-
泰国巨竹Gigantochloa tekserah属禾本科Poaceae竹亚科Bambusoideae巨竹属Gigantochloa,主产东南亚及南亚次大陆,多生于热带雨林中,中国主要分布于云南地区。泰国巨竹竹竿高大直立,竹材用途广,竹笋营养丰富,是笋材两用竹种[1]。竹类植物的组织培养起步于1968年,迄今已对刚竹属Phyllostachys,牡竹属Dendrocalamus,箬竹属Indocalamus等20余属70多个竹种进行了愈合组织诱导、微体繁殖等研究[2],未见泰国巨竹的组织培养方面的报道。本研究通过探索不同植物生长调节物质组合对泰国巨竹试管快繁的影响,成功建立了其组织培养体系,能够实现方便地将基因导入需要改良的竹子材料中,进行遗传转化得到竹子新品种。同时,试管苗免受季节限制,可以根据科研者的需要随时进行取材,为科学研究提供实验材料。
HTML
-
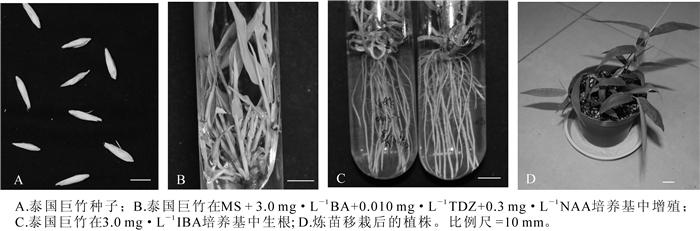
泰国巨竹成熟种子采自云南省。种子乳白色,长圆形,千粒质量为71.2 g,大小如图 1A。
-
将泰国巨竹的种子在自来水下冲洗12 h左右,用体积分数约2.5%的次氯酸钠(NaClO)溶液真空抽滤20 min,无菌水冲洗5~6次,在超净工作台上体视显微镜(OLYMPUS SZ61)下剥离种胚,于MS培养基中进行丛生芽增殖培养。约4周后分化出芽,从而获得泰国巨竹的无菌苗。将诱导出的长势一致的芽作为增殖试验的材料。
-
基本培养基为MS(Murashige and Skoog)培养基,同时添加30 g·L-1蔗糖和8 g·L-1A型琼脂,pH 5.7,并设计了BA(6-苄基腺嘌呤)质量浓度(1.0,3.0,10.0 mg·L-1),TDZ(噻苯隆)质量浓度(0,0.001,0.010 mg·L-1),KT(激动素)质量浓度(0,0.3,1.0 mg·L-1)和NAA质量浓度(0,0.3,1.0 mg·L-1)的4因素3水平正交试验。试验材料选择高度约3 cm丛生芽,3个·从-1,1丛·管-1,20管·处理-1。1月后,观察芽的生长情况。
-
选取增殖良好的长势一致的丛生芽接种于生根培养基中:1/2 MS+IBA(吲哚丁酸)(0,1.0,3.0,10.0 mg·L-1),共4个处理,20管·处理-1。1月后,观察泰国巨竹生根状况。
-
泰国巨竹在根长4~5 cm左右时放在驯化室强光下(20 000 lx)炼苗1周,之后取出试管苗用温水(30 ℃左右)洗净根部残余的培养基后,移栽于泥炭、蛭石、珍珠岩的混合基质中并套袋,7 d左右后开始剪袋,15 d左右完全脱袋。期间进行养护管理,1个月后统计移栽成活率。
-
培养室温度为(25±2)℃,光照强度2 400 lx,光照时间16 h·d-1。运用SPSS 17.0与DPS 9.50进行数据分析,方差分析采用Duncan法。增殖系数=新生总芽数/接种芽数:试管苗根诱导率=(诱导出根的试管苗/试管苗总数)×100%
1.1. 试验材料
1.2. 试验方法
1.2.1. 无菌苗的获得
1.2.2. 增殖培养
1.2.3. 生根
1.2.4. 练苗与移栽
1.2.5. 培养条件与统计分析
-
不同植物生长调节物质组合对泰国巨竹芽的增殖产生不同的影响。由R值可看出:BA在芽增殖中作用最大(0.60),KT作用最小(0.02)。由表 1可见:当BA质量浓度从1.0 mg·L-1增加到3.0 mg·L-1时,芽增殖系数升高,但当增加到10.0 mg·L-1时,增殖系数下降:KT的添加对芽增殖效果不显著,但随着KT的质量浓度增大,芽褐化较严重,叶片发黄:NAA的添加亦是随着质量浓度的增加,增殖系数先升高后下降,在质量浓度为0.3 mg·L-1时,芽生长良好,绿色、健壮:随着TDZ的质量浓度升高,芽的增殖系数呈先升高后降低的趋势。综上所述,处理6时,芽的增殖系数为2.21,与其他处理差异显著,芽生长健壮、叶片鲜绿,几乎没有褐化,为最适合的培养基,即泰国巨竹的最佳增殖培养基为MS + 3.0 mg·L-1 BA+0.010 mg·L-1 TDZ+0.3 mg·L-1 NAA。
处理 植物生长调节物质/(mg_L-1) 增殖系数 长势 BA TDZ KT NAA 1 1 0 0 0 1.02±0.120 9 de 芽不伸长 2 1 0.001 0.3 0.3 1.78±0.196 9 ab 叶片偏黄 3 1 0.01 1 1 1.56±0.110 3 bc 叶片黄绿 4 3 0 0.3 1 1.42±0.096 3 bc 芽簇生 5 3 0.001 1 0 1.62±0.167 1 ab 芽簇生 6 3 0.01 0 0.3 2.21±0.244 0 a 芽簇生,生长健壮 7 10 0 1 0.3 1.21±0.097 1 cd 苗枯黄 8 10 0.001 0 1 1.12±0.085 6 d 苗易褐化 9 10 0.01 0.3 0 1.11±0.174 5 d 生长差 x1 1.45 1.21 1.45 1.26 x2 1.75 1.63 1.44 1.73 x3 1.15 1.51 1.46 1.37 R 0.6 0.42 0.02 0.47 说明:表中增殖系数代表平均值±标准误; 多重比较采用Duncan法, 数值后有相同字母表示差异不显著(P < 0.05)。x为各因素在同一水平实验指标(增殖系数)的平均数。R=xmax-xmin Table 1. Circumstances of different plant growth regulators on buds multiplication of Gigantochloa tekserah
-
泰国巨竹的生根状况受IBA浓度的影响(表 2),在没有添加IBA的1/2 MS培养基中,只有2管生根,且根纤细,根数1~2条:IBA质量浓度为1.0 mg·L-1时,生根率升高,但根仍纤细,较短。IBA质量浓度为3.0 mg·L-1时,生根率达到最大,为70%,生根数为10条,根较粗壮,植株生长良好(图 1C)。但是,在添加10.0 mg·L-1 IBA的培养基中所诱导出的根粗短、基部膨大,为畸形根,且植株发黄,易褐化,长势较差。综上所述,泰国巨竹最优生根培养基为1/2 MS + 3.0 mg·L-1 IBA。
IBA/(mg-L-1) 接种数/管 生根率/L 生根数/条 根状况 0 20 10 1.50±0.500 a 纤细,较短 1.0 20 15 4.00±1.000 a 纤细,较短 3.0 20 70 10.00±1.000 b 粗壮,较长 10.0 20 40 8.13±1.125 b 粗短,膨大 说明:表中生根数代表平均值±标准误;多重比较采用Duncan法,同一栏中有相同字母表示差异不显著(P < 0.05)。 Table 2. Circumstances of different concentrations of IBA on roots induction of Gigantochloa tekserah
-
将生根的泰国巨竹组培苗在强光下练苗1周,移栽于等配制比的泥炭、蛭石、珍珠岩的混合基质中,期间进行观察、浇水等养护管理,1个月后苗成活率达100%(图 1D)。
2.1. 植物生长调节物质对泰国巨竹丛生芽增殖的影响
2.2. IBA对泰国巨竹生根诱导的影响
2.3. 移栽
-
一般认为内源激素的平衡决定植物器官分化的倾向,外源激素则通过改变内源激素平衡从而产生作用。为了让内源细胞分裂素和生长素达到一定平衡,外加的细胞分裂素以及生长素必须达到一定的质量浓度和比例,才能使器官分化达到预期目的[3-4]。细胞分裂素可有效地促进芽的萌发与不定芽增殖,较低浓度的生长素促进茎的伸长生长。本研究运用正交试验讨论了BA,TDZ,KT,NAA的组合对泰国巨竹试管繁殖的影响。在一定范围内,高质量浓度的BA能够促进植株的增殖: BA的质量浓度愈大,对增殖的影响会越明显。但当质量浓度为10 mg·L-1时,芽易褐化,叶片发黄,生长不健康。较低质量浓度的BA既能在一定程度上促进增殖,但效果不明显。这与卓仁英[5]的研究结果一致,即当BA质量浓度过高时可能会对竹类植物生长有一定的抑制或毒害作用。TDZ在植物组织培养中可以独立使用,或者与其他植物生长调节物质共同使用,实现对植物细胞的诱导和调节作用,具有很强的促进细胞分裂的活性,能够高效诱导离体材料的再生生长。但若浓度过高则会抑制生长,导致苗过细、偏黄、生长状况差。陈意涵等[6]、张铁等[7]和张桂和[8]对花秆绿竹Bambusa osdhami f. striata及麻竹Dendrocalamns latiflorus等研究发现,TDZ活性明显优于BA,添加一定质量浓度的TDZ芽的增殖系数显著增加。本实验中选取添加0.01 mg·L-1 TDZ的处理,增殖系数为2.11,苗长势良好,对泰国巨竹植株的增殖生长和伸长生长最有利,为最适合质量浓度。通过泰国巨竹丛芽诱导实验发现,培养基中添加KT对泰国巨竹的芽增殖生长没有太大意义,这与王曙光等[9]的研究结果一致。一般研究认为,在试管快速繁殖中,添加一定质量浓度的生长素更有利于诱导生根[10]。本实验中,泰国巨竹在未添加植物生长调节物质的1/2 MS中诱导的根数量少,较纤细,对于移栽成活不利[6, 11]。在培养基中添加3 mg·L-1 IBA时,试管苗生根率最高,根粗壮、发达,生长最好。但质量浓度过高10 mg·L-1时,所诱导的根基部膨大、较短,为畸形根,植株长势较差,易褐化,这与刘倩倩等[11]的研究结果相同。练苗移栽在试管快繁技术中是很重要的一环,练苗是否过关直接限制了工厂化育苗能否成功运行。同时,练苗成活率直接影响繁殖系数,最终影响商业化生产规模。本实验将生根的泰国巨竹组培苗在强光下练苗1周,移栽于等配制比的泥炭、蛭石、珍珠岩的混合基质中,成活率达100%。
本研究利用正交实验研究了泰国巨竹芽的增殖状况,之后进行生根诱导,从而成功获得完整植株,对于其他巨竹属的组织培养研究具有一定参考性。竹子种子很难获得,且通过种胚快繁产生幼苗的变异性较大,优良性状难以保持。采用变异性小的幼枝节段进行快速繁殖,还需进一步研究。










 DownLoad:
DownLoad: