-
叶绿素是光合作用中支持植物生长的必需物质,也是各种环境胁迫对叶片功能和健康损害的敏感指标[1-2]。酸雨直接通过破坏质膜、降解叶绿素、破坏叶绿体超微结构和使植物关键酶失活而抑制植物的生长发育[3]。酸雨可使植物细胞中氢离子(H+)浓度升高[4],引起过氧化氢(H2O2)和超氧阴离子自由基(O2·-)等活性氧(reactive oxygen species, ROS)的过度积累,高水平的ROS会对细胞结构造成包括脂质过氧化在内的显著损害[5-6]。植物抗氧化系统,包括抗氧化酶和非酶抗氧化剂,是ROS的清除和细胞膜稳定性的维持重要的防御机制[7]。其中超氧化物歧化酶(superoxide dismutase, SOD)是抗氧化的第1道防线,它催化超氧阴离子歧化生成过氧化氢和氧气,过氧化氢酶(catalase, CAT)是公认的第2道防线,能够清除细胞中产生的过氧化氢[8],过氧化物酶(peroxidase, POD)能在高浓度氧的情况下保持较高的氧化还原活性,保护细胞免受高浓度氧的伤害[9]。非结构性碳水化合物(nonstructural carbohydrate, NSC)主要包括果糖、葡萄糖、蔗糖和淀粉等,是重要的渗透调节物质和能量物质[10]。李天红等[11]对苹果Malus domestica研究发现:适度水分胁迫处理使叶片和根系中蔗糖含量升高,淀粉含量显著降低;BOLOURI-MOGHADDAM等[12]研究认为葡萄糖代谢可以通过清除ROS从而防止细胞程序性死亡。环境胁迫导致初级代谢紊乱,影响可溶性糖的积累。可溶性糖除了作为代谢物质和能量来源外,还充当信号分子,协调合成代谢和分解代谢过程,促进植物生长发育。酸雨被认为是一种世界性的污染物,尤其是在北欧、北美和中国。中国酸雨污染面积占国土面积的40%以上,强酸雨区(pH≤4.5)面积居世界三大酸雨区之首,其中长江以南地区已成为全球强酸雨中心[13-14]。长江中下游地区受副热带高压影响,梅雨季节过后常常伴随着高温,导致该地区植物易遭受酸雨和高温双重胁迫。桂花品种‘杭州黄’Osmanthus fragrans ‘Hangzhou Huang’是木犀科Oleaceae木犀属Osmanthus的常绿乔木,是新培育的珍贵品种。为了研究‘杭州黄’对酸雨高温双重胁迫的响应,模拟酸雨高温胁迫对非结构性碳质量分数、ROS及抗氧化酶活性的影响,揭示‘杭州黄’抵御酸雨高温胁迫的生理生化指标响应机制,以及‘杭州黄’抗酸雨高温胁迫的适应对策,同时为植物多样性保护、园林植物合理利用及可持续发展提供参考。
HTML
-
2016年6月,将生长良好、长势基本一致的2年生‘杭州黄’实生苗(购于浙江杭州画境种业有限公司,苗高约50 cm)栽植于盛有培养土[V(泥炭):V(沙土):V(蛭石)=1:2:1,干质量为1.5 kg]花盆中(φ=25 cm,h=30 cm),1株·盆-1,共计40盆。盆栽苗常规管理,光照条件为自然光,缓苗期间,用自来水进行浇灌。充分缓苗1个月,再实施酸雨高温处理。
-
选取盆栽苗40盆,随机分为8组,其中4组用作酸雨处理,另4组酸雨处理后再进行高温处理。酸雨处理:母液根据浙江省杭州市临安区酸雨监测分析资料,按照酸雨中x[硫酸根离子(SO42-)]:x[硝酸根离子(NO3-)]=4:1(摩尔比)的比例配制。设置pH 5.6(对照),pH 4.0,pH 3.0,pH 2.0等4个处理梯度,酸雨喷施量根据临安区月均降水量,5 d喷施1次,喷施5 mm·次-1,连续处理40 d。处理结束后,选取桂花枝条从上至下第3对完整的功能叶片,取样3.0 g·盆-1(约10个叶片),一部分新鲜叶片立即用做光合色素的测定,另一部分样品用液氮迅速冷冻后保存于-80 ℃超低温冰箱中待测定。高温处理:将对照组和每个酸雨处理后的苗木,放入人工气候箱(昼/夜为40 ℃ /28 ℃,湿度为40%,光合光子照度为400.0 μmol·m-2·s-1),胁迫2 d。将对照组进行高温胁迫作为高温组,其他作为酸雨高温协同处理组。5盆·处理-1,每盆作为1个独立实验,共5次重复。
-
O2·-测定参照李忠光等[17]的研究方法并稍作改进。过氧化氢测定参照CHANDRA等[18]的方法并略作改进。MDA测定参照HODGES等[19]的方法。
-
酶液提取方法:称取0.5 g冷冻样品,用液氮磨碎,加入9.0 mL磷酸缓冲溶液(0.1 mol·L-1,pH 7.4)研磨成匀浆,10 000 × g离心15 min(4 ℃)。取上清液用于SOD,POD和CAT活性的测定。SOD活性测定参照南京建成生物科技有限公司生产的A001-1型SOD试剂盒进行测定和计算。CAT和POD活性测定参照KUMARI等[20]的方法。
-
取植物材料0.5 g,用液氮磨碎,加入10.0 mL蒸馏水研磨成匀浆,100 ℃水浴10 min,冷却,3 000 r·min-1离心5 min,上清液为可溶性糖提取液。向沉淀中加体积分数为80%乙醇冲洗1遍,加蒸馏水3.0 mL,搅拌均匀,放入沸水浴中糊化15 min。冷却后,加入4.0 mL冷的9.2 mol·L-1高氯酸,搅拌,提取20 min后加蒸馏水至25.0 mL,混匀,离心10 min,上清液为淀粉提取液。蔗糖、果糖和葡萄糖参照南京建成生物科技有限公司生产的试剂盒进行测定和计算。淀粉采用蒽酮比色法测定。
-
所有数据均为5次重复的平均值±标准误,利用Origin 9.0软件进行数据处理、图表制作和统计分析,统计方法采用单因素方差分析(one way ANOVA)进行Turkey多重比较。
1.1. 试验材料
1.2. 试验处理
1.3. 试验方法
1.3.1. 光合色素质量分数的测定
1.3.2. ROS和丙二醛(MDA)质量摩尔浓度的测定
1.3.3. 抗氧化酶活性的测定
1.3.4. 非结构性碳质量分数的测定
1.4. 数据处理
-
由表 1看出:随着酸雨强度的增加,叶绿素a(Chl a)质量分数,总叶绿素(Chl t)质量分数和Chl a/b均呈降低趋势,pH 2.0处理分别比对照降低了16.9%,16.0%和4.8%(P<0.05)。高温胁迫后,叶绿素a质量分数,总叶绿素质量分数和Chl a/b分别比对照降低了12.4%,10.8%和7.7%(P<0.05)。酸雨高温协同胁迫后,叶绿素a质量分数,总叶绿素质量分数和Chl a/b进一步降低,pH 2.0时分别比对照降低了27.7%,23.8%和19.5%(P<0.05)。
T/℃ pH值 wChl a/(mg·g-1) wChl b/(mg·g-1) wChl t/(mg·g-1) Chl a/b 28 5.6(ck) 1.24 ± 0.03 A 0.36 ± 0.05 A 1.60 ± 0.07 A 3.49 ± 0.37 A 4.0 1.17 ± 0.10 A 0.35 ± 0.02 A 1.52 ± 0.12 AB 3.30 ± 0.20 B 3.0 1.09 ± 0.11 B 0.33 ± 0.09 A 1.42 ± 0.16 AB 3.50 ± 0.81 B 2.0 1.03 ± 0.04 B 0.31 ± 0.01 A 1.33 ± 0.05 B 3.36 ± 0.04 B 40 5.6(ck) 1.09 ± 0.04 a 0.34 ± 0.01 a 1.43 ± 0.04 a 3.21 ± 0.16 a 4.0 0.98 ± 0.03 ab 0.31 ± 0.03 a 1.29 ± 0.03 ab 3.17 ± 0.34 a 3.0 0.97 ± 0.01 ab 0.32 ± 0.02 a 1.29 ± 0.02 ab 3.06 ± 0.15 a 2.0 0.90 ± 0.03 b 0.32 ± 0.03 a 1.22 ± 0.04 b 2.81 ± 0.25 b 说明:不同大写字母表示28 ℃时不同处理差异显著;不同小写字母表示40 ℃时不同处理差异显著 Table 1. Effects of acid rain and heat stress on pigment contents in leaves of Osmanthus fragrans 'Hangzhou Huang'
-
由表 2可见:随着酸雨强度的增加,过氧化氢和O2·-质量摩尔浓度逐渐增加,在pH 2.0时分别比对照增加了94.5%和140.9%(P<0.05);MDA先增加后降低,pH 3.0时比对照增加了75.0%(P<0.05)。高温胁迫后,过氧化氢、O2·-和MDA质量摩尔浓度分别比对照增加了87.3%,120.9%和75.8%(P<0.05)。酸雨高温协同胁迫后,随着pH值的增加,过氧化氢质量摩尔浓度呈先增加后降低的趋势,在pH 3.0时达最大值,比对照增加了180.1%(P<0.05);O2·-和MDA质量摩尔浓度呈上升趋势,pH 2.0时分别比对照增加了158.3%和142.0%(P<0.05)。
T/℃ pH值 bO2·-/(mol·g-1) bH2O2 bMDA/(nmol·g-1) 28 5.6(ck) 0.48 ± 0.06 C 24.36 ± 2.90 D 3.29 ± 0.25 C 4.0 0.55 ± 0.05 C 30.32 ± 1.03 C 4.00 ± 0.35 B 3.0 0.67 ± 0.07 B 42.48 ± 2.23 B 5.76 ± 0.28 A 2.0 1.17 ± 0.04 A 47.32 ± 3.17 A 5.62 ± 0.39 A 40 5.6(ck) 1.07 ± 0.07 b 45.61 ± 5.15 c 5.78 ± 0.25 c 4.0 1.13 ± 0.05 b 53.36 ± 7.67 b 7.05 ± 0.42 b 3.0 1.24 ± 0.07 a 68.16 ± 6.10 a 7.26 ± 0.35 b 2.0 1.28 ± 0.09 a 27.18 ± 2.10 d 7.96 ± 0.21 a 说明:不同大写字母表示28 ℃时不同处理差异显著;不同小写字母表示40 ℃时不同处理差异显著 Table 2. Effects of acid rain and heat stress on ROS and MDA contents in leaves of O. fragrans 'Hangzhou Huang'
-
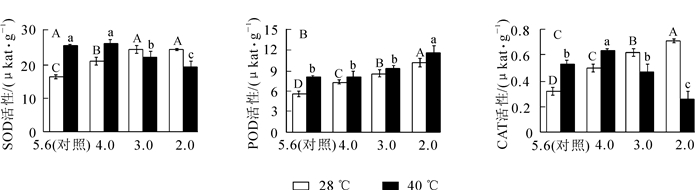
从图 1A可以看出:随着酸雨强度的增加,‘杭州黄’叶片的SOD活性先增加后降低,在pH 3.0时比对照增加了48.9%(P<0.05),随后略微降低。由图 1B和图 1C可知:POD和CAT活性随酸雨胁迫强度加强均逐渐增加,pH 2.0处理分别比对照增长了79.6%和118.2%(P<0.05)。高温胁迫后SOD,CAT和POD活性分别比对照增加了54.5%,64.1%和43.8%(P<0.05)。

Figure 1. Effects of acid rain and heat stress on activity of antioxidant enzymes in leaves of O. fragrans 'Hangzhou Huang'
酸雨高温协同胁迫下,随胁迫强度的增加,SOD和CAT活性呈先增加后降低趋势,均在pH 4.0处理达最大值,分别比对照增加了60.3%和92.9%(P<0.05);POD活性仍逐渐增强,pH 2.0处理比对照增加了107.3%(P<0.05),说明酸雨高温胁迫后主要靠POD发挥抗氧化作用减少自由基对植物造成的伤害。
-
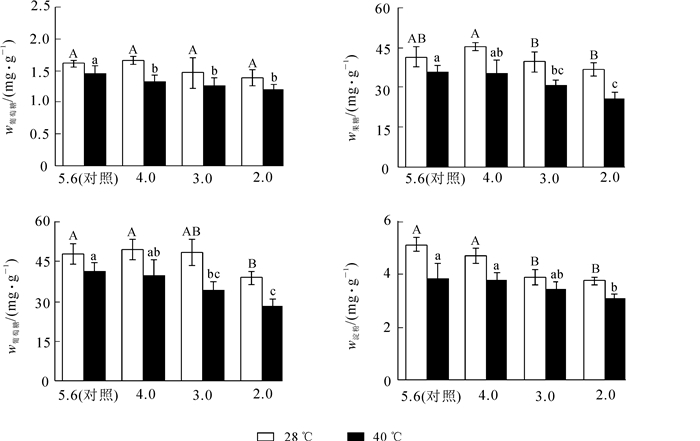
由图 2可见:‘杭州黄’叶片葡萄糖质量分数在酸雨胁迫后变化不显著;高温胁迫后,葡萄糖质量分数比对照降低了10.2%(P<0.05);酸雨高温协同胁迫后,葡萄糖质量分数随着酸雨强度的增加而减小,pH 2.0处理比对照降低了22.4%(P<0.05)。

Figure 2. Effects of acid rain and heat stress on non-structural carbohydrate contents in leaves of Osmanthus fragrans 'Hangzhou Huang'
果糖质量分数随着pH值的降低呈先升高后降低趋势,pH 2.0处理较pH 4.0降低了18.6%(P<0.05);高温胁迫后,果糖质量分数比对照降低了13.5%(P<0.05);酸雨高温协同胁迫后,果糖质量分数随着酸雨强度的增加而减小,pH 2.0处理比对照降低了38.1%(P<0.05)。可以看出,胁迫程度较轻时,果糖质量分数较稳定,重度胁迫后则显著降低。
蔗糖质量分数在pH 2.0胁迫后显著降低,比对照减少了18.9%(P<0.05);高温胁迫后,蔗糖质量分数比对照降低了13.8%(P<0.05);酸雨高温协同胁迫后,蔗糖质量分数随着pH值的降低而减少,pH 2.0处理比对照降低了41.6%(P<0.05)。由此可见,单一酸雨和高温胁迫时,蔗糖质量分数降幅较低;随着协同胁迫程度的加深,蔗糖质量分数显著降低。
淀粉质量分数随pH值的降低呈下降趋势,pH 2.0处理比对照减少了26.8%(P<0.05);高温胁迫后,淀粉质量分数比对照降低了25.3%(P<0.05)。酸雨高温协同胁迫后,淀粉质量分数逐渐减少,pH 2.0处理比对照降低了40.1%(P<0.05)。
2.1. 酸雨高温胁迫对‘杭州黄’光合色素的影响
2.2. 酸雨高温胁迫对‘杭州黄’ROS和MDA质量摩尔浓度的影响
2.3. 酸雨高温胁迫对‘杭州黄’抗氧化酶活性的影响
2.4. 酸雨高温胁迫对‘杭州黄’非结构性碳质量分数的影响
-
植物叶片是捕获转换光能和制造有机物的最重要器官[21],植物叶绿素含量变化直接影响其光合作用过程[22]。干旱[23]、高温和酸雨[24]等非生物胁迫导致植物叶绿素减少。在酸雨pH≤3.5的环境条件下,龙眼Dimorcarpus longana ‘Wulongling’叶片总色素含量和Chl a/b均下降[25]。本研究结果显示:‘杭州黄’叶片叶绿素a质量分数及Chl a/b在酸雨或高温单一胁迫后均明显下降,协同胁迫后进一步降低,说明酸雨高温胁迫导致叶片光合色素结构破坏,影响植物光合作用能力,协同胁迫对叶绿素破坏更大。
植物受到伤害时会导致细胞内ROS大量积累[26],促进膜脂过氧化作用加强,产生大量MDA,最终导致细胞膜功能受损,质膜透性增加[27],因此MDA质量摩尔浓度的高低可迅速反映出植物受逆境伤害的程度[28]。吴永波等[29]研究发现:高温或干旱胁迫后构树Broussonetia papyrifera幼苗ROS和MDA质量摩尔浓度增加,复合胁迫后ROS和MDA质量摩尔浓度均显著高于单一胁迫。本实验结果表明:酸雨或高温单一胁迫后ROS和MDA质量摩尔浓度均显著增加,说明受到酸雨及高温不同胁迫后,‘杭州黄’体内产生大量的ROS对细胞膜造成伤害。酸雨高温协同胁迫后,O2·-和MDA质量摩尔浓度进一步增加,过氧化氢质量摩尔浓度先增加后减少,说明协同胁迫加剧对植物的伤害,与前人研究结果一致。
植物体内的SOD,CAT和POD等3种酶是细胞抵御活性氧伤害的重要保护性酶。它们在清除O2·-,过氧化氢和过氧化物,阻止和减少自由基的形成,从而保护膜系统免受损伤方面起着重要作用。研究表明:酸雨胁迫后,茶梅Camellia sasanqua叶片SOD,POD和CAT活性提高[30],以消除ROS维持体内的氧化还原平衡;牛远等[31]研究发现高温胁迫后华北落叶松Larix principis-rupprechtii幼苗SOD和POD活性呈先上升后下降的趋势,CAT活性呈降低趋势。本研究结果显示:随酸雨胁迫处理强度的增加,SOD,CAT和POD活性逐渐增强;高温胁迫后,3种酶活性显著增加,但SOD活性高于pH 2.0处理,CAT和POD低于pH 2.0处理。协同胁迫后,POD活性进一步增强,SOD和CAT活性先升高后降低,说明协同胁迫初期3种酶共同作用,使植物体内ROS代谢平衡维持在正常水平,防止发生进一步氧化损伤;POD活性在协同作用后期对过氧化氢清除起主导作用,在高强度的酸雨高温条件下,POD清除ROS的效率更高,而pH 3.0处理下SOD和CAT活性表达受到抑制,且过度胁迫导致一部分已经表达的CAT失活,说明抗氧化酶减轻膜脂过氧化伤害的作用是有限的。
植物光合作用产生的非结构性碳水化合物,既是植物生长发育必需的能量物质,也是细胞内重要的渗透调节物质[10]。近年来越来越多的研究表明碳水化合物也是一种信号分子,通过光合氮同化形成复杂的信号网络,对植物的基因表达、逆境响应、生长发育进行调控[32]。已有研究表明:各种胁迫引起的糖限制可能是ROS积累的重要基础[12, 33],BOLOURI-MOGHADDAM等[12]发现葡萄糖能够通过磷酸戊糖途径和产生非酶性抗氧化剂,有效清除活性氧,提高植物抵抗能力;蔗糖代谢不仅为植物发育提供能量和碳骨架,还通过提供己糖作为必需代谢物和信号分子来调节它们对非生物胁迫的响应[34];童贯和等[35]指出:5种不同酸雨处理下的小麦Triticum aestivum幼苗茎叶可溶性糖含量分别下降15.4%~29.4%,差异达极显著水平。本实验结果显示:酸雨胁迫后‘杭州黄’蔗糖和淀粉质量分数显著降低,而葡萄糖和果糖质量分数相对稳定,可能是因为植物遭受胁迫时呼吸代谢增强,蔗糖和淀粉分解为葡萄糖和果糖提供能量物质,增加抵抗能力。高温胁迫后非结构性碳质量分数均显著降低,可能是因为高温促使植物呼吸代谢加速,叶片非结构性碳消耗速率大于合成速率。协同胁迫后非结构性碳质量分数进一步降低,一方面因为叶绿体膜结构受损,光合作用能力下降,引起植物体内非结构性碳积累速度降低,另一方面有些糖类通过代谢途径和产生非酶抗氧化剂,清除过量的ROS,减轻胁迫造成的伤害。
综上所述,在酸雨胁迫后,‘杭州黄’叶片内产生大量ROS对细胞膜及光合能力造成伤害,植物抗氧化酶活性提高有效清除ROS,减轻胁迫造成的伤害;高温胁迫后呼吸作用加快,可溶性糖和淀粉降解,为抵御胁迫提供能量和碳源;协同胁迫后伤害进一步加深,SOD和CAT活性逐渐降低,POD保持较高活性继续发挥抗氧化功能,同时通过调节非结构性碳代谢参与应激反应,提供能量并消除过量的ROS,表现出较强的抵御酸雨高温胁迫的能力。









 DownLoad:
DownLoad: