-
人们越来越意识到使用化学农药的弊端,并开始积极寻求其他防治手段来替代或减少化学农药的使用。生物防治因其高效,对环境、生态及人类健康安全,无毒等优点得到了广泛重视和关注,并成为了目前植物病害防治的热点。目前,很多有益微生物及其代谢产物被成功应用于植物病害的生物防治中,包括真菌、细菌、放线菌、酵母等。在这些生防微生物中,芽孢杆菌属Bacillus是近年来生物防治研究的一个重点方向[1]。芽孢杆菌广泛分布于自然界各种不同环境中,包括土壤、水体、植物体表面及体内等。其生长速度快、营养需求简单、抗逆性强、易于在植物体表面定殖与繁殖、抑菌谱广、对人畜无害、不污染环境,且制剂生产的工艺简单、制剂稳定、储存时间长、施用方便,不仅符合人们对环境和健康的需求,而且为农林业的可持续发展提供了保障[1-2]。大量的研究表明:芽孢杆菌防治植物病害的作用方式多种多样,主要有拮抗作用、竞争作用、溶菌作用、诱导抗性和促进植物生长等[1]。拮抗作用是芽孢杆菌通过产生抑菌活性物质,抑制或杀死病原菌的现象,是芽孢杆菌最主要的作用方式,也是目前研究较为深入广泛的一种抑菌机制[3]。芽孢杆菌主要通过2种途径产生抑菌活性物质,分别是核糖体途径和非核糖体途径。核糖体合成的抑菌物质主要有活性蛋白类、酶类、细菌素等,如鞭毛蛋白、枯草菌素、葡聚糖酶、纤维素酶和几丁质酶等;非核糖体途径合成的抑菌物质主要有脂肽类、多肽类物质、挥发性抑菌物质等,如表面活性素(surfactins),伊枯草菌素(iturins),丰原素(fengycins),醇类,聚酮类,酚类,大环内酯类等[1-5]。菌株TJB-8是从杨树Populus根部土壤中分离得到的一株对杨树腐烂病菌Cytospora chrysosperma具有明显抑菌活性的拮抗细菌。本研究测定了菌株TJB-8无菌滤液的抑菌活性,鉴定了菌株,并初步分析了其抑菌活性物质。研究结果为该菌株的进一步工业开发和田间应用提供理论基础和科学依据。
HTML
-
供试拮抗菌株TJB-8均由甘肃省靖远县职业中等专业学校生物学实验室分离并保存,供试病原真菌均由中国林业微生物保藏中心提供。
-
拮抗菌株的培养和斜面保存均采用Luria-Bertani(LB)固体培养基;拮抗菌株种子液制备采用LB液体培养基;拮抗菌株的发酵培养采用发酵培养基[葡萄糖22.64 g,蛋白胨11.93 g,大豆粉5.00 g,磷酸二氢钾(KH2PO4) 1.00 g,硫酸镁(MgSO4·7H2O) 0.50 g,氯化铵(NH4Cl) 3.00 g,磷酸氢二钠(Na2HPO4) 1.00 g,酵母浸粉1.86 g,蒸馏水定容至1 000 mL,pH 7.0~7.2][6];病原真菌的培养和抑菌活性的检测均采用马铃薯葡萄糖琼脂培养基(PDA)。
-
挑取活化后的TJB-8单菌落,接种至盛有100 mL LB液体培养基的250 mL三角瓶中,于28 ℃,200 r·min-1的条件下振荡培养24 h得到TJB-8种子液。按1%的接种量将种子液接入装有100 mL发酵培养基的500 mL三角瓶中,于28 ℃,200 r·min-1条件下振荡培养4 d。4 ℃,10 000 g的条件下离心30 min后收集上清液,再用0.22 μm微孔滤膜过滤,得到无菌滤液[7]。
-
抑菌活性的测定采用菌丝生长速率法[8]。将无菌滤液以体积比1:10的比例与加热冷却至40~50 ℃的PDA混合,倒平板,在不同的平板中央分别接入直径6 mm的病原真菌菌饼,28 ℃倒置培养,隔12 h测量病原真菌菌落直径,直至对照组菌落直径长至培养皿直径的3/4以上为止。以不加无菌滤液的平板作为对照,设重复3个·处理-1,采用十字交叉法测量病原菌菌落直径,计算抑菌率。抑菌率=[(对照组菌落扩展直径-处理组菌落扩展直径)/对照组菌落扩展直径]×100%。其中:菌落扩展直径=菌落总直径-菌饼直径。
-
分别挑取1.3.2中对照组和处理组中杨树腐烂病菌的边缘菌丝观察TJB-8无菌滤液对杨树腐烂病菌菌丝形态的影响。
-
参照文献[9-10]的方法对菌株的形态和生理生化特征进行测定,包括革兰氏染色、接触酶、淀粉水解、厌氧生长、明胶液化等指标。
-
采用菌落聚合酶链式反应(PCR)法扩增16S rDNA[11]:挑取单菌落至装有200.0 μL无菌去离子水的离心(EP)管中,100 ℃煮沸10 min后,10 000 g,2 min离心取上清液,即为菌株的基因组DNA。以提取的菌株基因组DNA为模板,利用引物63f:5′-CAGGCCTAACACATGCAAGTC-3′,1387r:5′-GGGCGGWGTGTACAAGGC-3′[12],进行PCR扩增。扩增体系为20 μL,含有10×TaqE缓冲液2.0 μL,dNTP 1.6 μL,引物63f和1387r各1.0 μL,DNA Taq聚合酶0.1 μL,DNA模板1.0 μL,双蒸水补足至20.0 μL。扩增条件:94 ℃ 4.0 min;94 ℃ 1.0 min,55 ℃ 1.0 min,72 ℃ 1.5 min,35个循环;72 ℃ 10.0 min。引物合成和测序由英潍捷基(上海)贸易有限公司完成。采用BLAST方法在GenBank数据库中进行相似性搜索和同源性比较,采用ClustalX 1.8进行序列匹配分析,通过MEGA 5.0软件,采用Neighbor-Joining法构建系统发育,用Bootstrap(重复抽样1 000次)分析评估树的稳定性。
-
5种不同真菌细胞壁裂解酶包括β-1, 3-葡聚糖酶、纤维素酶、酯酶、蛋白酶和几丁质酶的检测分别采用文献[9, 13-15]中的方法。
-
按照1.3.1中的方法获得TJB-8的无菌滤液,逐渐加入硫酸铵至70%的饱和度,振荡混匀,于4 ℃静置过夜。在4 ℃,10 000 g的条件下离心30 min后收集沉淀,将沉淀溶于少量蒸馏水中,再置于透析袋(8 000~14 000 D)中透析脱盐,透析后收集透析袋中的蛋白提取物溶液,调节其pH至中性,并将其最终体积调整为原始无菌滤液体积的一半。最后再用0.22 μm微孔滤膜过滤,得到无菌蛋白提取物溶液,并置于4 ℃冰箱保存[7]。利用菌丝生长速率法测定蛋白提取物溶液对杨树腐烂病菌的抑菌活性。
-
按照1.3.1中的方法获得TJB-8的无菌滤液,用6.0 mol·L-1的盐酸调节无菌培养滤液的pH值至pH 2.0,于4 ℃静置过夜。在4 ℃,10 000 g的条件下离心30 min后收集沉淀,用甲醇萃取3次,合并萃取液,然后在旋转蒸发仪中减压蒸干得到脂肽类提取物[15]。用蒸馏水将脂肽类提取物溶解至最终体积为原无菌滤液体积的一半,再用0.22 μm微孔滤膜过滤,得到无菌脂肽类提取物溶液,4 ℃保存备用[7]。利用菌丝生长速率法测定脂肽类提取物溶液对杨树腐烂病菌的抑菌活性。
-
采用二分皿法测定挥发性气体对杨树腐烂病菌的抑菌活性[8]。隔12 h测量病原菌菌落直径,直至对照组菌落直径长至培养皿半径的3/4以上为止。计算抑菌率,重复3次·处理-1。
-
采用Excel 2010和SPSS 20.0软件对实验数据进行统计分析,对不同处理组间的差异进行单因素方差分析,应用Duncan法进行差异显著性检验。
1.1. 供试菌株
1.2. 供试培养基
1.3. 拮抗菌株TJB-8抑菌活性的探究
1.3.1. TJB-8无菌滤液的制备
1.3.2. TJB-8无菌滤液对杨树腐烂病菌及其他5种病原真菌的抑制活性
1.3.3. TJB-8无菌滤液对杨树腐烂病菌菌丝形态的影响
1.4. 拮抗菌株的鉴定
1.4.1. 形态及生理生化指标测定
1.4.2. 16S rDNA基因序列测定和比对分析
1.5. 拮抗菌株TJB-8抑菌物质的初步分析
1.5.1. TJB-8产真菌细胞壁裂解酶特性测定
1.5.2. 蛋白类抑菌物质提取及抑菌活性测定
1.5.3. 脂肽类抑菌物质提取及抑菌活性测定
1.5.4. 挥发性气体抑菌活性测定
1.6. 数据统计及分析
-
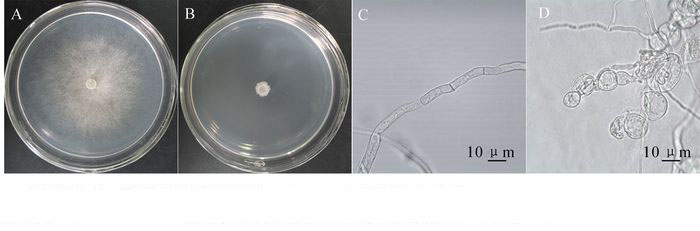
菌株TJB-8无菌滤液对杨树腐烂病菌具有明显的抑菌活性,培养12 h时,能有效地抑制杨树腐烂病菌菌丝生长;随着培养时间的延长,处理组的菌丝开始生长,至48 h后,处理组的菌丝停止生长。培养72 h后,抑制率达到97.42%(图 1A,图 1B,表 1)。无菌滤液对其他5种病原真菌也均有较好的抑菌活性。抑菌活性显示TJB-8无菌滤液对杨树腐烂病菌,细极链格孢菌Alternaria tenuissima,立枯丝核菌Rhizoctonia solani的抑菌活性最高,抑制率分别达到97.42%,96.65%和95.45%;对禾谷镰刀菌Fusarium graminearum的抑菌活性次之,抑菌率达到64.11%;对胶孢炭疽菌Colletotrichum gloeosporioides和尖孢镰刀菌Fusarium oxysporum的抑菌活性最低,但抑制率也分别达到58.40%和57.73%(表 2)。此外,通过观察杨树腐烂病菌对照组和处理组菌落边缘的菌丝发现,杨树腐烂病菌菌丝经过TJB-8无菌滤液作用后会产生局部膨大的畸形(图 1D),而对照组的菌丝生长正常、形态均匀(图 1C)。

Figure 1. Effect of sterile culture filtrate of strain TJB-8 on the growth and morphology of fungal hyphae
培养时间/h 菌落扩展直径/mm 抑制率/% 处理组 对照组 12 0 ± 0 14.83 ± 0.29 100 ± 0 a 24 1.00 ± 0 33.67 ± 0.58 97.03 ± 0 b 36 1.60 ± 0.36 53.33 ± 1.15 97.00 ± 0.68 b 48 2.17 ± 0.76 72.17 ± 0.76 97.00 ± 1.06 b 60 2.17 ± 0.76 84.00 ± 0 97.42 ± 0.91 b 72 2.17 ± 0.76 84.00 ± 0 97.42 ± 0.91 b 说明:表中数据为平均数±标准差。同列数据后无相同小写字母的表示差异显著(P<0.05)。 Table 1. ntifungal activity of sterile culture filtrate of strain TJB-8 against Cytospora chrysosperma
病原真菌 抑制率/% 杨树腐烂病菌Cytospora chrysosperma 97.42 ± 0.91 a 胶抱炭痕菌Colletotrichum gloeosporioides 58.40 ± 1.26 c 细极链格抱菌Alternaria tenuissima 96.65 ± 0.83 a 尖抱镰刀菌Fusarium oxysporum 57.73 ± 236 c 禾谷镰刀菌Fusarium graminearum 64.11 ± 2.87 b 立枯丝核菌Rhizoctonia solani 95.45 ± 1.10 a Table 2. Antifungal spectrum of sterile culture filtrate of strain TJB-8
-
菌株TJB-8为革兰氏阳性菌,杆状,菌体大小为(0.8~1.1) μm × (2.1~3.0) μm,有鞭毛,产芽孢,兼性厌氧型。在LB培养基平板上菌落呈灰白色,不透明,表面光滑干燥,形状不规则近圆形,中部隆起,不产色素。依据菌株的生理生化特征(表 3),并利用统计学方法将拮抗菌株TJB-8和芽孢杆菌属内各个种的形态和生理生化特征进行聚类分析,鉴定菌株TJB-8与蜡样芽孢杆菌Bacillus cereus最为相近。
测定指标 菌株特性 蜡样芽孢杆菌 TJB-8 接触酶 + + 厌氧生长 + + V-P测定 + + 氧化酶 + + 柠檬酸盐利用 + + 卵磷脂酶水解 + + 硝酸盐还原 + + 明胶液化 + + 吲哚产生 + - 苯丙氨酸脱氨酶 - - 淀粉水解 + + 葡萄糖产酸 + + D-木糖产酸 - - L-阿拉伯糖产酸 - - 甘露醇产酸 - - 生长pH 5.7 + + 说明: +表示阳性或生长; -表示阴性或不生长。蜡样芽孢杆菌生理生化特性参见参考文献[9]。 Table 3. Physiological and biochemical characteristics of strain TJB-8
-
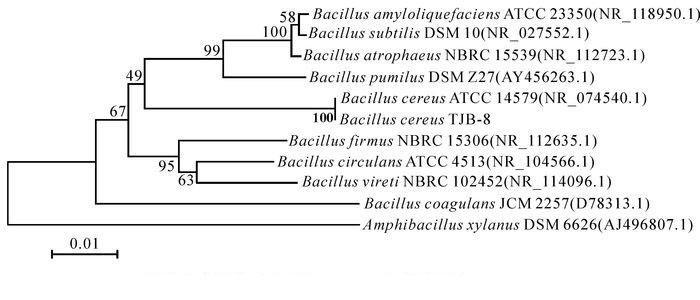
菌株TJB-8的16S rDNA PCR扩增得到一段大小约为1.3 kb的基因片段,回收测序后,测定的片段大小为1 258 bp,提交到GenBank,序列号为KX886805。用ClustalX和MEGA软件进行分析,Neibour-Joining法构建系统发育树(图 2)。该菌株与模式菌株Bacillus cereus ATCC 14579相似度达100%。综合菌株TJB-8的形态学特征、生理生化特性及16S rDNA序列分析的结果,最终将菌株TJB-8鉴定为蜡样芽孢杆菌。
-
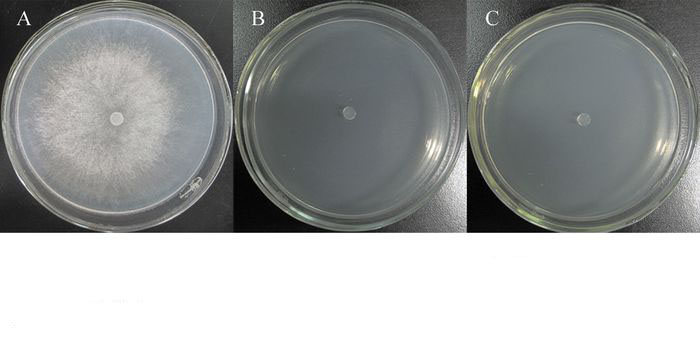
真菌细胞壁裂解酶特性的检测结果显示,只有β-1, 3-葡聚糖酶(图 3A)和蛋白酶(图 3B)的检测平板中菌体周围产生明显透明圈,而其他裂解酶检测平板中菌体周围未见透明圈或晕圈出现,表明TJB-8能产生β-1, 3-葡聚糖酶和蛋白酶,而不能产生几丁质酶、纤维素酶和脂酶。
-
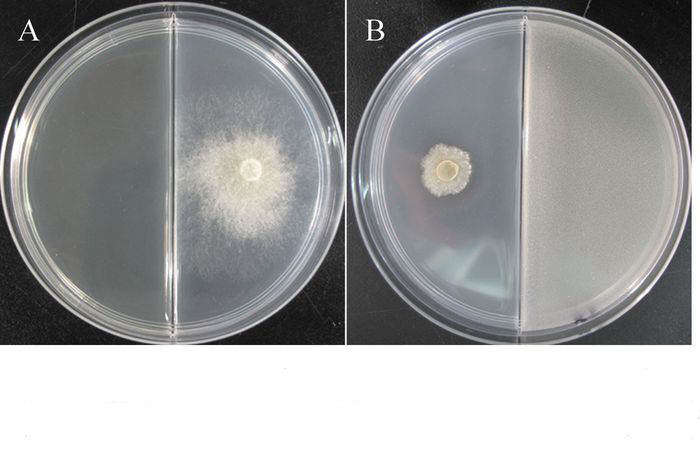
TJB-8蛋白类和脂肽类提取物的抑菌活性检测结果表明:TJB-8的蛋白类和脂肽类提取物均具有较强的抑菌活性(图 4)。蛋白类和脂肽类提取物都能完全抑制杨树腐烂病菌菌丝生长,且抑菌活性稳定,培养72 h后依然保持100%的抑制率(表 4)。
t/h 抑制率/% 蛋白类提取物 脂肽类提取物 挥发性气体 12 100 ± 0 a 100 ± 0 a 100 ± 0 a 24 100 ± 0 a 100 ± 0 a 78.26 ± 1.88 b 36 100 ± 0 a 100 ± 0 a 77.02 ± 1.08 b 48 100 ± 0 a 100 ± 0 a 76.08 ± 0.83 b 60 100 ± 0 a 100 ± 0 a 72 100 ± 0 a 100 ± 0 a Table 4. Antifungal activities of different antifungal substances produced by strain TJB-8 against Cytospora chrysosperma
-
TJB-8挥发性气体的抑菌活性检测发现,TJB-8的挥发性气体对杨树腐烂病菌具有很好的抑菌活性,能有效地抑制杨树腐烂病菌菌丝的生长(图 5)。挥发性气体的抑菌活性随着培养时间的延长而逐渐降低,但依然保持较高的抑菌率,培养48 h后其抑制率仍然达到76.08%(表 4)。
2.1. 拮抗菌株TJB-8无菌滤液对杨树腐烂病菌及其他5种病原真菌的抑菌活性
2.2. 拮抗菌株TJB-8的鉴定
2.2.1. 形态和生理生化特征
2.2.2. 16S rDNA序列测定及分析
2.3. 拮抗菌株TJB-8产真菌细胞壁裂解酶特性
2.4. 拮抗菌株TJB-8蛋白类和脂肽类提取物的抑菌活性
2.5. 拮抗菌株TJB-8挥发性气体的抑菌活性
-
蜡样芽孢杆菌广泛存在于自然界土壤中,和其他种类的生防芽孢杆菌一样都具有抑菌活性强、抑菌谱广的优点。蜡样芽孢杆菌能抑制包括镰刀菌属Fusarium,葡萄孢属Botrytis,腐霉属Pythium,梨孢属Piricularia,丝核菌属Rhizoctonia,假单胞菌属Pseudomonas等植物病原菌[16-19]。本研究发现菌株TJB-8具有较强的拮抗活性和较广的抑菌谱,其无菌滤液在PDA平板上能有效地抑制杨树腐烂病菌的生长,抑制率达到97.42%(72 h),且能使生长的杨树腐烂病病菌菌丝产生畸形膨大;同时TJB-8的无菌滤液还能有效抑制其他多种植物病原真菌菌丝生长,包括胶孢炭疽菌、细极链格孢菌、尖孢镰刀菌、禾谷镰刀菌和立枯丝核。通过结合菌株的形态学特征、生理生化特征和16S rDNA序列分析最终将TJB-8鉴定为蜡样芽孢杆菌。
通过产生抑菌活性物质来抑制或杀死病原菌是蜡样芽孢杆菌和其他种类芽孢杆菌表现出较强抑菌活性及较广抑菌谱的重要原因之一[3]。通过核糖体途径和非核糖体途径合成的蛋白类和脂肽类抑菌物质是蜡样芽孢杆菌产生的主要抑菌活性物质[3-4]。蛋白类物质主要有蛋白酶类物质和其他蛋白类物质。蛋白酶类物质常见的主要有几丁质酶、纤维素酶、蛋白酶、脂肪酶和葡聚糖酶等。这些真菌细胞壁裂解酶的作用机制主要是通过裂解病原菌细胞壁,导致病原菌细胞破裂死亡,从而抑制或杀死病原菌[20]。大部分的生防芽孢杆菌都能产生1种或多种细胞壁裂解酶。CHANG等[16]发现生防蜡样芽孢杆菌Bacillus cereus QQ308能产生多种抗真菌裂解酶,包括几丁质酶和蛋白酶。HUANG等[21]报道蜡样芽孢杆菌28-9能产生一种有效抑制百合灰霉病菌Botrytis elliptica孢子萌发的几丁质酶。本研究发现蜡样芽胞杆菌TJB-8能产生β-1, 3-葡聚糖酶和蛋白酶,这可能是TJB-8无菌滤液能使杨树腐烂病菌的菌丝产生畸形的重要原因之一。蜡样芽孢杆菌还能通过核糖体途径产生其他类型的蛋白类抑菌物质。郝变青等[22]研究发现蜡样芽孢杆菌BC98-1不仅能产生几丁质酶,还能产生能有效抑制黄瓜枯萎病菌尖孢镰刀菌的菌丝生长和孢子萌发的抑菌蛋白。本研究从TJB-8无菌滤液中提取蛋白类物质并测定其抑菌活性发现,其蛋白类提取物具有很强的抑菌活性,能100%抑制杨树腐烂病菌的生长。除了蛋白类抑菌物质,蜡样芽孢杆菌通过非核糖体途径还能产生多种类型的多肽类抑菌物质。SRIRAM等[23]报道蜡样芽孢杆菌NK1能产生具有抑菌活性的脂肽类物质。SILO-SUH等[24]首次从蜡样芽孢杆菌UW85中分离到了线形多肽类抑菌物质zwittermicin A,能有效抑制苜蓿疫霉根腐病菌Phytophthora medicagini的菌丝生长和孢子萌发。本研究发现TJB-8的脂肽类提取物也具有抑菌活性,有效抑制了杨树腐烂病菌的生长,抑制率达到100%。
挥发性气体也是芽孢杆菌产生的重要抑菌活性物质之一,这些具有抑菌活性的挥发性气体主要包括醇类、醛类、酸类、脂类和酮类等化合物[5, 25]。很多蜡样芽孢杆菌也被报道能产生挥发性抑菌气体。HUANG等[26]研究发现蜡样芽孢杆菌C1L产生的挥发性气体能有效抑制灰霉病菌Botrytis cinerea侵染烟草Nicotiana tabacum叶片,最后鉴定发现其挥发性气体中主要的抑菌物质是二甲基二硫(DMDS)。KAI等[27]报道蜡样芽孢杆菌的挥发性气体能抑制病原真菌菌丝生长和孢子萌发,并影响菌丝的形态。本研究的结果表明:菌株TJB-8的挥发性气体能很好的抑制杨树腐烂病菌的生长,抑制率达到76.08%。
蜡样芽孢杆菌TJB-8抑菌活性强、抑菌谱广且能产生多种类型的抑菌活性物质,显示出了较高的研究和应用价值。目前,对于蜡样芽孢杆菌TJB-8的不同类型的抑菌活性物质的具体种类及结构还不清楚,需要后期进一步的纯化与鉴定,在田间的实际应用效果也需要开展后续的验证。













 DownLoad:
DownLoad: