-
随着工业化的迅速发展,土壤的重金属污染已经成为了全球关注的问题。中国农田土壤镉污染面积已经超过2×105 hm2,生产镉含量超标的农产品达1.46×106 t·a-1[1]。镉通过食物链进入人体,引起了如“痛痛病”等慢性疾病。世界各国的土壤都存在重金属污染问题[2]。如何治理和修复污染土壤已经成为国际热点问题。20世纪80年代之后植物修复(phytoremediation)逐步发展起来,因其操作简便、成本低廉、环境友好等优点而日益受到人们的关注[3-4]。学术界公认的超富集植物需要满足2个条件:①植物地上部富集的重金属达到一定临界值,不同的重金属其富集界限不同;②地上部分富集的重金属含量高于地下部分。据报道,已鉴定的重金属超积累植物有500多种[5],但镉超积累的植物较少见,已发现的仅有遏蓝菜属Thlaspi的几种植物[6]。何冰等[7]发现了一种新型的锌、镉、铅超积累植物——矿山型东南景天Sedium alfredii。矿山型东南景天对土壤中的镉、锌、镍等重金属具有极强的超积累富集能力[8],更重要的是适量的重金属含量已成为其维持更好的生长状态所必需[9]。因此,查清东南景天体内涉及重金属离子吸收、转运、隔离、耐受等相关基因的分子机制,对林木重金属高抗新品种的培育具有重要理论指导意义。捕光蛋白复合体(LHC)能把捕获到的光能量迅速传导到光化学反应中心,光系统Ⅰ和光系统Ⅱ都有各自的LHC(LHC Ⅰ和LHCⅡ)。自LHCⅡ被发现以来[10],人们对其结构和功能的研究取得了较大的进展[11-12]。LHC Ⅱ含有Lhcb1-6等6种蛋白质,它们在类囊体膜中进行光能的吸收和传递,此外在维持类囊体膜的结构,调节激发能量在2个光系统之间的分配,光保护以及对各种环境的适应等过程中都起着重要的作用。林江波等[13]克隆和分析了中国水仙Narcissus tazetta var. chinensis叶绿素a/b结合蛋白,推测其可能属于Lhcb1类蛋白;高志民等[14]克隆了毛竹Phyllostachys edulis的a/b结合蛋白cab-PhE1,证明该基因在叶绿体的类囊体中表达;向太和等[15]分离出了水稻Oryza sativa捕光叶绿素a/b结合蛋白,分析显示该基因在叶片和茎中表达无差异,但是光对其表达有明显促进作用;Simon等[16]研究证明:叶绿素结合蛋白中的LI181家族和胁迫响应相关;张敏等[17]研究了2种生态型东南景天Lhcb2基因在镉和锌胁迫时的表达变化,并发现镉、锌胁迫处理能够提高转基因烟草Nicotiana tabacum中该基因的表达,且转基因烟草能够积累更多的镉。此外,菊花Chrysanthemum morifolium等植物中的Lhcb蛋白也已被克隆[18]。但这些研究主要集中于LHCB基因在不同光处理后的表达差异以及蛋白的结构和起源进化,对于Lhcb蛋白与植物重金属胁迫相关方面的研究还相对较少。本研究初步探讨了该基因在镉、铜、铅胁迫后的表达差异,结合前人研究推测东南景天LHCB基因可能是通过调节光合作用为重金属胁迫后的植物解毒机制提供能量,从而缓解植物体中毒的现象。
-
矿山型东南景天无性系采集于浙江省衢州市的一个铅/锌矿区,取回后在人工气候箱中水培处理。胁迫处理时在水中添加设定浓度的重金属离子,然后分别提取根、茎、叶的核糖核酸(RNA),用于后续研究。
RNA提取使用总RNA提取试剂盒(加拿大,NORGEN, Thorold),DNA凝胶回收试剂盒购自AXYGEN公司(中国上海),2×Goldstar Taq Master Mix购自北京康为世纪生物技术公司(中国北京),反转录试剂盒(Prime ScriptTM RT reagent Kit),大肠埃希菌Escherichia coli DH5α感受态细胞和荧光定量试剂盒SYBR Prime ScriptTM RT-PCR Kit均购自Takara公司(中国大连)。
-
以本实验室前期构建的cDNA文库为模板,利用转录组测序结果设计特异引物LHCB-F和LHCB-R,ORF-F和ORF-R,分别以LHCB-F和M13-以及LHCB-R和M13+从基因中间向两端扩增,以ORF-F和ORF-R扩增ORF进行验证(表 1)。20.0 μL扩增体系为:模板0.6 μL,引物1.0 μL,2×Goldstar Taq Master Mix10 μL,双蒸水(ddH2O)7.4 μL。聚合酶链式反应(PCR)程序为:94 ℃预变性10 min;94 ℃变性30 s,60 ℃ 30 s,72 ℃延伸1 min,32个循环;最后72 ℃延伸10 min。
表 1 引物名称及对应序列
Table 1. Name and sequence of primers
引物名称 引物序列(5′→3′) LHCB-F GTGGATCTTTTGACCCACTT M13R CAG GAA ACA GCT ATG ACC M13F TGT AAA ACG ACG GCC AGT LHCB-R TTCTCAACAGGTCCTTTTCC ORF-F ATGGCCACATCTGCTATCCAATC ORF-R TTATTTGCCGGGGACAAAGTTT LHCB-RT-F GAGGCGCACTGTGAAAAGCA LHCB-RT-R TTTCAGGGAAGACGCAGCCT UBC-F TGGCGTCGAAAAGGATTCTGA UBC-R CCTTCGGTGGCTTGAATGGAT T7 TAATACGACTCACTATAGGG SP6 ATTTAGGTGACACTATAG T7ter TGCTAGTTATTGCTCAGCGG LHCB-F-BamH Ⅰ CGCGGATCCTCATCCGCCTTTGCTGGC LHCB-R-Xho Ⅰ CCGCTCGAGTTATTTGCCGGGGACAAAGTTT -
采用十六烷基三甲基溴化铵(CTAB)法[19]提取东南景天叶片的基因组DNA,以其为模板,引物和扩增程序同cDNA序列克隆。10 g·kg-1琼脂糖凝胶电泳后回收目的片段,分光光度计测定其比例,将该片段连接到pGEM-T-easy载体上,热激转化大肠埃希菌DH5α感受态细胞,37 ℃过夜培养,挑取单克隆至800 μL LB液体培养基(LB/ampicillim),37 ℃,180 r·min-1,过夜培养,用通用引物T7和SP6进行菌液聚合酶链式反应(PCR)检测,阳性结果送测序。测序和引物合成由生工生物工程(上海)股份有限公司完成。
-
参考金晓芬[20]和桑健等[21]的研究数据,分别以400 μmol· L-1的氯化镉(CdCl2),硫酸铜(CuSO4)和硝酸铅[Pb(NO3)2]的水处理东南景天无性系植株0.5,6.0,12.0,24.0,48.0,72.0和96.0 h,以未处理的东南景天无性系为对照,按照NORGEN的RNA提取试剂盒说明,分别提取不同处理时间东南景天根、茎、叶的RNA,设置3个生物学重复。用NanoDrop 2000分光光度计(Thermo, Massachusetts, 美国)测定浓度,用10.0 g·kg-1的琼脂糖电泳分析完整性。反转录根据RNA的含量取1.5~4.0 μg RNA,使用主要scripttm逆转录试剂盒(Prime ScriptTM RT reagent Kit)反转合成第1链,稀释3倍后利用ABI 7300实时荧光定量PCR仪(Applied Biosystems, Foster City, 美国)进行qRT-PCR分析。根据LHCB基因cDNA序列用Primer 3设计实时荧光定量引物LHCB-RT-F和LHCB-RT-R。以东南景天泛素结合酶基因(ubiquitin conjugating enzyme 9)为内参,其特异性引物为UBC-F和UBC-R。实时荧光定量PCR扩增体系(10.0 μL)为:模板2.0 μL,特异引物(10 μmol· L-1)0.4 μL,2×SYBR Premix Ex TaqTM 10.0 μL,50×ROX Reference Dye 0.4 μL,双蒸水(ddH2O)6.8 μL。扩增条件:95 ℃预变性10 s,95 ℃变性5 s,60 ℃复性31 s,40个循环。做3个重复·样品-1。数据分析采用ΔΔCT法计算相对定量,目标基因相对定量=2-ΔΔCT[22]。
-
根据LHCB基因序列设计引物LHCB-F-BamH Ⅰ和LHCB-R-Xho Ⅰ,在其两端引入酶切位点BamH Ⅰ和Xho Ⅰ。以东南景天cDNA文库为模板进行扩增,利用限制性内切酶BamH Ⅰ和Xho Ⅰ处理PCR产物和pET-28a,回收目的片段后进行连接,获得重组原核表达载体pET-28a-LHCB。将pET-28a-LHCB基因重组质粒用热激转化的方法转入E. coli BL21(DE3)感受态细胞,挑选单克隆过夜培养后用载体上的通用引物T7和T7ter进行PCR检测插入片段的长度,阳性质粒送测序并用BamH Ⅰ和Xho Ⅰ 37 ℃处理3 h后电泳分析。在含有Kanamycin的LB液体培养基中培养细菌,当D(600)为0.6~0.8时,加入异丙基硫代半乳糖苷(IPTG,0.1 mmol· L-1)诱导培养,为了研究蛋白的具体定位,分离上清和包涵体,收集菌体进行蛋白质变性聚丙烯酰胺凝胶电泳(PAGE)分析。
-
用DNAMAN软件,DNAtools,SignalP 4.1 Server()和ExPASy(http://web.expasy.org/cgi-bin/compute_pi/pi_tool)等软件分析测定的DNA,cDNA及其编码的蛋白质结构特点,利用美国国家生物技术信息中心(NCBI)数据库信息进行比对(表 1)。
-
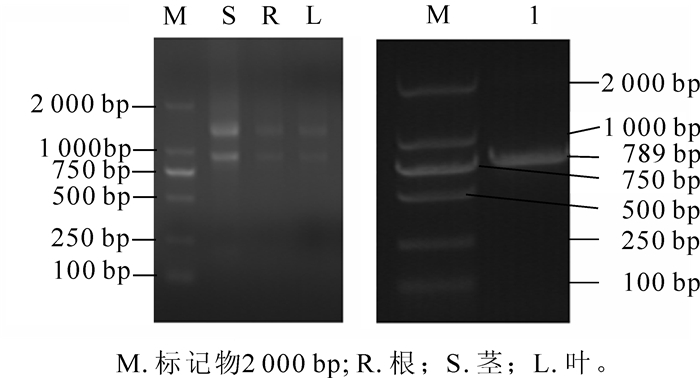
图 1是东南景天根、茎、叶的RNA电泳图及胶回收后的目的条带电泳图,从图 1可以看出:目的条带清晰无杂带、未降解,条带明亮,Nano Drop2000分光光度计检测根、茎、叶、总RNA质量浓度分别为147.1,299.8,97.3 mg· L-1,吸光度D(260/280)分别为2.16,2.30和2.02,电泳显示根、茎中总RNA略有降解,但都能满足后续反转录聚合酶链式反应(RT-PCR)试验要求。

图 1 东南景天根、茎、叶RNA电泳图(左)和胶回收电泳图(右)
Figure 1. Electrophoresis figure of RNA in root stem leaf (left) and agarose gel DNA purification (right)
从东南景天文库中分离出cDNA全长,测序后将序列提交到GenBank中,登陆号为KF806555。SaLhcb2基因cDNA全长929 bp(图 2a),其中开放阅读框长度为798 bp,5′端含非编码区48 bp,3′端含非编码区83 bp,其中Poly(A)17 bp。该基因的开放阅读框编码1个265个氨基酸的蛋白,将它命名为SaLhcb2。以基因组DNA为模板分离的基因组序列和cDNA序列比对,发现该基因在147~222 bp含有1个74 bp的内含子(图 2b)。
对SaLhcb2编码的蛋白进行分析,预测其分子量大小为28.5 kd,等电点为5.69,总平均亲水性为-0.097,脂溶指数为78.83。信号肽分析表明该蛋白含有1个信号肽,在第20个和第21个氨基酸之间有切割位点。跨膜结构分析表明该蛋白具有3个明显的跨膜区。二级结构预测,SaLhcb的环状结构占60.75%,螺旋结构占39.25%。软件分析预测该蛋白位于叶绿体中。将SaLhcb2与其他物种的该基因编码蛋白进行同源比对,结果显示:SaLhcb2和辽东楤木Aralia elata该蛋白同源性最高(94.28%),和蓖麻Ricinus communis的同源性相对最低(92.08%)(图 3)。
-
重组产物送测序并用BamH Ⅰ和Xho Ⅰ双酶切电泳检测(图 4)。图 4中可以看出:重组后的质粒经过双酶切之后电泳结果出现载体片段和800 bp左右的目的基因,结合测序结果证明:SaLhcb2和表达载体pET-28a重组成功。图 5中泳道2~5分别是诱导前和诱导4.0,5.0和6.0 h后的蛋白电泳结果,红色框内为目的蛋白条带。可以看出:经IPTG诱导后,随着诱导时间的延长,目的片段蛋白浓度逐渐增加,即SaLhcb2表达量升高,说明重组蛋白能够在原核系统中高效表达,泳道6和泳道7分别是上清和包涵体中的目的蛋白条带,可以看出,蛋白主要位于包涵体中,因此,需要在后续实验进行蛋白复性以用于功能研究。
-
以东南景天泛素结合酶基因为内参,借助实时荧光定量,分析在镉、铜、铅不同胁迫时间后根、茎、叶中SaLhcb2基因的表达量。从图 6中可以看出:镉处理后,根中SaLhcb2基因的表达量呈现先下调后上调的趋势,在48.0 h后其表达量显著上调,达到对照的4.45倍;在茎中除了6.0 h SaLhcb2基因胁迫后的表达量都明显上调外,在0.5 h时为最高,为对照的5.86倍;叶中表达量先降低后升高,在12.0 h后表达量上调,72.0 h达到最高,为对照的3.91倍。铜处理后,0.5 h根中该基因表达量就显著上调,6.0 h时茎中的表达量也显著上调,分别为对照的12.78倍和54.39倍,随后其表达量都急剧降低;在叶片中表达量一直偏低,且变化不明显。铅处理后,根中SaLhcb2基因胁迫后0.5~72.0 h一直显著降低,96.0 h时才出现上调,表达量接近未处理时;茎中SaLhcb2基因的表达量显著上调,6.0 h时达到最高,为对照的2.93倍;但叶片中的表达量低于对照,48.0 h时略有上升,为对照的0.98倍。说明SaLhcb2能够响应重金属镉、铜、铅的胁迫,但是响应模式和响应速度不同。3种重金属处理后,从地下部位——根部的基因表达变化分析结果可以看出:镉、铅胁迫使得SaLhcb2基因表达量在一定时期内(24.0 h和72.0 h)受到抑制,而铜胁迫后,根中表达量迅速上调(0.5 h),表明根部对3种重金属都较为敏感;从茎部受胁迫后表达量看:镉、铅胁迫后,该基因表达量明显上调;铜处理后,在6.0 h左右该基因表达量显著上调,随后一直表达量较低。从叶片表达量来看:镉胁迫后,目的基因表达量在12.0 h后都显著上调,而铜、铅胁迫后表达量都基本上都比对照水平低。从超积累植物的特性可知,地下部位积累量很少,主要超富集重金属的部位是地上部位。推测东南景天的SaLhcb2对3种重金属响应模式不同,已有的研究结论显示:东南景天能够超富集镉,能够耐受一定程度的铅,但是目前未有研究证明其能超富集铜,从基因表达分析结果可以看出,铜胁迫后根部响应最快,随后茎部响应,这与离子运输的过程相符合,推测镉胁迫后茎叶部位能够超积累镉。
-
本研究利用本实验室前期构建的东南景天cDNA文库,克隆获得了SaLhcb2基因,构建了pET-28a-LHCB原核表达载体,初步研究了SaLhcb2基因和镉、铜、铅的相关性,为进一步研究重金属胁迫下SaLhcb2基因的调控机理奠定了基础。
Jun等[23]研究了光系统Ⅱ中捕光蛋白的结构和功能,指出LHCB基因编码LHCⅡ蛋白,它是光系统Ⅱ的主要捕光叶绿素a/b结合蛋白,其主要功能是光能的吸收和传递,提高捕光效率,此外,LHC Ⅱ还能够调节光能的分配。光系统Ⅱ中的捕光蛋白复合物主要由3种基因编码,分别是Lhcb1,Lhcb2和Lhcb3[24]。在这3种蛋白中Lhcb1和Lhcb2含量最丰富,当植物体内Lhcb1和Lhcb2的表达量受到抑制时,植物体内构成LHC Ⅱ复合物的亚单元将缺失[25]。镉胁迫致使LHCⅡ复合体构象发生变化,这种变化可能是由于镉胁迫使得Lhcb1和Lhcb2蛋白分子量降低[26]。田生科[27]和张晓玲[28]的研究显示:超积累型东南景天植株中的镉分布特点是叶中最高,茎次之,根中最低;铅则是根中最多,茎叶中含量较低。张敏等[17]分析了2种不同生态型东南景天LHCB基因对锌/镉胁迫的响应,证明LHCB基因的超表达能够增加超积累型东南景天根和茎的生物量,并提高镉在植物体内的积累量。
qRT-PCR结果显示,镉处理后,根部的SaLhcb2基因在24.0 h内表达量均降低,48.0 h后表达量上调,在茎、叶中12.0 h后该基因表达都上调。SaLhcb2基因在镉处理0.5 h时茎中显著升高,12.0 h时叶片中显著升高,推测其对镉胁迫相对敏感,为前期响应基因。在叶片中响应速度相比茎中较慢,推测这可能和根、茎对镉的隔离作用有关,使得叶片中镉的积累相对较慢。SaLhcb2基因可能并不直接参与东南景天受到镉胁迫之后的解毒机制,但是能够为其提供能量,使得地上绿色部分光能的吸收效率提高,并传递能量到茎、叶中。张玉秀等[29]的研究表明:重金属锌或镉胁迫能够增强印度芥菜Brassica juncea重金属ATP酶的表达,说明重金属富集与能量代谢相关。我们推测在茎和叶中由于积累的镉离子较多从而相对需要更多的能量,所以该基因的表达量较高,而根部镉积累量较少,所以SaLhcb2基因表达量较低,这与田生科等[27]的研究结果一致。铜处理后0.5 h根中表达量就极显著上调,6.0 h时茎中表达量极显著上调,叶片中表达量一直较低,可能在0.5 h根中能够积累和吸收部分铜,6.0 h时转移到茎中,但是叶片中不能够积累铜,目前报道并未显示东南景天能够超富集铜。铅处理后,该基因在根中表达量一直较低,到96.0 h才上调,而胁迫0.5 h后茎中该基因的表达就迅速上调随后其表达量一直较对照高,而叶片中表达量在96.0 h内都较低。东南景天铅积累的特点是在茎和叶片中积累较少,而根部积累较多[27],推测可能SaLhcb2定位于叶绿体中,所以在根部该基因本身的表达量偏低,SaLhcb2基因可能为后期铅胁迫响应基因。此外,3种重金属胁迫后根部该基因显著上调的时间点不同,说明东南景天对这3种重金属的敏感度有差异;一般重金属富集主要是在地上部位,在茎中,可以看出长时间处理后(96.0 h),镉、铅处理过的植株该基因表达量相比铜更高,说明植株茎中富集镉和铅的能力强于铜,同理可看出在叶片中富集镉的能力强于铜和铅。
Characterization of a light-harvesting chlorophyll a/b binding protein (LHCB) gene, SaLhcb2, in Sedum alfredii
-
摘要: LHCB基因对植物适应各种环境胁迫的过程中起着重要作用。序列分析显示:东南景天Sedum alfredii的SaLhcb2基因全长929 bp, 其中开放阅读框(ORF)为798 bp, 含有1个74 bp的内含子。通过蛋白序列比对, SaLhcb2基因编码的蛋白与多种植物的蛋白序列同源性都很高(92%以上)。分析400 μmol·L-1镉离子(Cd2+), 铜离子(Cu2+)和铅离子(Pb2+)胁迫处理后的东南景天, 结果显示:镉处理后SaLhcb2基因在茎、叶中表达量快速上升(12.0 h内), 根中表达量到48.0 h后才上调。铜处理后0.5 h根中表达显著上调, 胁迫6.0 h后茎中表达量显著上调, 随后一直降低, 叶片中该基因表达量一直较低。铅处理后, 根中表达量降低, 96.0 h左右比对照略微上调, 而茎中96.0 h内表达量相比对照都上调, 叶片中96.0 h内都降低。研究结果表明:SaLhcb2与东南景天的镉、铜、铅胁迫抗性有密切的相关性。
-
关键词:
- 植物学 /
- 镉、铜、铅 /
- 叶绿素a/b结合蛋白(LHCB) /
- 荧光定量PCR /
- 东南景天
Abstract: The light-harvesting chlorophyll a/b binding protein (LHCB) gene plays an important role in plants adapting to various environments. Sedum alfredii is a new Zn/Cd hyper-accumulator and whether LHCB in this interesting species was related to the heavy-metal tolerance interested us a lot. In the present study, we isolated a cDNA using homologous cloning and designated it as SaLhcb2 which encoded a light-harvesting chlorophyll a/b binding protein in Sedum alfredii. A prelimary sequence analysis was conducted and homologous comparison was also performed using MegAlign. In order to identify the gene expression profiles of SaLhcb2 response to heavy-metal stress, seedlings of Sedum alfredii were treated by 400 μmol·L-1 Cd2+, Cu2+, and Pb2+ stresses with those cultured in water as a control. Roots, stems and leaves were dissected and promptly frozen in liquid nitrogen for RNA extraction and the followed real-time PCR. Sequence analysis showed that the coding sequence of SaLhcb2 was 929 bp and the open reading frame was 798 bp. The deduced protein, SaLHCB2, consisted of 266 amino acids and had a high homology (above 92%) with LHCB2 of other plants via homologous comparison. For the Cd2+, Cu2+, and Pb2+ stress treatments, the level of SaLhcb2 was elevated dramatically in stems and leaves within 12.0 h. However, with the Cd2+ treatment, the expression of SaLhcb2 in roots did not display a tendency of up-regulation until 48.0 h. For the Cu2+ treatment, in roots, the expression of SaLhcb2 showed a prompt response of up-regulation at 0.5 h and then decreased weirdly. With the Pb2+ treatment, the expression of SaLhcb2 in roots displayed a profile of down-regulation except the point of 96.0 h. Based on the above findings, it could be concluded that the expression of SaLhcb2 in Sedum alfredi was influenced by heavy-metal treatment and may function in the process of plants combating heavy-metal stress. -
表 1 引物名称及对应序列
Table 1. Name and sequence of primers
引物名称 引物序列(5′→3′) LHCB-F GTGGATCTTTTGACCCACTT M13R CAG GAA ACA GCT ATG ACC M13F TGT AAA ACG ACG GCC AGT LHCB-R TTCTCAACAGGTCCTTTTCC ORF-F ATGGCCACATCTGCTATCCAATC ORF-R TTATTTGCCGGGGACAAAGTTT LHCB-RT-F GAGGCGCACTGTGAAAAGCA LHCB-RT-R TTTCAGGGAAGACGCAGCCT UBC-F TGGCGTCGAAAAGGATTCTGA UBC-R CCTTCGGTGGCTTGAATGGAT T7 TAATACGACTCACTATAGGG SP6 ATTTAGGTGACACTATAG T7ter TGCTAGTTATTGCTCAGCGG LHCB-F-BamH Ⅰ CGCGGATCCTCATCCGCCTTTGCTGGC LHCB-R-Xho Ⅰ CCGCTCGAGTTATTTGCCGGGGACAAAGTTT -
[1] 柳絮, 范仲学, 张斌, 等.我国土壤镉污染及其修复研究[J].山东农业科学, 2007, 39(6):94-97. LIU Xu, FAN Zhongxue, ZHANG Bin, et al. Soil pollution and repair of Cd in our country[J]. Shandong Agric Sci, 2007, 39(6):94-97. [2] 郑喜珅, 鲁安怀, 高翔, 等.土壤中重金属污染现状与防治办法[J].土壤与环境, 2002, 11(1):79-84. ZHENG Xishen, LU Anhuai, GAO Xiang, et al. Contamination of heavy metals in soil present situation and method[J]. Soil Environ Sci, 2002, 11(1):79-84. [3] 陈同斌, 韦朝阳, 黄泽春, 等.砷超富集植物蜈蚣草及其对砷的富集特征[J].科学通报, 2002, 47(3):207-210. CHEN Tongbin, WEI Chaoyang, HUANG Zechun, et al. Enrichiment characteristics of arsenic in Pteris vittata L.:a kind of arsenic hyperaccumulation plants[J]. Chin Sci Bull, 2002, 47(3):207-210. [4] SAIFULLAH, MEERS E, QADIR M, et al. EDTA-assisted Pb phytoextraction[J]. Chemosphere, 2002, 74(10):1279-1291. [5] 何启贤.镉超富集植物筛选研究进展[J].环境保护与循环经济, 2013, 33(1):46-49. HE Qixian. Research advance in screening of cadmium hyperaccumulation plants[J]. Environ Prot & Crcular Econ, 2013, 33(1):46-49. [6] 熊愈辉.镉污染土壤植物修复研究进展[J].安徽农业科学, 2007, 35(22):6876-6878. XIONG Yuhui. Research advance in soil phytoremediation polluted by cadmium[J]. J Anhui Agric Sci, 2007, 35(22):6876-6878. [7] HE Bing, YANG Xiao'e, NI Wuzhong, et al. Sedum alfredii:a new lead-accumulating ecotype[J]. Acta Bot Sin, 2002, 44(11):1365-1370. [8] 杨肖娥, 龙新宪, 倪吾钟, 等.东南景天Sedum alfredii:一种新的锌超级累植物[J].科学通报, 2002, 47(13):1003-1006. YANG Xiao'e, LONG Xinxian, NI Wuzhong, et al. Sedum alfredii:a new zinc-accumulating ecotype[J]. Chin Sci Bull, 2002, 47(13):1003-1006. [9] LIU Fengjie, TANG Yetao, DU Ruijun, et al. Root foraging for zinc and cadmium requirement in the Zn/Cd hyperaccumulator plant Sedum alfredii[J]. Plant Soil, 2010, 327(1):365-375. [10] THORNBER J P, ALBERTE R S. The Organization of Chlorophyll in vivo[M]. Beilin:Springer, 1977:574-582. [11] BASSI R, RIGONI F, GIACOMETTI G M. Chlorophyll binding proteins with antenna function in higher plants and green algae[J]. Photochem & Photobiol, 1990, 52(6):1187-1206. [12] BASSI R, DAINESE P A. Supramolecular light-harvesting complex from chloroplast photosystem(Ⅱ) membranes[J]. Eur J Biochem, 1992, 204(1):317-326. [13] 林江波, 王伟英, 邹晖, 等.中国水仙叶绿素a/b结合蛋白基因Ntcab1的克隆与序列分析[J].福建农业学报, 2013, 28(5):463-467. LIN Jiangbo, WANG Weiying, ZOU Hui, et al. Cloning and sequence analysis on gene of chlorophyll a/b-binding protein from Narcissus tazetta var. chinensis[J]. Fujian J Agric Sci, 2013, 28(5):463-467. [14] 高志民, 刘颖丽, 彭镇华.毛竹PS Ⅱ大量捕光天线蛋白基因克隆及其表达分析[J].植物科学学报, 2012, 30(1):64-71. GAO Zhimin, LIU Yingli, PENG Zhenhua. Cloning and molecular characterization of the major light harvesting antenna protein genes of PS Ⅱ in Phullostachys edulis[J]. Plant Sci J, 2012, 30(1):64-71. [15] 向太和, 王利琳, 庞基良.水稻(Oryza sativa L.)捕光叶绿素a/b结合蛋白全长cDNA的克隆和特性分析[J].作物学报, 2005, 31(9):1227-1232. XIANG Taihe, WANG Lilin, PANG Jiliang. Cloning and characterization of a full-length cab gene encoding the light-harvesting chlorophyll a/b-binding proteins in rice (Oryza sativa L.)[J]. Acta Agron Sin, 2005, 31(9):1227-1232. [16] DITTAMI S M, MICHEL G, COLLÉN J, et al. Chlorophyll-binding proteins revisited-a multigenic family of light-harvesting and stress proteins from a brown algal perspective[J]. BMC Evol Biol, 2010, 10(11):365-378. [17] ZHANG Min, SENOURA T, YANG Xiao, et al. Lhcb2 gene expression analysis in two ecotypes of Sedum alfredii subjected to Zn/Cd treatments with functional analysis of SaLhcb2 isolated from a Zn/Cd hyperaccumulator[J]. Biotechnol Lett, 2011, 33(9):1865-1871. [18] 韩霜, 刘瑞霞, 张兆和, 等.菊花叶绿素a/b结合蛋白基因CmLhcb1及其启动子的克隆和表达分析[J].园艺学报, 2013, 40(6):1119-1128. HAN Shuang, LIU Ruixia, ZHANG Zhaohe, et al. cloning of chlorophyll a/b binding protein CmLhcb1 and promoter from Chrysanthemum morifolium and expression analysis[J]. Acta Hortic Sin, 2013, 40(6):1119-1128. [19] 王关林, 方红筠.植物基因工程[M]. 2版.北京:科学出版社, 2002:744. [20] 金晓芬.镉超级累植物东南景天谷胱甘肽代谢特征及比较蛋白质组学研究[D].杭州:浙江大学, 2008. JIN Xiaofen. Glutathione Metabolisms in Cadmium Hyperaccumulator Sedum alfredii Hance and its Proteomics Analysis[D]. Hangzhou:Zhejiang University, 2008. [21] SANG Jian, HAN Xiaojiao, LIU Mingying, et al. Selection and validation of reference gene for real-time quantitative PCR in hyperaccumulating ecotype of Sedum alfredii under different yeavy metals stresses[J]. PLOS one, 2013, 8(12):1-10. [22] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data use real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4):402-408. [23] MINAĠAWA J, TAKAHASHI Y. Structure, function and assembly of photosystem Ⅱ and its light-harvesting proteins[J]. Photosynth Res, 2004, 82(3):241-263. [24] LUCINSKI R, JACKOWSKI G. The structure, functions and degradation of pigment-binding proteins of photosystem Ⅱ[J]. Acta Biochim Pol, 2006, 53(4):693-708. [25] JENNY A, MARK W, ROBIN G, et al. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem (Ⅱ) effects on photosynthesis, granna stacking and fitness[J]. Plant J, 2003, 35(3):350-361. [26] JANIK E, MAKSYMIEC W, MAZUR R, et al. Structual and functional modifications of the major light-harvesting complex Ⅱ in cadmium-or copper-treated Secale cereal[J]. Plant Cell Physiol, 2010, 51(8):1330-1340. [27] 田生科.超级累东南景天(Sedum alfredii Hance)对重金属(Zn/Cd/Pb)的解毒机制[D].杭州:浙江大学, 2012. TIAN Shengke. Mechanisms Behind Detoxification of Heavy Metals(Zn/Cd/Pb) in the Hyperaccumulatoor Sedum alfredii Hance[D]. Hangzhou:Zhejiang University, 2012. [28] 张晓玲.东南景天古银矿生态型超级累镉的生理机制与调控[D].杭州:浙江大学, 2008. ZHANG Xiaoling. Physiological Mechanisms of Cd Hyperaccumulation by the ASME of Sedum alfredii H. and its Regulation[D]. Hangzhou:Zhejiang University, 2008. [29] 张玉秀, 张媛雅, 柴团耀.印度芥菜重金属ATP酶基因的分离与表达[J].中国科学院研究生院学报, 2011, 28(4):551-555. ZHANG Yuxiu, ZHANG Yuanya, CHAI Tuanyao. Isolation and expression of heavy metal ATPase in Brassica juncea L.[J]. J Grad Univ Chin Acad Sci, 2011, 28(4):551-555. [30] 张圆圆, 窦春英, 姚芳, 等.氮素营养对重金属超积累植物东南景天吸收积累锌和镉的影响[J].浙江林学院学报, 2010, 27(6):831-838. ZHANG Yuanyuan, DOU Chunying, YAO Fang, et al. Nitrogen application to enhance zinc and cadmium uptake by the hyperaccumulator Sedum alfredii[J]. J Zhejiang For Coll, 2010, 27(6):831-838. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2014.06.003






 下载:
下载: