejiang A&F University, 2025, 42(5): 967−974 doi: 10.11833/j.issn.2095-0756.20250493 |










 DownLoad:
DownLoad: