-
扦插是目前繁育无性系砧木的常用方式,效率高,成本低,且能保持砧木的优良性状。对于难生根树种的无性繁殖,扦插时往往需使用植物生长调节剂进行诱导;研究发现生长素处理插穗可诱生根原基进而诱导扦插枝条生根[1-2]。生长素类物质,如吲哚丁酸(IBA)和萘乙酸(NAA),外施于插穗基部时能够明显提高不定根的发生率[3-4],显著影响和改变内源生长素水平;激素水平是影响扦插不定根发生的重要因素[5-6],内源激素的含量变化,会刺激不定根发生的启动。胡桃Juglans又名核桃,其属植物普遍难生根,复幼处理可以使插穗具备生根能力,但在扦插时还必须施加外源生长素方能生根[7]。本试验以经复幼处理的核桃品种‘中宁盛’‘Zhongningsheng’嫩枝插穗为试材,分析施加IBA处理对插条生根的影响,测定扦插生根过程中插穗基部的内源激素变化,初步探讨内源激素动态变化及相互作用与不定根形成的关系,为研究核桃嫩枝扦插繁殖和生根生理机制提供参考。
-
供试材料为经复幼处理的核桃品种‘中宁盛’半木质化嫩枝插穗,要求长势一致、无病虫害。
参考宋晓波[8]方法,试验组用5.0 g·L-1的IBA溶液速蘸插穗基部10 s,对照组以清水速蘸,扦插枝条50根·处理-1,并重复3次。扦插基质以V(黄土):V(牛粪):V(河沙)=1:1:1配置混合,扦插前5 d装入营养钵,并用质量浓度为0.5%的高锰酸钾喷淋消毒后备用。插穗制备好后放置数分钟待基部表面溶液挥发后,竖直插于营养钵中,深度5 cm。扦插后浇水,并隔7 d喷洒质量浓度为1.0 g·L-1的高锰酸钾溶液消毒。保持扦插环境温度为18~35 ℃,相对湿度70%以上。
扦插前0 d和扦插后1,3,5,7,9 d随机采集插穗,3条·处理-1,重复3次,共取样6次。迅速用蒸馏水将插穗基部冲洗干净并用滤纸擦干,迅速截取穗条基部底端茎段约1 cm,用锡纸包裹放入液氮冷冻保存。样品转移至实验室后,加入液氮快速研磨并冷冻干燥,-80 ℃下保存备用。
-
扦插后,1次·d-1观察插穗形态变化,拍照,记录生根进程。扦插至20 d时,检测插穗的生根数量和生根情况,统计生根率。
-
扦插后每隔1 d取样,重复3次,共取样8次。截取插穗基部1 cm长的茎段用甲醛-醋酸-乙醇固定液(FAA)固定,参照叶宝兴等[9]的方法并作改动,制作石蜡切片。番红固绿染色,加拿大树胶封片,OLYMPUS光学显微镜观察并拍照。
-
采用高效液相色谱-四级杆离子阱串联质谱仪(HPLC-MS/MS Q-TRAP)测定。内源激素提取参照贾鹏禹等[10]方法略作改动,称取冻干粉0.1 g·样本-1,萃取获得上清液用于激素质量分数(鲜质量)测定。数据用Analyst 1.5数据处理软件(美国Applied Biosystem公司)处理与分析。
-
应用Excel 2007软件进行数据整理和绘图,使用SPSS 18.0软件进行t检验分析。激素检测值以取平均值±标准差表示。
-
扦插20 d后,测量核桃复幼插穗生根情况。由表 1可知:试验组生根率高达98.1%,对照完全没有生根,且大部分死亡。说明有无IBA处理,切口愈伤率、植株死亡率和插穗的生根率差异显著。
表 1 IBA处理对核桃嫩枝扦插生根的影响
Table 1. Effects of IBA on the rooting of walnut soft-wood cuttings
处理 切口愈伤率/% 死亡率/% 生根率/% 对照组 0 b 75.5 b 0 b 试验组 93.5 a 0 a 98.1 a -
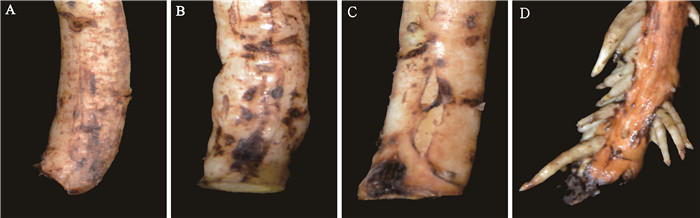
扦插后3 d,插穗基部部分表皮褐化(图 1A);扦插后5 d,试验组部分插穗基部膨大,个别皮孔有外凸甚至开裂迹象(图 1B);扦插后7 d至9 d,试验组插穗基部膨大,切口附近沿茎开裂,开口处有白色絮状组织,不定根开始发生(图 1C);扦插至15 d时,不定根突破表皮,开始伸长生长(图 1D)。对照组插穗扦插至15 d时,插穗基部表皮褐化,未生根。
-
相比于对照,经IBA诱导的嫩枝插穗基部组织结构变化明显。图 2为试验组插穗基部横切片,可以发现扦插后3 d,插穗基部形成层开始迅速分裂,并明显增厚(图 2A);扦插后5 d,部分增厚的形成层细胞进一步发育,在韧皮部形成团状的分生组织(图 2B);扦插至7 d时,根原基分生组织进一步分裂分化成初始根原基细胞(图 2C),扦插至9 d时,初始根原基细胞在韧皮部发育为圆顶状的根原基(图 2D);至扦插15 d时,不定根形成并突破表皮(图 2E)。
-
嫩枝扦插后插穗中IAA质量分数迅速上升。由图 3A可知:对照组在1 d时,IAA质量分数达到峰值,为857.0 ng·g-1,此后下降。试验组IAA质量分数在扦插5 d时升到峰值,为1 395.0 ng·g-1;此时,插穗基部增粗,出现开裂,说明诱导不定根原基生成。
-
嫩枝扦插后插穗中ABA质量分数变化如图 3B所示。扦插后插穗的内源ABA质量分数迅速下降,但对照组在3 d时开始回升,并在之后一直保持上升趋势;试验组则持续下降,至5 d时达到最低之后迅速上升,并在7 d时达到峰值56.6 ng·g-1,至9 d后逐渐下降。
-
扦插后7 d插穗的内源GA3质量分数变化趋势大体呈“升高—降低”趋势(图 3C),并在5 d时达到峰值,试验组最高为17.1 ng·g-1,对照组最高为11.1 ng·g-1。不同的是试验组在7 d后GA3质量分数再次上升,而对照则持续下降。
-
ZT质量分数的变化图 3D所示。扦插后3 d,试验组和对照组内源ZT质量分数变化不明显;3 d后试验组ZT质量分数开始上升,9 d时达到298.5 ng·g-1,而对照组一直无明显变化,只在7 d后略微升高,9 d时仅达到30.9 ng·g-1。
-
由图 4A可知:试验组wIAA/wABA比值在扦插后升高,至5 d时达129.1,之后急剧下降,但7 d后又缓慢上升;对照组wIAA/wABA比值在扦插后1 d时达到最高值为43.5,而后持续下降。
-
由图 4B可知:试验组wIAA/wGA3比值呈现“M”型变化趋势,并在3 d时达第1个峰值为103.9,7 d达第2个峰值为108.5。与试验组相比,对照组wIAA/wGA3比值在扦插后1 d时达到最高值为119.7,此后迅速下降,至3 d时后下降速度放缓。
-
从图 4C可以看出:试验组wIAA/wZT呈现先升高后降低变化趋势,扦插后3 d时wIAA/wZT达峰值为60.1,之后至9 d时持续下降。对照组wIAA/wZT比值也呈现同样的变化趋势,不同之处在扦插后1 d时即达到峰值42.4,后转为下降。
-
外源生长素的诱导可以调节内源激素的水平,间接影响不定根的形成及根系的形成和发育[11-12];而内源激素水平的高低则直接与插穗生根能力密切相关[13],同时多激素的相互作用也是调控生根的关键[14]。核桃是难生根树种,早先研究表明:核桃成龄嫩枝扦插几乎不能生根[7-8]。本研究以经过复幼处理的嫩枝为试材,用外源IBA诱导其生根,发现不定根发生率达到98.1%,而对照生根率为0,表明外源生长素的诱导对核桃嫩枝发根至关重要。
大量试验表明:生长素是促进不定根发生的主要因素[15-17],生长素峰值的出现往往会诱导根原基的发生[16]。本研究结果发现:外源IBA诱导下的插穗在扦插5 d后IAA鲜质量分数出现高峰(图 3A),结合显微观察发现5 d时根分生组织发生(图 2B),7 d时初始根原基细胞团形成(图 2C),9 d时不定根原基产生(图 2D),说明施加外源生长素明显提高了内源IAA的水平,从而促进了不定根原基细胞的形成。
脱落酸通常被认为是不定根发生的抑制剂[18],其调控不定根的发生是通过与IAA的平衡共同起作用,较高的wIAA/wABA能促进不定根的形成[19]。本研究发现无论试验组对照组,受切伤和水分胁迫所致,扦插初期插穗ABA水平较高,扦插后迅速下降,根原基产生后又有所升高;wIAA/wABA峰值出现在根原基发生阶段,说明相对较低水平的脱落酸可以促进不定根的形成。
有研究认为:较高水平内源赤霉素对不定根发生有抑制作用[20],赤霉素高表达后可通过影响生长素的运输而抑制不定根发生[21]。本研究发现:IBA诱导后的插穗自扦插伊始至根原基产生,期间GA3与IAA呈协同上升的趋势,第3天时达到第1个峰值,此时形成层开始增厚(图 2A),第7天达第2峰值,此时根原基初始细胞开始生长(图 2C);同时发现两者的比值在不定根发生阶段始终高于对照组(图 3C,图 4D);由此认为核桃复幼插穗中相对较高水平的wIAA/wGA3,可能在不定根形成前期有利于形成层增厚和脱分化,而在后期对不定根原基的细胞生长有促进作用。
关于ZT等细胞分裂素对植物不定根形成的影响,有研究认为其主要作用是抑制根原基的分化和形成,同时促进不定根的发育[22]。细胞分裂素的作用主要是调控生长素的变化;较高水平的细胞分裂素能抑制生长素极性运输载体PIN1的表达[23]。因此认为当ZT水平较高时,wIAA/wZT比值上升促进不定根原基的发生。本研究发现:经IBA诱导的插穗在扦插前期ZT质量分数相对较低,根原基开始产生后则迅速上升,与对照组差异显著;wIAA/wZT比值前高后低,至第3天达到高峰,此时正是形成层分裂增厚时期。该结果表明:核桃复幼插穗中ZT质量分数较低,外源生长素的诱导可能对ZT的影响不明显,高比值的wIAA/wZT与形成层分裂有关;而根原基发生后ZT明显增加,此时wIAA/wZT降低,说明比值下降对根的分化和发育起着促进作用。
综上所述,施加外源吲哚丁酸可以诱导核桃复幼嫩枝不定根的发生,同时改变了内源激素的含量。然而,关于外源生长素改变内源激素含量及内源激素对生根的作用机制,还有待进一步的探索和研究。
Adventitious root formation with IBA and endogenous hormones dynamics in walnut soft-cuttings
-
摘要: 植物激素是调控不定根形成的重要因素。通过测定胡桃Juglans复幼嫩枝扦插过程内源激素的质量分数变化,分析植物生长调节剂诱导对内源激素的影响,为探讨植物激素对不定根发生提供参考。以胡桃品种‘中宁盛’‘Zhongningsheng’复幼插穗为试验材料,利用高效液相色谱串联质谱法,测定外源吲哚丁酸(IBA)诱导处理下插穗基部内源激素变化,并统计生根情况。结果表明:外源IBA诱导处理对插穗生根影响显著。经IBA诱导的嫩枝插穗生根率高达98.1%,对照处理没有生根,死亡率达75.5%;发根过程经历了形成层增厚、分生组织细胞团产生、根原基初始细胞发生、根原基形成和根的发育等阶段,外部形态表现为增粗、开裂;与对照组相比,扦插后内源激素变化明显,吲哚乙酸(IAA)先升高,并于第5天达到峰值,之后降低;赤霉素(GA3)变化趋势与IAA一致;脱落酸(ABA)则是先降低后上升;玉米素(ZT)前期没有明显变化,根原基诱导后明显升高。外源生长素的诱导提高了嫩枝插穗IAA的水平,改变了内源激素质量分数,促进了不定根的形成。Abstract: Phytohormones are an important factor in regulating adventitious root formation. To provide a reference for discussing the role of phytohormones on adventitious root formation in walnut, the effect of exogenous hormones on endogenous hormones was analyzed by measuring the level of endogenous hormones during the cutting process of a rejuvenated softwood, Juglans(walnut) 'Zhongningsheng'. The rejuvenated softwood was first induced by exogenous indole-3-butyric acid (IBA) and then, using High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS), the endogenous hormones:Indole-3-acetic acid (IAA), Gibberellic acid (GA3), Abscisic Acid (ABA), and Zeatin (ZT); basal segments of soft-cuttings were measured followed by measurement of the rooting situation, student's test were used for the treatment of the data. Results showed strong differences between samples induced and not induced by IBA. The rooting rate of rejuvenated softwood induced by IBA was up to 98.1%; whereas, in the control the rooting rate was 0 and the mortality rate was 75.5%. The rooting process experienced several stages, such as thickening of the cambium, meristem cell formation, root primordia initial cell formation, root primordia formation, and root development through observation of the anatomic structure. Additionally, the external morphology showed thickening and cracking. During softwood cutting, endogenous hormone levels in rejuvenated soft shoots induced by IBA changed noticeably. Also, IAA increased first and reached its peak at day 5, then decreased as did GA3. However, ABA decreased first and then increased. In the early stages, ZT changed little but did increase after root primordia induction. The level of IAA in softwood induced by IBA improved; however, the effects on ZT were small. So the changes of endogenous hormones induced by IBA promoted adventitious root formation.
-
Key words:
- botany /
- walnut /
- softwood cutting /
- adventitious root /
- HPLC-MS /
- phytohormones
-
表 1 IBA处理对核桃嫩枝扦插生根的影响
Table 1. Effects of IBA on the rooting of walnut soft-wood cuttings
处理 切口愈伤率/% 死亡率/% 生根率/% 对照组 0 b 75.5 b 0 b 试验组 93.5 a 0 a 98.1 a -
[1] 王紫阳, 徐建华, 李火根, 等.中山杉优良无性系302, 118, 405扦插生根能力比较[J].浙江农林大学学报, 2015, 32(4):648-654. WANG Ziyang, XU Jianhua, LI Huogen, et al. Rooting capabilities for Taxodium 'Zhongshanshan' 302, 118, and 405[J]. J Zhejiang A & F Univ, 2015, 32(4):648-654. [2] 白磊, 李荣生, 尹光天, 等.米老排扦插生根因子及优化[J].浙江农林大学学报, 2016, 33(3):543-550. BAI Lei, LI Rongsheng, YIN Guangtian, et al. Rooting factors and optimization for propagation of Mytilaria laosensis cuttings[J]. J Zhejiang A & F Univ, 2016, 33(3):543-550. [3] 董宁光, 齐建勋, 陈永浩, 等.沙藏埋枝处理对促进平欧杂种榛绿枝扦插生根的机制分析[J].果树学报, 2016, 33(12):1510-1516. DONG Ningguang, QI Jianxun, CHEN Yonghao, et al. Physiological and biochemical basis of hazelnut rhizogenesis promoted by embedded stock etiolation[J]. J Fruit Sci, 2016, 33(12):1510-1516. [4] SWAMY S L, PURI S, SINGH A K. Effect of auxins (IBA and NAA) and season on rooting of juvenile and mature hardwood cuttings of Robinia pseudoacacia and Grewia optiva[J]. New For, 2002, 23(2):143-157. [5] NEGISHI N, NAKAHAMA K, URATA N, et al. Hormone level analysis on adventitious root formation in Eucalyptus globules[J]. New For, 2014, 45(4):577-587. [6] 吴文浩, 曹凡, 刘壮壮, 等. NAA对薄壳山核桃扦插生根过程中内源激素含量变化的影响[J].南京林业大学学报(自然科学版), 2016, 40(5):191-196. WU Wenhao, CAO Fan, LIU Zhuangzhuang, et al. Effects of NAA treatment on the endogenous hormone changes in cuttings of Carya illinoinensis during rooting[J]. J Nanjing For Univ Nat Sci Ed, 2016, 40(5):191-196. [7] 裴东.核桃等树种不定根发生及其无性繁殖[M].北京:中国环境科学出版社, 2009. [8] 宋晓波. 复幼提高核桃不定根发生能力的多基因作用机制[D]. 北京: 北京林业大学, 2016. SONG Xiaobo. Role of Multi-genes during Rooting Promoting by Rejuvenation in the Adventitious Rooting of Walnut[D]. Beijing:Beijing Forestry University, 2016. [9] 叶宝兴, 毕建杰, 孙印石.植物组织与细胞研究方法[M].北京:化学工业出版社, 2011. [10] 贾鹏禹, 曾明飞, 冯乃杰, 等.在线固相萃取-高效液相色谱-串联质谱法同时检测大豆不同部位的4种植物激素[J].分析化学, 2014, 42(12):1743-1749. JIA Pengyu, ZENG Mingfei, FENG Naijie, et al. An automated dual-gradient liquid chromatography MS/MS method for simultaneous determination of four kinds of plants hormones in different parts of soya beans and its application to a real analysis[J]. Chin J Analyt Chem, 2014, 42(12):1743-1749. [11] HENRIQUE A, CAMPINHOS E N, ONO E O, et al. Effect of plant growth regulators in the rooting of Pinus cuttings[J]. Braz Arch Biol Technol, 2006, 49(2):189-196. [12] OUYANG Fangqun, WANG Junhui, LI Yue. Effects of cutting size and exogenous hormone treatment on rooting of shoot cuttings in Norway spruce[Picea abies, (L.) Karst.] [J]. New For, 2015, 46(1):91-105. [13] NORDSTRÖM A C, ELIASSON L. Levels of endogenous indole-3-acetic acid and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings[J]. Physiol Plant, 1991, 82(4):599-605. [14] WOLTERS H, JÜRGENS G. Survival of the flexible:hormonal growth control and adaptation in plant development[J]. Nat Rev Genet, 2009, 10(5):305-317. [15] SAINI S, SHARMA I, KAUR N, et al. Auxin:a master regulator in plant root development[J]. Plant Cell Rep, 2013, 32(6):741-757. [16] PACURAR D I, PERRONE I, BELLINI C. Auxin is a central player in the hormone cross-talks that control adventitious rooting[J]. Physiol Plant, 2014, 151(1):83-96. [17] de ALMEIDA M R, de BASTIANI D, GAETA M L, et al. Comparative transcriptional analysis provides new insights into the molecular basis of adventitious rooting recalcitrance in Eucalyptus[J]. Plant Sci, 2015, 239(7):155-165. [18] STEFFENS B, WANG J X, SAUTER M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice[J]. Planta, 2006, 223(3):604-612. [19] DAVIES P J. The plant hormones:their nature, occurrence, and functions[G]//DAVIES P J. Plant Hormones.[s.l.]:Springer Netherlands, 2010:1-12. [20] BUSOV V, MEILAN R, PEARCE D W, et al. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus[J]. Planta, 2006, 224(2):288-299. [21] MAURIAT M, PETTERLE A, BELLINI C, et al. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport[J]. Plant J Cell Mol Biol, 2014, 78(3):372-384. [22] LAPLAZE L, BENKOVA E, CASIMIRO I, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation[J]. Plant Cell, 2007, 19(12):3889-3900. [23] MARHAVÝ P, DUCLERCQ J, WELLER B, et al. Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis[J]. Curr Biol, 2014, 24(9):1031-1037. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2017.06.010







 下载:
下载: