-
在亚热带低山丘陵森林植被退化地区,芒萁Dicranopteris dichotoma和五节芒Miscanthus floridulus等[1-2]以极强的扩散定居能力率先成为草本层的绝对优势种,形成单优草本层片[3]。芒萁是里白科Gleicheniaceae芒萁属Dicranopteris多年生常绿蕨类植物。野外调查发现,芒萁自然生长于杉木Cunninghamia lanceolata,马尾松Pinus massoniana和毛竹Phyllostachys edulis单优群落下层、杨梅Myrica rubra,茶园和林缘的土壤深厚肥沃立地上,也可生长于土层瘠薄全光地段和水土流失严重的红壤侵蚀区[4],适应于从暖性针叶群落下层遮光到全光、从植被退化后土壤资源剩余到资源流失等多种生境[5],被认为是亚热带森林退化植被的“标志种”[6]。光强和氮素是影响植物光合生理过程的重要生态因子,植物通过形态结构可塑和生理可塑响应光强和氮素的变化[7]。蔡建国等[8]发现:为适应全光照条件,绣球Hydrangea macrophylla var. macrophylla可通过提高叶片吸收光能向热耗散等PSⅡ调节性能量耗散途径的分配,削弱反应中心过量激发能的积累。万宏伟等[9]在羊草Leymus chinensis等的栽培土壤中添加氮素,发现植物叶绿素相对含量增加,光合作用能力提高。活地被物层优势种过度发育繁茂,会形成“生态筛(ecological filter)”[10-11]效应,即对林下植物多样性产生“过滤”,造成活地被物的植物物种多样性下降[12],并阻碍乔木树种种子更新[13-14],导致森林植被进展演替受阻。在此背景下,芒萁单优层片形成的原因成为有趣的生态问题。芒萁为强耐荫且具有一定喜光特性的多年生草本植物[5, 15],本项研究以盆栽芒萁为材料,从光强变化和氮素添加角度出发,模拟暖性针叶单优群落下层遮荫与土壤较为肥沃、灌草丛全光与土壤贫瘠等不同生境斑块下芒萁光合生理可塑性变化的规律,揭示芒萁对光强和氮素添加的光合生理响应特征,为亚热带低山丘陵区芒萁单优草本层片的调控、促进森林植被的进展演替提供理论依据。
HTML
-
选取长势基本一致的1年生芒萁克隆分株苗,各株保留长10 cm左右的根状茎,采用盆栽法,于2017年3月栽植于规格为40 cm × 21 cm × 17 cm的花盆内,1株·盆-1;盆栽土壤为当地红黄壤,于双层遮光棚内缓苗2月。于2017年5月选取长势基本一致的盆栽芒萁分株,随机分成6组,10盆·组-1,分别置于3个遮光棚内,2组·棚-1,分别对2组进行施氮(N1)和不施氮(N0)处理。
遮光棚分别覆盖1,2,3层黑色尼龙遮阳网,设置不同光强处理:透光率(35.96±0.04)%(L1),(13.00±0.01)%(L2)和(4.75±0.01)%(L3),以无遮光处理(透光率为100%)为对照(ck)。施氮量根据前人对草本植物氮沉降的研究设定[16-17],称取3 g硫酸铵[(NH4)2SO4]分析纯溶解于200 mL纯水中,均匀喷入盆内;不施氮处理组喷入等量的水。处理时间为芒萁快速生长期(5-7月),高温期间适当浇水以保证试验材料免受干旱影响。
-
利用便携式光合测定系统Li-6800(Li-COR,美国)测定光响应曲线。于2017年9月晴天8:00-11:00,选取长势一致的功能叶片,3盆·组-1,重复3次取平均值;设定光合有效辐射梯度为2 000,1 500,1 200,1 000,800,500,300,100,50,30,10和0 μmol·m-2·s-1。测定净光合速率Pn(μmol·m-2·s-1)、气孔导度Gs(mmol·m-2·s-1)、胞间二氧化碳摩尔分数Ci(mmol·mol-1)和蒸腾速率Tr(mmol·m-2·s-1)。利用测定光响应曲线时获得的数据进行气体交换特征的比较,用直角双曲线修正模型对光响应进行拟合[18],根据模型计算最大净光合速率Pnmax(μmol·m-2·s-1)、光饱和点PLS(μmol·m-2·s-1)、光补偿点PLC(μmol·m-2·s-1)、表观量子效率AQY(mmol·mol-1)和暗呼吸速率Rd(μmol·m-2·s-1)。
-
选择测定光响应曲线的叶片,先进行40 min的暗适应处理;利用YZQ-500动态荧光仪(翼鬃麒科技公司)测定叶片快速叶绿素荧光诱导动力学曲线(O-J-I-P曲线),得到初始荧光(Fo)、最大荧光(Fm)。并根据SRIVASTAVA等[19]方法计算得到可变荧光(Fv)、PSⅡ潜在活性(Fv/Fo)、PSⅡ最大光化学效率(Fv/Fm)以及以吸收光能为基础的性能指数(Piabs)。
-
将测定叶绿素荧光的叶片摘下,丙酮浸提得到芒萁提取液,利用分光光度计测定浸提液在波长470,663和645 nm下光密度值,按照LICHTENTHALERD[20]的方法计算得到叶绿素a(mg·g-1)、叶绿素b(mg·g-1)和类胡萝卜素(mg·g-1)质量分数,总叶绿素(mg·g-1)以叶绿素a与叶绿素b的总和表示。
-
所有数据均利用Excel,SPSS进行处理,利用Origin Pro 8.0制图。采用单因素方差分析(one-way ANOVA)和最小显著差法(LSD)比较不同数据组间的差异性。利用双因素方差分析(two-way AVOVA)检验光强、氮素及其交互作用对光合特征参数、各色素质量分数及荧光参数的影响。试验所有数据用平均值±标准误表示。
1.1. 材料与试验方案
1.2. 研究测定方法
1.2.1. 气体交换特征和光合作用-光响应曲线的测定
1.2.2. 叶绿素荧光参数的测定
1.2.3. 叶绿素质量分数的测定
1.3. 数据分析
-
不同光强和氮素处理,盆栽芒萁最大净光合速率(Pnmax)、光饱和点(PLS)、光补偿点(PLC)、暗呼吸速率(Rd)及表观量子效率(AQY)变化规律表现不一(表 1)。未施氮组中,盆栽芒萁Pnmax和PLS随光强增加先升后降,且均在L2光强下出现最大值;PLC和Rd随光强增加呈“N”字形变化;PLC,Rd和AQY在L2光强时出现最小值。施氮和未施氮,L1和L2光强下Pnmax均显著高于L3(P<0.05),但L3和对照差异不显著(P>0.05)。施氮组与未施氮组相比,所有光强下盆栽芒萁的Pnmax均有所提高,且L1和L2下盆栽芒萁PLS上升,L3下降,但差异不显著(P>0.05);L1下盆栽芒萁的PLC,Rd及AQY均降低,L2下均上升,L3下无统一变化规律。
处理 Pnmax/(μmol·m-2·s-1) PLS/(μmol·m-2·s-1) PLC/(μmol·m-2·s-1) Rd/(μmol·m-2·s-1) AQY/(mmol·mol-1) N0 L1 10.24 ± 0.32 a 1 158.06 ± 0.72 a 19.14 ± 0.78 a 1.21 ± 0.04 a 0.068 ± 0.00 a L2 10.52 ± 0.24 a 1 233.66 ± 20.01 a 5.39 ± 0.30 d 0.30 ± 0.00 c 0.057 ± 0.00 a L3 6.27 ± 0.41 b 1 174.26 ± 9.29 a 8.25 ± 0.77 cd 0.42 ± 0.08 c 0.058 ± 0.00 a ck 7.47 ± 0.23 b 1 008.87 ± 40.05 b 16.87 ± 0.96 ab 1.06 ± 0.04 a 0.069 ± 0.00 a N1 L1 12.03 ± 0.54 a 1 172.00 ± 14.65 a 13.29 ± 0.66 abc 0.84 ± 0.05 ab 0.067 ± 0.00 a L2 11.23 ± 0.25 a 1 255.00 ± 9.77 a 11.24 ± 1.18 bcd 0.62 ± 0.07 bc 0.058 ± 0.00 a L3 6.43 ± 0.29 b 1 158.22 ± 15.96 a 11.68 ± 1.27 bcd 0.45 ± 0.04 bc 0.038 ± 0.00 b ANOVA L *** ** ** *** * N ns ns ns ns ns L×N ns ns * * ns 说明:*、**和***分别表示P<0.05,P<0.01和P<0.001,ns表示不显著。同列不同字母表示处理间差异显著(P<0.05) Table 1. Changes in photosynthetic characteristics of potted D. dichotoma under different light intensityies and nitrogen applications
双因素方差分析表明(表 1):氮素对盆栽芒萁光合特征参数均影响不显著(P>0.05),光强对芒萁光合特征参数均影响显著(P<0.05),光强和氮素对盆栽芒萁光补偿点和暗呼吸速率具有显著性交互影响(P<0.05),对其他光合特征参数交互作用不显著。
-
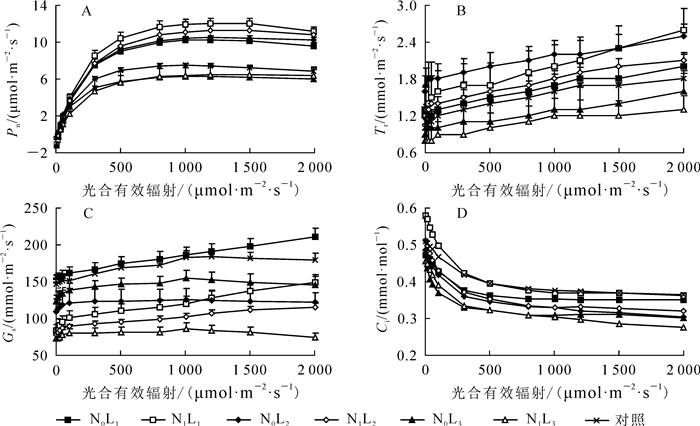
随着光合有效辐射的增加,盆栽芒萁Pn总体呈增加趋势。L1和L2光强下,施氮组盆栽芒萁Pn在光合有效辐射为1 500 μmol·m-2·s-1时,未施氮组在1 200 μmol·m-2·s-1时达到最大,随后下降(图 1A),L3下则未出现下降趋势。未施氮组盆栽芒萁Pn变化顺序为L2>L1>对照>L3,施氮组为L1>L2>L3。随光合有效辐射增加,芒萁Tr总体呈上升趋势;与未施氮相比,施氮组L2和L3光强下盆栽芒萁Tr降低。未施氮组盆栽芒萁Tr大小顺序为L2>L1>对照>L3,施氮组为L1>L2>L3(图 1B)。随光合有效辐射增加,盆栽芒萁Ci总体呈降低趋势,与未施氮相比,施氮组L1和L2光强下盆栽芒萁Ci升高。未施氮组盆栽芒萁Ci大小变化顺序为对照>L1>L2>L3,施氮组为L1>L2>L3(图 1C)。盆栽芒萁Gs随光合有效辐射的增加变化较为复杂(图 1D),L3光强下,盆栽芒萁Gs在光合有效辐射为1 000 μmol·m-2·s-1时达到最大,随后下降;未施氮组Gs大小变化为L1>对照>L3>L2;施氮组为L1>L2>L3。3种光强下,未施氮组盆栽芒萁Gs均高于施氮组。
-
随光强降低,未施氮组盆栽芒萁叶片的叶绿素a、叶绿素b、总叶绿素和类胡萝卜素质量分数均增加(图 2),L2和L3光强下叶绿素a、叶绿素b、总叶绿素和类胡萝卜素质量分数均显著高于L1和对照(P<0.05)。叶绿素a/b随光强降低先升后降,且差异显著(P<0.05)。L1光强下,施氮组盆栽芒萁叶绿素a、叶绿素b、总叶绿素和类胡萝卜素质量分数均显著高于未施氮组(P<0.05),但L2和L3条件下,施氮组低于未施氮组。施氮组盆栽芒萁叶绿素a、叶绿素b、总叶绿素和类胡萝卜素质量分数及叶绿素a/b大小顺序均为L1>L3>L2,且差异达显著水平(P<0.05)。

Figure 2. Pigment content of potted D. dichotoma under different light intensities and nitrogen treatments
双因素方差分析结果表明(表 2):光强、氮素及光强×氮素处理对芒萁叶绿素a,叶绿素b,总叶绿素及类胡萝卜素质量分数的影响均达显著水平(P<0.05)。
处理 叶绿素a 叶绿素b 总叶绿素 类胡萝卜素 光强 25.7*** 24.2*** 25.3*** 24.0*** 氮素 46.5*** 38.6*** 44.6*** 42.2*** 光强×氮素 94.0*** 90.2*** 93.3*** 89.5*** 说明:*、**和***分别表示P<0.05,P<0.01和P<0.001 Table 2. Effect of different light intensity (L), nitrogen (N) and light × nitrogen (L × N) on the pigment content of potted D. dichotoma
-
由表 3可知:与未施氮组相比,施氮组3种光强下盆栽芒萁Fo均显著降低(P<0.05);L1和L3的Fm,L3的Fv/Fm,Fv/Fo及Piabs降低;L2的Fm,L1和L2的Fv/Fm,Fv/Fo和Piabs升高。未施氮组盆栽芒萁Fo,Fm,Fv/Fm及Fv/Fo的大小顺序表现均为对照<L1<L2<L3,Piabs表现为对照>L3>L2>L1;施氮组盆栽芒萁Fo和Fm表现为L2>L3>L1;Fv/Fm,Fv/Fo和Piabs表现为L2>L1>L3。
处理 Fo Fm Fv/Fm Fv/Fo Piabs N0 L1 4 041.3 ± 50.2 bc 12 865.7 ± 105.9 bc 0.69 ± 0.00 ab 2.18 ± 0.06 bc 0.90 ± 0.03 c L2 4 244.3 ± 53.4 b 13 575.3 ± 389.2 b 0.69 ± 0.01 ab 2.20 ± 0.10 bc 0.93 ± 0.07 bc L3 4 845.3 ± 202.1 a 16 377.7 ± 340.1 a 0.70 ± 0.00 ab 2.37 ± 0.09 abc 1.00 ± 0.08 bc ck 2 059.7 ± 136.9 e 6 233.7 ± 554.3 d 0.67 ± 0.00 b 2.02 ± 0.08 c 1.22 ± 0.06 ab N1 L1 3 087.3 ± 77.5 d 11 056.3 ± 401.8 c 0.72 ± 0.00 a 2.58 ± 0.08 ab 1.22 ± 0.05 ab L2 3 795.0 ± 36.8 c 14 253.7 ± 340.2 b 0.73 ± 0.00 a 2.76 ± 0.11 a 1.31 ± 0.08 a L3 3 709.0 ± 75.4 c 11 423.0 ± 1071.4 c 0.67 ± 0.04 b 2.09 ± 033 bc 0.88 ± 0.19 c ANOVA L *** *** ns ns ns N *** ** ns ns * L×N * ** * * * 说明:*、**和***分别表示P<0.05,P<0.01和P<0.001,ns表示不显著。同列不同字母表示处理间差异显著(P<0.05) Table 3. Changes of chlorophyll fluorescence parameters of potted D. dichotoma with different light intensity and nitrogen application
双因素方差分析结果表明(表 3):光强、氮素及光强×氮素均显著影响了盆栽芒萁的初始荧光和最大荧光(P<0.05),但对PSⅡ最大光化学效率影响未达到显著水平(P>0.05),光强对吸收光能的性能指数无显著影响(P>0.05),氮素及光强×氮素则对其影响显著(P<0.05)。
2.1. 光强和氮素对盆栽芒萁光合-光响应特征参数的影响
2.2. 光强和氮素对盆栽芒萁光合气体交换参数的影响
2.3. 光强和氮素对盆栽芒萁叶绿素及类胡萝卜素质量分数的影响
2.4. 光强和氮素对盆栽芒萁叶片叶绿素荧光参数的影响
-
光照和氮素是植物生长发育的重要影响因子[21]。植物对光强改变最直接的生理响应就是光合作用能力的改变[22]。氮素影响植物的叶绿素质量分数,间接影响植物光合作用[23]。光合特征参数可反映植物光合作用和光能利用能力。本研究发现:光强对芒萁光合特征参数影响显著,但氮素对光合特征参数均影响不显著,而光强和氮素的交互作用对各色素质量分数影响极显著。无论施氮与否,L2下芒萁光饱和点(PLS)出现最大值,光补偿点(PLC)出现最小值,表明芒萁在此光强下具有较宽的光生态幅。研究可知,L3下芒萁Pnmax小于对照,显著小于L1,Rd值显著小于对照和L1,认为芒萁通过降低呼吸消耗积累有机物质,体现了芒萁对弱光环境的适应。表观量子效率(AQY)反映了植物对弱光的利用能力。自然条件下长势良好的植物AQY为0.04~0.07[27]。本研究中,施氮组L3处理下芒萁AQY低于正常值,说明此处理下芒萁受到一定的胁迫。
施氮提高了各光强下芒萁的Pnmax,说明施氮一定程度上提高了芒萁的光合作用能力。施氮组中L1芒萁的Pnmax,叶绿素a和叶绿素b质量分数均显著高于L2和L3,说明施氮提高了植株对光照的捕获能力和利用能力[24];L1较高的类胡萝卜素可吸收过多的光能,保护芒萁的光合器官免受伤害。叶绿素a/b提示了植物弱光条件下的捕光效率[26]。本研究发现:随光强降低,芒萁叶绿素a/b呈降低趋势,但施氮组L3的叶绿素a/b有所上升,说明芒萁能够通过改变叶绿素a和叶绿素b质量分数来适应弱光环境。早先研究认为施氮有利于增加植物叶片的叶绿素含量[23, 25]。本研究发现:相比于未施氮组,施氮组在L2和L3光强下,芒萁叶片叶绿素质量分数降低,可能是此光强不利于芒萁对氮素的吸收利用,出于对弱光的生理适应,芒萁需通过提高其他生理功能以提高对光能的吸收、传递和转换。
植物光合速率的提高得益于气孔导度Gs和蒸腾速率Tr的提高[29],而植物光合速率的降低有气孔限制和非气孔限制两方面的因素,若Pn下降且Gs和Ci同时下降,则为气孔限制,而非气孔限制因素主要是叶肉细胞羧化能力的降低造成[28]。本研究中施氮组芒萁Pn均为气孔限制,而未施氮组2种因素均存在。
与“表观性”的气体交换指标相比,荧光测量参数具有反映“内在性”的特点,是无损研究光合作用的重要手段[30],同时能够反映植物对生长环境的适应和耐受能力[31]。Fo为叶片暗适应后再完全开放、未发生光化学反应状态下的参数,Fo降低,植物热耗散增加;Fm为PSⅡ光体系完全关闭下的荧光产量,Fv/Fo表示捕获光能和热耗散能量的比例。本研究中Fo,Fm和Fv/Fo随光强的增加而降低,表明芒萁增加了热耗散启动光保护机制,同时降低了PSⅡ电子传递能力。Fv/Fm和Piabs均能反映PSⅡ的功能[32],但Piabs主要反映植株的整体功能,Fv/Fm下降则植物受到光抑制。本研究中Fv/Fm随光强的增加而降低,Piabs在全光时出现最大值,说明光强增加使芒萁受到了光抑制作用;全光下植株启动体内其他防御系统从而保证植株的整体功能。李伟成等[33]研究发现:施氮能降低植物发生光抑制的可能;本研究发现:施氮使得L1和L2光强下芒萁的Fv/Fm和Fv/Fo均增加,表明盆栽芒萁捕获光能增加的同时热耗散也增加,光抑制减弱。但是L3光强下施氮组各荧光参数均降低,可能是芒萁为偏阳性植物,虽然有一定的耐荫性,但极度弱光条件下施氮不能补偿光照的不足,反而降低了光合氮利用率,造成光合色素含量降低,从而降低了荧光辐射的可能。
综上所述,光强对盆栽芒萁光合作用有显著影响,氮素主要影响光合色素质量分数。L2光强(透光率为13.00%)时盆栽芒萁光生态幅较宽;强光(L1)及较强光(L2)下盆栽芒萁出现光抑制,施氮能调整光能分配,增加热耗散减轻光抑制伤害;L3光强(透光率为4.75%)时,盆栽芒萁通过调整各光合色素质量分数来提高对弱光的吸收、传递和转换,较低的暗呼吸速率有利于积累光合有机物质;相比于未施氮组,施氮出现负补偿现象。未施氮盆栽芒萁净光合速率降低的原因有气孔限制和非气孔限制,而施氮盆栽芒萁净光合速率降低的原因均为气孔限制。










 DownLoad:
DownLoad:
