-
石漠化是中国西南脆弱岩溶基底基础上形成的一种特有荒漠化生态现象[1],如何恢复该地区植被是一个亟待解决的瓶颈问题[2]。丛枝菌根(AM)真菌作为广布于土壤的一类非专一性有益微生物,能与绝大多数植物根系耦合形成共生体[3]。丛枝菌根真菌通过“菌丝—根系—土壤”之间的耦合,能够显著影响植物水、碳、氮、磷等营养物质代谢及光合生理生化过程[4],进而直接或间接地促进石漠化生境的植物生长[5]。丛枝菌根真菌共生能够提高菌根浸染率,形成菌丝桥改变根系形态学特征,直接或间接促进寄主植物对水分与养分的吸收与利用[6],进而为植物光反应与暗反应供给物质与能量。丛枝菌根真菌还可通过分泌细胞分裂素,降低叶绿素分解速率,促进叶绿素肽链所需酶的合成,增加叶绿素含量与叶面积,从而间接地提高植物的光合速率[7-8]。同时,丛枝菌根真菌共生能够改善宿主植物营养状况,从而显著促进植株树高和胸径生长及根、茎、叶生物量的积累[9]。陈良华等[10]研究发现:丛枝菌根真菌与美洲黑杨Populus deltoides共生,可显著提高植物光合作用、水分利用效率以及降低蒸腾速率,进而促进植物生长。
白枪杆Fraxinus malacophylla为木犀科Oleaceae梣属Fraxinus植物,广布于石灰岩为主的中国西南山地次生林中,具有喜光、耐贫瘠、速生等特点[11]。目前,白枪杆作为云南石漠化恢复的首选阔叶物种,造林成活率高,植被恢复效果好,被广泛应用于该地区的植被恢复。然而,如何选择对白枪杆具有较高亲和度的优势菌种,仍然是菌根技术运用中的关键科学问题。因此,基于“植物根系—丛枝菌根真菌—土壤”相互作用的理论,以3种丛枝菌根真菌为研究对象,探究丛枝菌根真菌与白枪杆共生对白枪杆树高和胸径生长、根和叶生物量积累、光合色素与叶绿素合成、光合荧光特征等的影响,以期筛选石漠化植被恢复的优势菌种,为丛枝菌根技术在石漠化植被恢复中的运用提供参考数据。
-
研究区位于云南省红河州弥勒县西一镇(24°44′N,103°38′E),该区属亚热带季风气候区,夏季高温多雨,冬季温和少雨。年均降水量为1 070 mm,年均气温为17 ℃,年均日照时间为2 322 h。选择国家林业和草原局石漠化治理技术推广示范基地的白枪杆群落为样地。样地恢复年限约5 a,海拔为1 600 m,土壤为砂质红壤,上覆盖凋落物厚度约0.5 cm,坡度较缓,郁闭度为35%。主要树种有白枪杆、车桑子Dodonaea viscosa、紫茎泽兰Eupatorium adenophora、长波叶山蚂蝗Desmodium sequax、胡枝子Lespedeza bicolor、地果Ficus tikoua、艾纳香Blumea balsamifera等。
-
随机设置3个白枪杆群落重复样地(40 m×40 m,相距500 m)进行田间试验。供试丛枝菌根真菌分别为摩西斗管囊霉Funneliformis mosseae、幼套近明球囊霉Claroideoglomus etunicatum、根内根孢囊霉Rhizophagus intraradices。每个重复样地中,均设置接种摩西斗管囊霉+农林生物肥(MN)、幼套近明球囊霉+农林生物肥(YN)、根内根孢囊霉+农林生物肥(GN)、农林生物肥(对照,ck)共4个处理。农林生物肥为大豆Glycine max饼肥与油茶Camellia oleifera饼肥1∶1(质量比)混合的有机肥。每种菌剂接种量为40 g·株−1(孢子数约144个·g−1),菌剂均购自北京市农林科学院的植物营养与资源研究所丛枝菌根真菌种质资源库(BGC)。
-
2020年9月,测定样地内所有白枪杆植株生长指标,采用钢卷尺测量树高,游标卡尺测定胸径,株高为整个地上部高度,胸径测定地面上5~10 cm处横径,取平均值;采用Li-6400XT便携式光合仪(Licor-6400,美国),于晴朗无风的天气9:00—16:00,选择4株生长良好的白枪杆,从顶叶向下数第2、3、4片展开功能叶片,测定净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)和胞间二氧化碳摩尔分数(Ci)、水分利用效率(Ewu),重复3次,每个样地上午每隔1 h随机测定1次,下午每隔2 h测定1次,共测定3 d。
光合测定结束后,用叶面积仪测定白枪杆单叶平均面积(cm2);采用天平(精度0.1 mg)称取植株叶片和根系鲜质量,然后将洗净的植株组织放入烘箱,105 ℃杀青后,75 ℃烘干至恒量,用天平称取叶片和根系干质量。采用丙酮法测定叶绿素质量分数[8]。其中光合测定时,室外环境光源有效辐射为1 000 μmol·m−2·s−1,气体流速为500 μmol·s−1。水分利用效率为净光合速率与蒸腾速率的比值。
-
采用Excel 2010进行数据整理,计算平均值±标准差。借助SPSS 23.0软件,采用单因素法(one-way ANOVA)对不同处理进行差异性检验。利用Origin 2018制图。
-
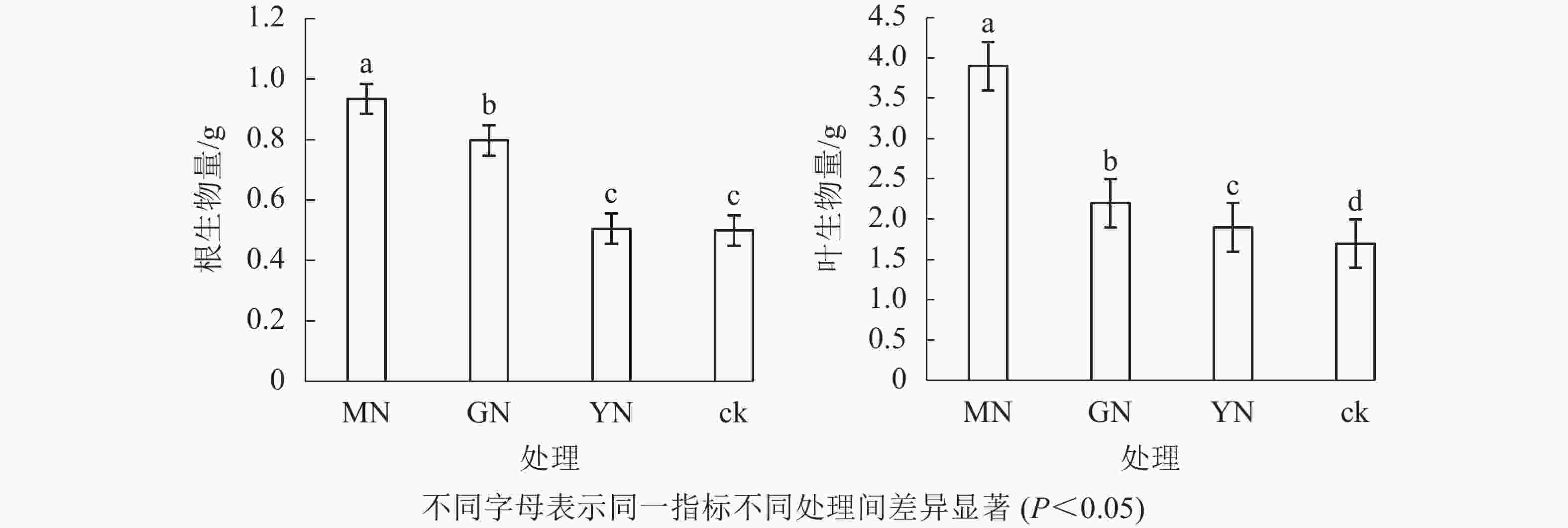
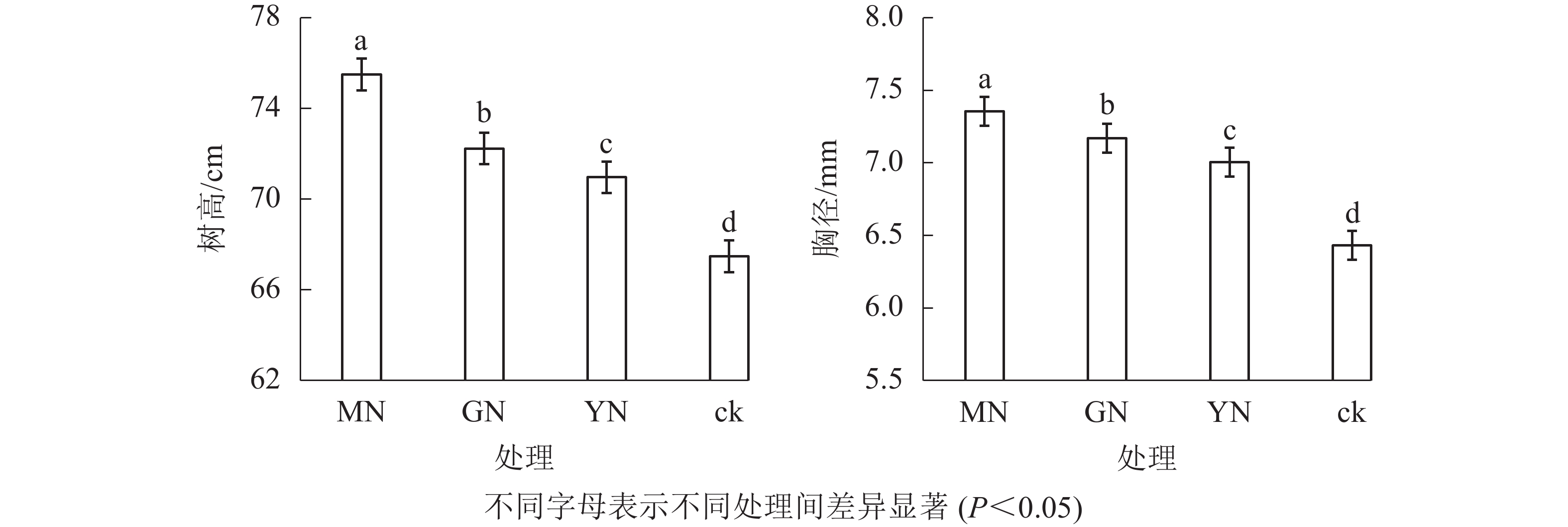
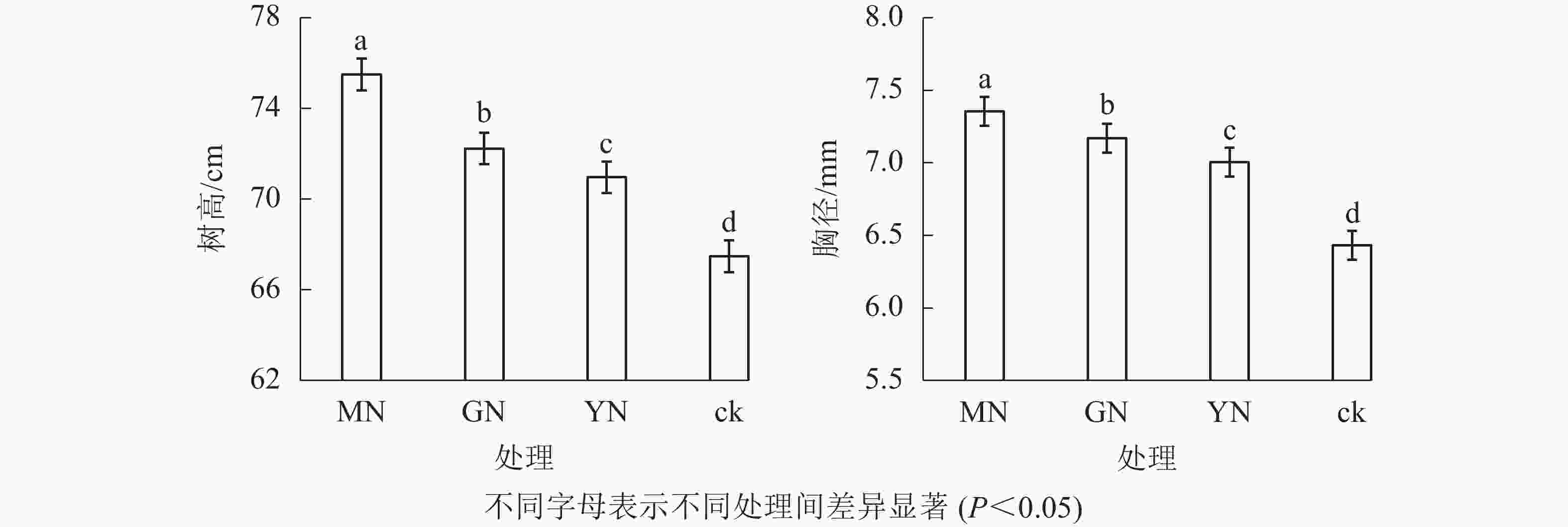
相较于对照,接种3种丛枝菌根真菌显著提高了白枪杆的树高和胸径(图1,P<0.05)。3种真菌处理下白枪杆树高和胸径的增幅分别为1.05~1.12倍和1.08~1.14倍,其中摩西斗管囊霉菌接种的增幅最大。3种真菌处理间白枪杆根和叶生物量均存在显著差异(图2,P<0.05)。相较于对照,接种摩西斗管囊霉、幼套近明球囊霉、根内根孢囊霉处理的生物量平均增长率分别为119%、31%和10%。
-
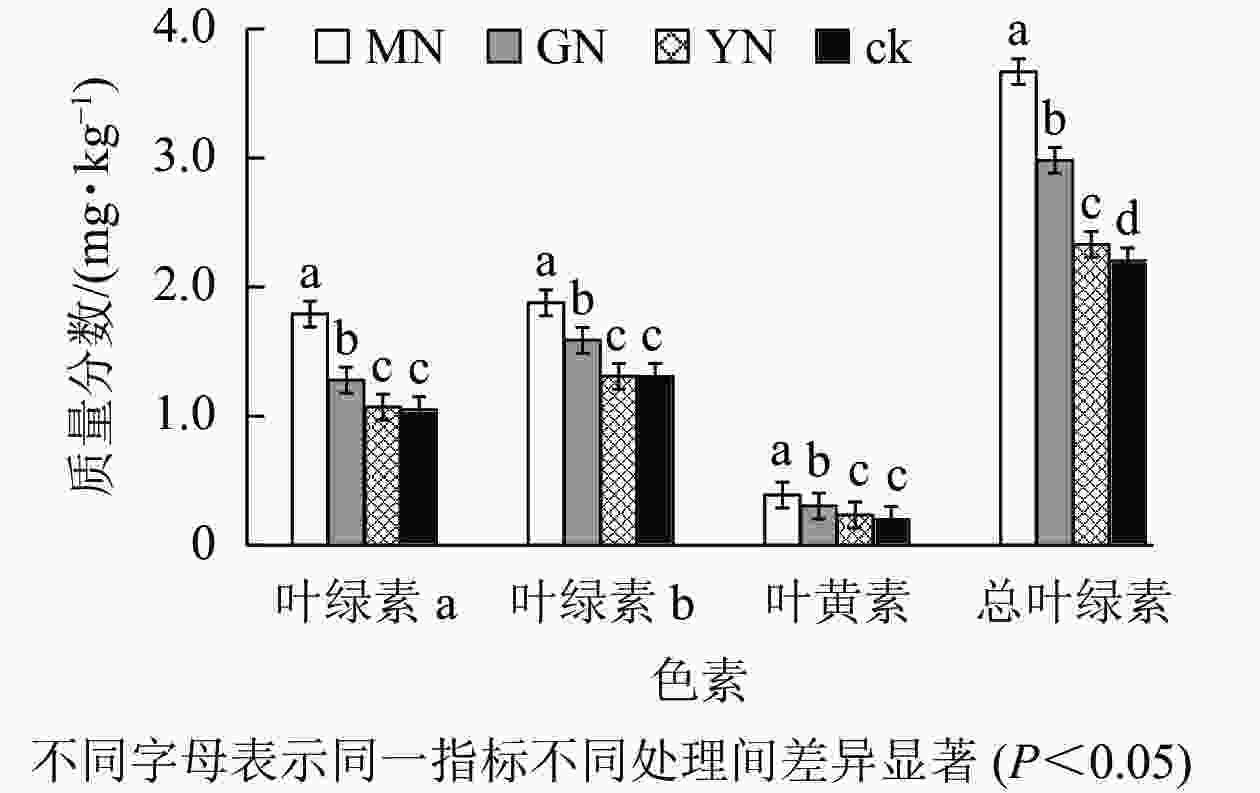
如图3所示:丛枝菌根真菌接种均显著提高白枪杆叶绿素a、叶绿素b、叶黄素及总叶绿素质量分数(P<0.05)。相较于对照组,3种丛枝菌根真菌处理下的叶绿素a、叶绿素b、叶黄素及总叶绿素增幅分别为1.02~1.71、1.12~1.44、1.17~1.94、1.06~1.67倍。
-
如图4所示:白枪杆净光合速率、气孔导度和蒸腾速率日变化规律均呈双峰曲线特征,3个指标均在10:00达第1个峰值,为日变化的最大值,并在14:00达第2个峰值。白枪杆胞间二氧化碳摩尔分数日变化呈W型双波谷变化规律,在9:00—10:00胞间二氧化碳摩尔分数逐渐降低;10:00后气孔关闭,胞间二氧化碳摩尔分数有所回升,在12:00时达到峰值之后,胞间二氧化碳摩尔分数再次下降;14:00后,胞间二氧化碳摩尔分数持续上升,至16:00达最高。
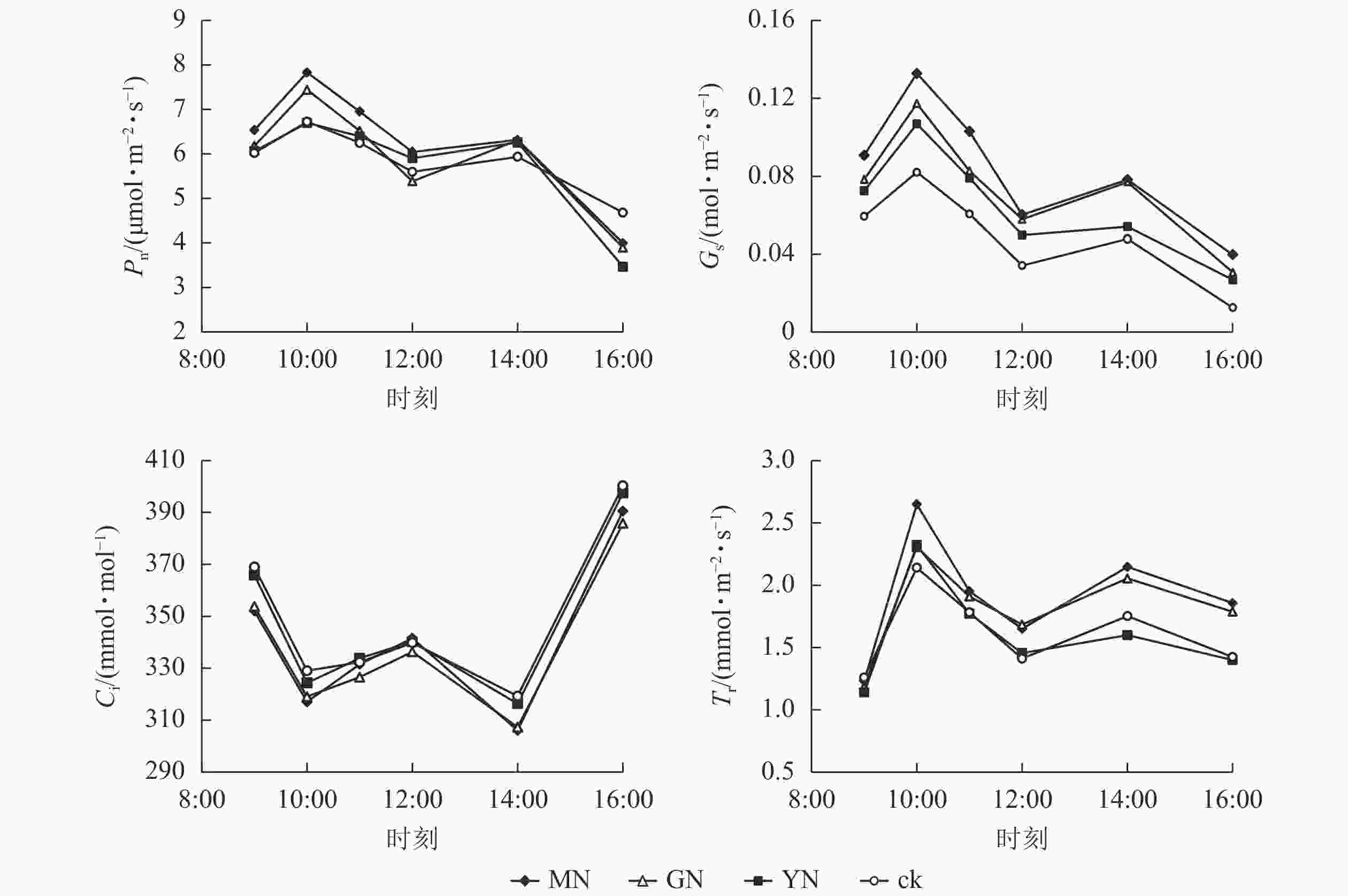
图 4 不同丛枝菌根真菌处理下白枪杆叶片光合特征的日变化
Figure 4. Daily changes of leaf photosynthetic characteristics of F. malacophylla under different arbuscular mycorrhizal fungi treatments
丛枝菌根真菌接种显著提高白枪杆叶片的光合速率、气孔导度和蒸腾速率(P<0.05),光合速率较对照组提高了12.3%~17.5%。其中,不同丛枝菌根真菌接种对净光合速率、气孔导度和蒸腾速率的提升率从大到小依次为摩西球囊霉菌、根内囊球霉菌、幼套球囊霉菌、对照处理。调节叶片与周围环境间物质交换的气孔导度较对照组提高了20%~40%;作为植物光反应和暗反应的主要原料,胞间二氧化碳摩尔分数与净光合速率的变化呈相反的变化规律,表明植物光合作用的荧光参数中,胞间二氧化碳摩尔分数可能不是净光合速率主控因子。
-
从表1可知:各光合参数间均存在显著相关关系,其中净光合速率与气孔导度呈极显著正相关,且相关系数最高,为0.950;胞间二氧化碳摩尔分数与其他参数均呈负相关;蒸腾速率与净光合速率及气孔导度呈正相关(P<0.05);水分利用效率与蒸腾速率呈极显著负相关(P<0.01)。
表 1 白枪杆光合特征参数间的相关关系
Table 1. Correlation among photosynthetic characteristic parameters of F. malacophylla
指标 Pn Gs Ci Tr Ewu Pn 1 0.950** −0.690** 0.562** 0.087 Gs 1 −0.565** 0.512* 0.081 Ci 1 −0.675** 0.160 Tr 1 −0.690** Ewu 1 说明:*表示显著相关(P<0.05);**表示极显著相关 (P<0.01) 由表2可知:白枪杆净光合速率与叶黄素、树高、根干质量、叶鲜质量、叶干质量、叶面积呈极显著正相关(P<0.01),与叶绿素b、总叶绿素以及根鲜质量呈显著正相关(P<0.05),而与叶绿素a、胸径的相关性不显著。
表 2 白枪杆光合参数与叶片色素、生长指标之间的关系
Table 2. Correlation among photosynthetic parameters, leaf pigments and plant growth indices of F. malacophylla
指标 净光合
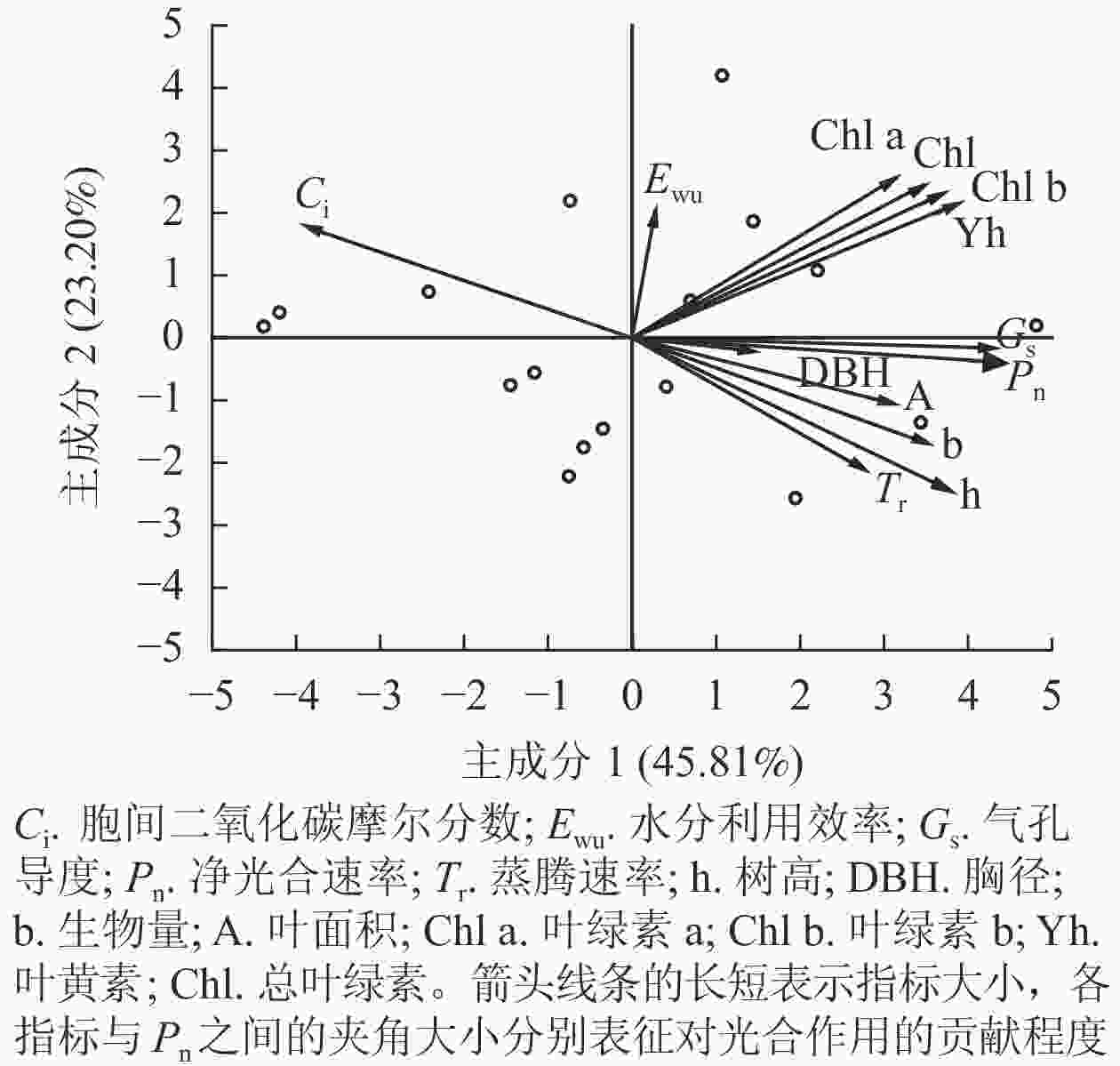
速率叶绿素a 叶绿素b 总叶绿素 叶黄素 胸径 树高 根鲜质量 根干质量 叶鲜质量 叶干质量 叶面积 净光合速率 1 叶绿素a 0.359 1 叶绿素b 0.466* 0.928** 1 总叶绿素 0.494* 0.968** 0.989** 1 叶黄素 0.603** 0.723** 0.805** 0.819** 1 胸径 0.203 0.123 0.012 0.141 −0.036 1 树高 0.769** −0.003 0.125 0.120 0.171 0.148 1 根鲜质量 0.496* 0.152 0.312 0.264 0.404 −0.086 0.583** 1 根干质量 0.553** 0.078 0.261 0.238 0.353 −0.252 0.652** 0.918** 1 叶鲜质量 0.615** 0.199 0.331 0.319 0.468* −0.143 0.607** 0.910** 0.873** 1 叶干质量 0.559** 0.238 0.330 0.316 0.508* −0.234 0.499* 0.759** 0.686** 0.900** 1 叶面积 0.597** 0.062 0.227 0.165 0.279 −0.078 0.787** 0.819** 0.884** 0.858** 0.716** 1 说明:*表示显著相关(P<0.05);**表示极显著相关(P<0.01) 图5所示:主成分1和主成分2对净光合速率的贡献率分别为45.81%、23.20%。按箭头夹角来看,气孔导度、树高、叶黄素与净光合速率的夹角较小,说明它们是促进净光合速率的主控因子;叶绿素b、生物量、总叶绿素对净光合速率的影响次之;胞间二氧化碳摩尔分数与净光合速率的夹角为钝角,呈负相关关系,表现出抑制效应。
-
本研究表明:丛枝菌根真菌接种均显著提高了石漠化地区白枪杆树高和胸径的生长,显著增加了根和叶生物量。这可能是丛枝菌根真菌的接种显著提高了寄主根系的侵染率和菌丝密度,提高了根系吸收水分与养分的效率,进而促进了植物的地上与地下生长[12]。岳辉等[13]与袁丽环等[14]的研究结果也表明:丛枝菌根真菌能显著促进紫穗槐Amorpha fruticosa、翅果油树Elaeagnus mollis的生长。这是因为丛枝菌根真菌与寄主植物根系形成菌丝共生体后建立了互利共惠关系,宿主植物能够为丛枝真菌生长提供所需的光合产物[15-16],而丛枝菌根真菌通过其菌丝网络结构扩大了植物根系的表面积,并改善石漠化土壤团聚体结构,有利于植物根系从土壤中吸收水分及碳、氮、磷等营养物质[17],并为植物叶片与叶面积数量增长提供充足的养分供给,进一步通过增加光能接收场所来提高植物光合效率,从而促进植物地上(树高、胸径、叶生物量等)及地下(根系生物量)的生长。
不同丛枝菌根真菌对寄主植物的促生效应存在一定差异。本研究中,摩西斗管囊霉的促生效果显著高于幼套近明球囊霉、根内根孢囊霉。王邵军等[12]与马仕林等[18]研究也表明:不同菌种因侵染能力不同而对植物的促生效应不同。岳辉等[13]也报道摩西斗管囊霉对紫穗槐树高和胸径生长具有最大的促进作用,但何跃军等[15]研究表明:摩西斗管囊霉对石漠化基质上构树Broussonetia papyrifera生长的促进作用显著低于其他2种丛枝菌根菌种。这可能是因为不同菌根真菌的侵染能力及宿主植物的反应特异性存在一定差异,从而对寄主植物生长发育产生不同的调控效应。
-
植物叶片色素能够捕获和转换光能,决定着植物的光合效率。叶绿素a和叶绿素b是植物光合最直接的参与者,能调控植物光合能量的积累。叶黄素作为辅助色素,它形成的叶黄素循环和类胡萝卜素的清除途径可耗散过剩热能,是植物重要的光保护机制[19]。本研究表明:接种丛枝菌根真菌对叶绿素a、叶绿素b、叶黄素及总叶绿素的提高率达10%~60%,但低于孙佳琦等[20]对槲树Quercus dentata接种真菌后的叶绿素a和总叶绿素的提升效果(22%~93%)。然而,袁丽环等[14]接种丛枝菌根真菌对翅果油树幼苗叶绿素b质量分数的影响表现出负效应。可见,不同丛枝菌根真菌共生对干旱胁迫生境中植物总叶绿素与叶黄素质量分数的提升率存在差异,这可能与石漠化生境理化状况、树种年龄以及菌种适应能力密切相关[21]。
丛枝菌根真菌共生能够显著促进退化生境寄主植物叶片光合色素质量分数。主要是由于丛枝菌根真菌能够提高光合色素合成过程中的水、蛋白质、酶等物质的循环效率,从而显著增加叶绿素a和叶绿素b质量分数。丛枝菌根真菌共生能够提高叶片色素质量分数,增强植物叶片对光谱中红、蓝光的捕捉能力,增加单位面积有活性反应中心数量,提高叶片光系统线性电子传递速率、实际光化学效率以及光化学猝灭系数,进而提升寄主植物的光能利用率[22]。另外,丛枝菌根真菌共生促进叶黄素参与三大循环,间接地影响植物的光合作用[23]。因此,本研究中丛枝菌根真菌接种显著提升了白枪杆叶片光合色素含量,有助于理解丛枝菌根真菌共生对寄主植物光合作用的影响机制。
-
丛枝菌根真菌共生通过刺激白枪杆的树高和胸径生长及根系生物量积累,促进植物的光合作用,这与赵华等[24]和王志刚等[25]的研究结果相似。可能是由于丛枝菌根真菌共生能够在植物根系与土壤中形成菌丝桥,在根皮部形成丛枝结构,有效地改善通气通水条件,提高土壤养分的可利用性[26],促进植物对营养物质的吸收与固碳循环,从而提高植物生长及光合效率。丛枝菌根真菌定殖还可引起根际分泌和周围土壤微生物群落的变化,有利于某些细菌属(如偶氮螺菌Azospirillum、假单胞菌Pseudomonas)的增长[27],影响土壤养分的循环效率,从而直接或间接促进植物的生长与光合作用。特别是植物高度与胸径能够影响植物对弱光的接受效率,所产生的联动效应[28]影响植物光合作用。
叶绿素是植物光合作用过程中最主要、最敏感的色素,其含量变化能够调控光合作用的效率。本研究发现:接种摩西球囊霉菌、根内球囊霉菌和幼套球囊霉菌显著提高了白枪杆叶片叶绿素质量分数和净光合速率。崔令军等[29]研究证实:接种丛枝菌根真菌对植物叶片光合色素质量分数具有促进作用,并显著提高了植物净光合速率。可能是由于丛枝菌根真菌共生有利于光合作用叶绿素的内囊体以及碳同化酶(如二磷酸核酮糖羧化酶)活性的合成[30],从而增加光合色素质量分数以及叶中的碳源,并直接提高了光系统Ⅰ和Ⅱ对红蓝光的吸收效率,有利于植物将激发能转化为生物化学能,从而提高光合过程中的转能效率及净光合速率。
丛枝菌根真菌共生通过影响寄主植物光合荧光特征而提高植物光合速率。气孔导度、蒸腾速率、胞间二氧化碳摩尔分数、水分利用效率等是影响光合作用强弱的重要参数。研究发现:接种丛枝菌根真菌后植物的光合作用增加,这主要取决于植物叶片气孔通透性和胞间二氧化碳摩尔分数及水分利用效率[31]。本研究中,接种丛枝菌根真菌后,增加了白枪杆叶片的气孔导度、蒸腾速率、水分利用效率,降低了叶片的胞间二氧化碳摩尔分数,从而提高了植物的净光合速率,且叶片光合色素及生长指标呈正相关关系。这与宋会兴等[32]对旱生境中三叶鬼针草Bidens pilosa接种丛枝菌根真菌后,气孔导度显著增加,胞间二氧化碳摩尔分数显著降低,从而提高植物的净光合速率的结果相似。这可能是因为丛枝菌根真菌共生能够引起植物叶片气孔导度的增大,使得外界环境中更多二氧化碳进入叶肉细胞,降低了胞间二氧化碳摩尔分数,这为叶片光合作用提供了充足的原料,加速了光反应体系的效率,从而提高了植物的光合效率。
在石漠化地区,丛枝菌根真菌共生能够有效地增加白枪杆叶片蒸腾速率和水分利用效率,从而提高植物光合速率。可能是由于丛枝菌根真菌改善植物水分代谢状况与抗旱性,保障了净光合作用的水分供给。郑亚茹等[33]接种丛枝菌根菌根真菌对盐胁迫下桑树Morus alba光合特性的影响研究也表明:接种真菌处理下桑树叶片蒸腾速率与净光合速率呈显著正相关,说明丛枝菌根真菌能够解除非气孔限制(蒸腾速率、水分利用效率),从而提高净光合速率。因此,石漠化生境胁迫条件下,丛枝菌根真菌共生能够刺激植物地上与地下生长、色素合成及叶片荧光特征变化,进而调控植物的光合作用过程及效能。
Effects of arbuscular mycorrhizal fungi symbiosis on growth and photosynthetic characteristics of Fraxinus malacophylla in
-
摘要:
目的 探究石漠化生境丛枝菌根真菌共生对白枪杆Fraxinus malacophylla生长及光合特征的影响,为植被恢复选取优势菌种提供参考。 方法 设置摩西斗管囊霉Funneliformis mosseae+农林生物肥(MN)、幼套近明球囊霉Claroideoglomus etunicatum+农林生物肥(YN)、根内根孢囊霉Rhizophagus intraradices+农林生物肥(GN)、农林生物肥(ck)共4个处理,测定不同处理下白枪杆生长(树高、胸径、根和叶生物量、叶面积、叶片色素及叶绿素)及光合特征(净光合速率、气孔导度、胞间二氧化碳摩尔分数、蒸腾速率、水分利用效率等)的变化。 结果 ①接种丛枝菌根真菌显著促进了白枪杆的生长与叶、根生物量积累(P<0.05)。②接种摩西斗管囊霉和根内根孢囊霉显著提高了白枪杆叶绿素a、叶绿素b、叶黄素的相对含量(P<0.05),提升率达6%~67%。③接种丛枝菌根真菌显著提高了白枪杆的净光合速率、气孔导度、蒸腾速率与水分利用效率(P<0.05),显著降低了胞间二氧化碳摩尔分数(P<0.05)。④主成分分析表明:气孔导度、树高、叶黄素是提高净光合速率的主控因子,平均贡献率达45.81%,叶绿素b、生物量和总叶绿素的影响次之。 结论 丛枝菌根真菌共生主要通过促进植株生长、光合色素含量,显著提高白枪杆净光合速率,其中摩西斗管囊霉为最优菌种。图5表2参33 Abstract:Objective This study aims to explore the effects of arbuscular mycorrhizal (AM) fungal symbiosis on growth and photosynthetic characteristics of Fraxinus malacophylla in rocky desertification habitats, so as to provide data reference for selecting dominant AM fungal species for vegetation restoration. Method An experiment was designed with four treatments: Funneliformis mosseae+agroforestry biofertilizer (MN), Claroideoglomus etunicatum+agroforestry biofertilizer (YN), Rhizophagus intraradices+agroforestry biofertilizer (GN) and agroforestry biofertilizer (ck). The changes in F. malacophylla growth (tree height, diameter at breast height, leaf and root biomass, leaf area, leaf pigment and chlorophyll) and photosynthetic characteristics (net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, transpiration rate, and leaf water use efficiency, etc.) were measured under different treatments. Result (1) Inoculation with AM fungi significantly promoted the growth of F. malacophylla and biomass accumulation of leaf and root(P<0.05). (2) Inoculation with MN and GN significantly increased the relative contents of chlorophyll a, chlorophyll b and lutein in plant leaves (P<0.05), and the increase rate was 6%−67%. (3) Inoculation with AM fungi significantly increased the net photosynthetic rate, stomatal conductance, transpiration rate, and water use efficiency of F. malacophylla (P<0.05), but significantly decreased the intercellular CO2 concentration (P<0.05). (4) Principal component analysis indicated that the stomatal conductance, tree height, and lutein were the key factors to increase the net photosynthetic rate, with an average contribution rate of 45.81%, followed by chlorophyll b, biomass and total chlorophyll. Conclusion AM fungal symbiosis can significantly improve the net photosynthetic rate of F. malacophylla by promoting plant growth and photosynthetic pigment content. The optimal strain is F. mosseae. [Ch, 5 fig. 2 tab. 33 ref.] -
表 1 白枪杆光合特征参数间的相关关系
Table 1. Correlation among photosynthetic characteristic parameters of F. malacophylla
指标 Pn Gs Ci Tr Ewu Pn 1 0.950** −0.690** 0.562** 0.087 Gs 1 −0.565** 0.512* 0.081 Ci 1 −0.675** 0.160 Tr 1 −0.690** Ewu 1 说明:*表示显著相关(P<0.05);**表示极显著相关 (P<0.01) 表 2 白枪杆光合参数与叶片色素、生长指标之间的关系
Table 2. Correlation among photosynthetic parameters, leaf pigments and plant growth indices of F. malacophylla
指标 净光合
速率叶绿素a 叶绿素b 总叶绿素 叶黄素 胸径 树高 根鲜质量 根干质量 叶鲜质量 叶干质量 叶面积 净光合速率 1 叶绿素a 0.359 1 叶绿素b 0.466* 0.928** 1 总叶绿素 0.494* 0.968** 0.989** 1 叶黄素 0.603** 0.723** 0.805** 0.819** 1 胸径 0.203 0.123 0.012 0.141 −0.036 1 树高 0.769** −0.003 0.125 0.120 0.171 0.148 1 根鲜质量 0.496* 0.152 0.312 0.264 0.404 −0.086 0.583** 1 根干质量 0.553** 0.078 0.261 0.238 0.353 −0.252 0.652** 0.918** 1 叶鲜质量 0.615** 0.199 0.331 0.319 0.468* −0.143 0.607** 0.910** 0.873** 1 叶干质量 0.559** 0.238 0.330 0.316 0.508* −0.234 0.499* 0.759** 0.686** 0.900** 1 叶面积 0.597** 0.062 0.227 0.165 0.279 −0.078 0.787** 0.819** 0.884** 0.858** 0.716** 1 说明:*表示显著相关(P<0.05);**表示极显著相关(P<0.01) -
[1] 曹建华, 袁道先, 童立强. 中国西南岩溶生态系统特征与石漠化综合治理对策[J]. 草业科学, 2008, 25(9): 40 − 50. CAO Jianhua, YUAN Daoxian, TONG Liqiang. Features of karst ecosystem and integrating measure for rock desertification in southwest China [J]. Pratacultural Sci, 2008, 25(9): 40 − 50. [2] 袁道先. 岩溶石漠化问题的全球视野和我国的治理对策与经验[J]. 草业科学, 2008, 25(9): 19 − 25. YUAN Daoxian. Global view on karst rock desertification and integrating control measures and experiences of China [J]. Pratacultural Sci, 2008, 25(9): 19 − 25. [3] 孙吉庆, 刘润进, 李敏. 丛枝菌根真菌提高植物抗逆性的效应及其机制研究进展[J]. 植物生理学报, 2012, 48(9): 845 − 852. SUN Jiqing, LIU Runjin, LI Min. Advances in the study of increasing plant stress resistance and mechanisms by arbuscular mycorrhizal fungi [J]. Plant Physiol J, 2012, 48(9): 845 − 852. [4] 王邵军. “植物—土壤”相互反馈的关键生态学问题: 格局、过程与机制[J]. 南京林业大学学报(自然科学版), 2020, 44(2): 1 − 9. WANG Shaojun. Key ecological issues in plant-soil feedback: pattern, process and mechanism [J]. J Nanjing For Univ Nat Sci Ed, 2020, 44(2): 1 − 9. [5] 谌诺君, 李辉, 赵子豪, 等. 丛枝菌根真菌提高植物抗逆性研究进展[J]. 河南农业科学, 2014, 43(10): 1 − 5. CHEN Nuojun, LI Hui, ZHAO Zihao, et al. Research progress on arbuscular mycorrhizal fungi improving plant stress resistance [J]. Henan Agric Sci, 2014, 43(10): 1 − 5. [6] 王邵军, 李霁航, 陆梅, 等. “AM真菌-根系-土壤”耦合作用机制研究进展[J]. 中南林业科技大学学报, 2019, 39(12): 1 − 9. WANG Shaojun, LI Jihang, LU Mei, et al. Advance on the mechanism of coupling interactions among AM fungi, roots and soils [J]. Cent South Univ For Technol, 2019, 39(12): 1 − 9. [7] MOUSTAKAS M, BAYCU G, SPERDOULI I, et al. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem Ⅱ photochemistry[J/OL]. Plants, 2020, 9(8): 962[2021-10-20]. doi: 10.3390/plants9080962. [8] NIKOLAEVA M K, MAEVSKAYA S N, SHUGAEV A G, et al. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity [J]. Russ J Plant Physiol, 2010, 57(1): 87 − 95. [9] 刘欢, 姚拓, 刘婷, 等. 不同丛枝菌根真菌对苜蓿生长的影响[J]. 草原与草坪, 2017, 37(4): 61 − 67, 73. LIU Huan, YAO Tuo, LIU Ting, et al. Effect of different arbuscular mycorrhizal fungi on the growth of Medicago sativa [J]. Grassland Turf, 2017, 37(4): 61 − 67, 73. [10] 陈良华, 赖娟, 胡相伟, 等. 接种丛枝菌根真菌对受镉胁迫美洲黑杨雌、雄株光合生理的影响[J]. 植物生态学报, 2017, 41(4): 480 − 488. CHEN Lianghua, LAI Juan, HU Xiangwei, et al. Effects of inoculation with arbuscular mycorrhizal fungi on photosynthetic physiology in females and males of Populus deltoides exposed to cadmium pollution [J]. Chin J Plant Ecol, 2017, 41(4): 480 − 488. [11] 李燕燕, 段华超, 李世民, 等. 3种外源植物激素对白枪杆幼苗生长特性的影响[J]. 贵州农业科学, 2021, 49(4): 112 − 117. LI Yanyan, DUAN Huachao, LI Shiming, et al. Effect of there exogenous plant hormones on growth characteristics of Fraxinus malacophylla seedlings [J]. Guizhou Agric Sci, 2021, 49(4): 112 − 117. [12] 王邵军, 左倩倩, 曹乾斌, 等. 云南寻甸石漠化土壤易氧化碳对丛枝菌根真菌共生的响应[J]. 南京林业大学学报(自然科学版), 2022, 46(1): 7 − 14. WANG Shaojun, ZUO Qianqian, CAO Qianbin et al. Response of readily oxidized carbon to AM fungi inoculations in rocky desertification soils, Xundian, Yunnan [J]. J Nanjing For Univ Nat Sci Ed, 2022, 46(1): 7 − 14. [13] 岳辉, 毕银丽, ZHAKYPBEK Y, 等. 接种菌根对神东矿区采煤沉陷地的生态修复效应[J]. 科技导报, 2012, 30(36): 56 − 60. YUE Hui, BI Yinli, ZHAKYPBEK Y, et al. Ecological reclamation effect of arbuscualr mycorrhizal inoculum on subsided land in the area of shendong coal mine [J]. Sci Technol Rev, 2012, 30(36): 56 − 60. [14] 袁丽环, 王文科. 接种AM菌根对翅果油树幼苗生长及叶片光合作用的影响[J]. 西北林学院学报, 2011, 26(4): 33 − 35, 127. YUAN Lihuan, WANG Wenke. Influence of AM fungion seedling growth and photosythsis of Elaeagnus mollis [J]. Northwest For Univ, 2011, 26(4): 33 − 35, 127. [15] 何跃军, 钟章成, 刘锦春, 等. 石灰岩土壤基质上构树幼苗接种丛枝菌根(AM)真菌的光合特征[J]. 植物研究, 2008, 28(4): 452 − 457. HE Yuejun, ZHONG Zhangcheng, LIU Jinchun et al. Photosynthetic characteristics of Broussonetia papyrifera seedlings inoculated AM fungus in limestone soil substratum [J]. Bull Bot Res, 2008, 28(4): 452 − 457. [16] 姚娟, 王茂胜, 王通明, 等. 接种丛枝菌根真菌对烤烟叶片光合特性的影响[J]. 中国烟草科学, 2013, 34(4): 30 − 35. YAO Juan, WANG Maosheng, WANG Tongming, et al. Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics in leaves of flue-cured tobacco [J]. Chin Tob Sci, 2013, 34(4): 30 − 35. [17] 陈永亮, 陈保冬, 刘蕾, 等. 丛枝菌根真菌在土壤氮素循环中的作用[J]. 生态学报, 2014, 34(17): 4807 − 4815. CHEN Yongliang, CHEN Baodong, LIU Lei, et al. The role of arbuscular mycorrhizal fungi in soil nitrogen cycling [J]. Acta Ecol Sin, 2014, 34(17): 4807 − 4815. [18] 马仕林, 曹鹏翔, 张金池, 等. 盐胁迫下AMF对榉树幼苗生长和光合特性的影响[J]. 南京林业大学学报(自然科学版), 2022, 46(1): 122 − 130. MA Shilin, CAO Pengxiang, ZHANG Jinchi, et al. Effects of AMF on the growth and photosynthetic characteristics of Zelkova serrata under salt stress [J]. J Nanjing For Univ Nat Sci Ed, 2022, 46(1): 122 − 130. [19] 靳红磊, 明宇, 王宏斌. 阴生和阳生植物在光合结构及功能中的差异概述[J]. 中山大学学报(自然科学版), 2021, 60(6): 1 − 8. JIN Honglei, MING Yu, WANG Hongbin. An overview of the differences between shade and sun plants in photosynthetic structure and function [J]. Acta Sci Nat Univ Sunyatseni, 2021, 60(6): 1 − 8. [20] 孙佳琦, 曹文琪, 冷平生, 等. 接种4种外生菌根真菌对槲树幼苗生长、光合及营养元素含量的影响[J]. 中南林业科技大学学报, 2021, 41(10): 67−74, 101. SUN Jiaqi, CAO Wenqi, LENG Pingsheng, et al Effect of four ectomycorrhizal fungi inoculation on the growth, photosynthesis, and nutrient element content of Quercus dentata seedlings[J]. J Cent South Univ For Technol, 2021, 41(10): 67−74, 101. [21] 喻志, 梁坤南, 黄桂华, 等. 丛枝菌根真菌对植物抗旱性研究进展[J]. 草业科学, 2021, 38(4): 640 − 653. YU Zhi, LIANG Kunnan, HUANG Guihua, et al. Research progress on the mechanisms of arbuscular mycorrhizal fungi on drought resistance in plants [J]. Pratacultural Sci, 2021, 38(4): 640 − 653. [22] 许绍欢, 许忠顺, 杜飞, 等. 混合接种球孢白僵菌与摩西球囊霉对烟草促生抗逆影响[J]. 菌物学报, 2021, 40(8): 2191 − 2200. XU Shaohuan, XU Zhongshun, DU Fei, et al. Effects of mixed inoculation of Beauveria bassiana and Glomus mosseae on tobacco growth and stress resistance [J]. Mycosystema, 2021, 40(8): 2191 − 2200. [23] 刘建新, 胡浩斌, 王鑫. 外源一氧化氮供体对镉胁迫下黑麦草幼苗活性氧代谢、光合作用和叶黄素循环的影响[J]. 环境科学学报, 2009, 29(3): 626 − 633. LIU Jianxin, HU Haobin, WANG Xin. Effects of an exogenous nitric oxide donor on active oxygen metabolism, photosynthesis and the xanthophyll cycle in ryegrass(Lolium perenne L. ) seedlings under cadmium stress [J]. Acta Sci Circumstantiae, 2009, 29(3): 626 − 633. [24] 赵华, 任晴雯, 王熙予, 等. 丛枝菌根真菌对盐胁迫下番茄抗氧化酶活性和光合特性的影响[J]. 浙江农业学报, 2021, 33(11): 2075 − 2084. ZHAO Hua, REN Qingwen, WANG Xiyu, et al. Effects of arbuscular mycorrhizal fungi on antioxidant enzymes activities and photosynthetic characteristics of Solanum lycopersicum L. under salt stress [J]. Acta Agric Zhejiang, 2021, 33(11): 2075 − 2084. [25] 王志刚, 毕银丽, 李强, 等. 接种AM真菌对采煤沉陷地复垦植物光合作用和抗逆性的影响[J]. 南方农业学报, 2017, 48(5): 800 − 805. WANG Zhigang, BI Yinli, LI Qiang, et al. Effects of arbuscular mycorrhizal fungus on photosynthesis and stress resistance of reclamation plants in coal mining subsidence areas [J]. J Southern Agric, 2017, 48(5): 800 − 805. [26] VÁZQUEZ M M, CÉSAR S, AZCÓN R, et al. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants [J]. Appl Soil Ecol, 2000, 15(3): 261 − 272. [27] BALESTRINI R, BRUNETTI C, CHITARRA W, et al. Photosynthetic traits and nitrogen uptake in crops: which is the role of arbuscular mycorrhizal fungi[J/OL]. Plants, 2020, 9(9): 1105[2021-10-20]. doi: 10.3390/plants9091105. [28] FIORILLI V, VANNINI C, ORTOLANI F, et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat [J]. Sci Rep, 2018, 8(1): 1 − 18. [29] 崔令军, 刘瑜霞, 林健, 等. 盐胁迫下丛枝菌根真菌对桢楠根系生长和激素的影响[J]. 南京林业大学学报(自然科学版), 2020, 44(4): 119 − 124. CUI Lingjun, LIU Yuxia, LIN Jian, et al. Effects of arbuscular mycorrhizal fungi on roots growth and endogenous hormones of Phoebe zhennan under salt stress [J]. J Nanjing For Univ Nat Sci Ed, 2020, 44(4): 119 − 124. [30] MASUMOTO C, ISHII T, HATANAKA T, et al. Mechanism of high photosynthetic capacity in BC2F4 lines derived from a cross between Oryza sativa and wild relatives O. rufipogon [J]. Plant Prod Sci, 2005, 8(5): 539 − 545. [31] HUBBARD R M, RYAN M G, STILLER V, et al. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine [J]. Plant Cell Environ, 2001, 24(1): 113 − 121. [32] 宋会兴, 彭远英, 钟章成. 干旱生境中接种丛枝菌根真菌对三叶鬼针草(Bidens pilosa L. )光合特征的影响[J]. 生态学报, 2008, 28(8): 3744 − 3751. SONG Huixing, PENG Yuanying, ZHONG Zhangcheng. Photosynthetic responses of AMF-infected and AMF-free Bidens pilosa L. to drought stress conditions [J]. Acta Ecol Sin, 2008, 28(8): 3744 − 3751. [33] 郑亚茹, 唐明. 丛枝菌根真菌对盐胁迫下桑树生长及光合特性的影响[J]. 蚕业科学, 2020, 46(6): 669 − 677. ZHENG Yaru, TANG Ming. Effects of arbuscular mycorrhizal fungi on the growth and photosynthetic characteristics of mulberry tree under salt stress [J]. Acta Sericologica Sin, 2020, 46(6): 669 − 677. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20210740






 下载:
下载: