-
兴安落叶松Larix gmelinii是中国东北地区三大针叶树种之一[1],20世纪70年代成为该地区主要造林树种,但由此也带来了林分结构简单、群落物种多样性降低与森林地力衰退等一系列问题[2]。森林土壤养分含量的增加依赖于地表凋落物[3]和地下有机物的输入,以及微生物进行的分解利用[4],因此,森林生态系统的物质生产能力和树种组成则是调控落叶松林土壤质量与养分利用状态的关键生物因子[5]。研究清楚土壤养分含量及决定其周转的微生物胞外酶的活性随群落中兴安落叶松所占比例的变化动态,对全面衡量东北地区针阔混交林在气候变化情景下的演替趋势具有重要的生态学意义。土壤酶是生化反应的催化剂[6],土壤中生化反应的进行需要酶的参与[7]。土壤酶不仅是检验土壤质量变化的指标[8],也是影响土壤碳(C)、氮(N)、磷(P)循环的主要限制因子[9]。土壤酶化学计量比反映土壤微生物对养分需求的差别,可以在一定程度上反映土壤养分的有效性[10]。与土壤C、N、P循环相关的酶主要有β-1,4-葡萄糖苷酶[β-1,4-glucosidase(BG)]、β-1,4-N-乙酰氨基葡萄糖苷酶[β-1,4-N-acetylglucos-aminidase(NAG)]、亮氨酸氨基肽酶[leucine aminopeptidase(LAP)]、酸性磷酸酶[acid or alkaline phosphatase(AP)]、α-纤维素酶[α-cellulases (CBH)],其中BG、CBH与纤维素降解有关,NAG与蛋白质水解有关。有效性氮的升高会导致NAG和LAP活性的降低,提高对其他养分元素分解酶的投入[11],LAP与几丁质和肽聚糖降解有关。AP与有机磷矿化有关。在土壤酶活性的基础上,SINSABAUGH等[12]采用ln(xBG+xCBH)∶ln(xNAG+xLAP)∶ln(xAP) (x为酶活性)表示土壤酶化学计量比对土壤C∶N∶P化学计量比和土壤C、N、P循环的影响。土壤C∶N∶P化学计量比与土壤C、N、P循环有关[13],土壤化学计量比可以反映土壤元素调节机制[14],进而对植物生长和生理机能进行调控。前人研究大多集中在不同林龄、不同林型对土壤理化性质、土壤化学计量比等方面,例如:随林龄的增加,土壤C∶P、N∶P增大,P成为限制因子[15]。土壤微生物通过分泌胞外酶从土壤中获取需要的养分[16],土壤微生物数量随林龄增大而降低[17]。华北落叶松Larix principis-rupprechtii-白桦Betula platyphylla混交林土壤有机质、全氮、全钾、全磷含量高于华北落叶松纯林[18],但对华北落叶松所占不同比例的针阔混交林的土壤酶化学计量比的研究较少。土壤中C、N、P等养分的有效性主要取决于与其矿化相关的水解酶的强弱。有研究表明:微生物胞外酶活性[8]及其化学计量比[12]是衡量土壤微生物和森林生态系统功能的重要生化指标。在森林生态系统中,土壤理化性质[19]、土壤酶活性[20]、土壤微生物群落结构及其功能[21]和土壤养分有效性[22]又受到树种组成的影响。尽管大兴安岭地区森林群落结构相对简单,但其优势树种兴安落叶松和白桦在物质生产能力、凋落物性状等方面存在较大的差异,随着群落中兴安落叶松所占比例的变化,量化不同群落的土壤养分状况、土壤酶活性及其生态计量比,并以此为基础探讨兴安落叶松所占比例与土壤生化性状间的内在驱动机理,为客观了解东北地区寒温带针阔混交林的演替趋势提供理论依据。
HTML
-
研究区域位于内蒙古自治区根河市根河国家湿地森林公园(50°25′30″~51°17′00″N,120°41′30″~122°42′30″E), 属寒温带大陆性气候,昼夜温差大,冬长夏短,年平均气温−5.3 ℃。土壤为酸性棕色针叶林土,土层浅,砾石含量高,且存在永冻层[23],年降水量为450.0 ~550.0 mm[24]。主要乔木为兴安落叶松、白桦。主要灌木为红豆越橘Vaccinium vitis-idaea、山刺玫Rosa davurica、杜香Ledum palustre、兴安杜鹃Rhododendron dauricum、笃斯越橘Vaccinium uliginosum等。主要草本为鹿蹄草Pyrola calliantha、地榆Sanguisorba officinalis、山芹Ostericum sieboldii等。
-
2018年7月,为研究东北地区退化森林演替规律,在50°56.662 5′~51°00.748 3′N的范围,从北向南,按照兴安落叶松的长势,对该区域林龄相近的兴安落叶松群落进行了调查。每个地点调查3个20 m×20 m样方,样方之间间距为20 m。对布设样地进行了每木检尺,测量了群落内胸径大于5 cm乔木的胸径、树高、冠幅,以及灌木、草本的盖度、株数、高度等信息。按《中国立木材积表》计算每个森林群落内的树木材积所占比例,并按兴安落叶松占整个群落的材积比(70%、75%、80%、85%、90%、95%)把调查样地分为了6个梯度,每个样方内挖取3个剖面,取0~5、5~20 cm土样,并在实验室过2 mm筛。一部分风干测定土壤理化性质,一部分冷冻保存测定土壤酶活性和微生物量。
采用96微孔酶标板荧光分析法测定β-1,4-葡糖苷酶(BG)、α-纤维素酶(CBH)、β-1,4-N-乙酰氨基葡萄糖苷酶(NAG)、亮氨酸氨基肽酶(LAP)、酸性磷酸酶(AP)活性[25]。称取1 g鲜土放在1 000 mL烧杯中,加入125 mL 50 mmol·L−1的醋酸钠缓冲液(pH=5), 涡旋振荡1 min, 使用移液器向96微孔酶标板中分别对应加入250 μL缓冲液、200 μL土壤匀浆样品、50 μL标物、50 μL底物。在培养箱以25 ℃黑暗条件下培养4 h 后,加入10 μL 1 mol·L−1的氢氧化钠终止反应。采用多功能酶标仪(Spectramax 190) 测定其荧光度。
根据《土壤农业化学分析方法》测定土壤理化性质[26]。土壤pH用pH计测定(m土∶m水=1.0∶2.5);土壤有机碳(SOC)采用高温外热重铬酸钾氧化容量法测定,使用硫酸亚铁溶液滴定;全氮(TN)、碱解氮(AHN)使用凯氏定氮仪测定;全磷(TP)采用高氯酸-硫酸(HClO4-H2SO4)消煮-钼锑抗比色法测定;易氧化碳(EOOC)采用高锰酸钾氧化法测定;土壤微生物量的测定采用氯仿熏蒸浸提法[26-27]。
-
数据统计在R 3.5.3中完成, 使用R 3.5.3和SigmaPlot 12.5软件作图。土壤酶化学计量比采用ln(xBG+xCBH)∶ln(xNAG+xLAP)∶ln(xAP)(x为酶活性)表示。采用Pearson相关性分析土壤酶活性、土壤酶化学计量比与土壤理化性质之间的关系。采用Canoco 5软件进行冗余分析(RDA)。
1.1. 研究区概况
1.2. 研究方法
1.3. 数据分析
-
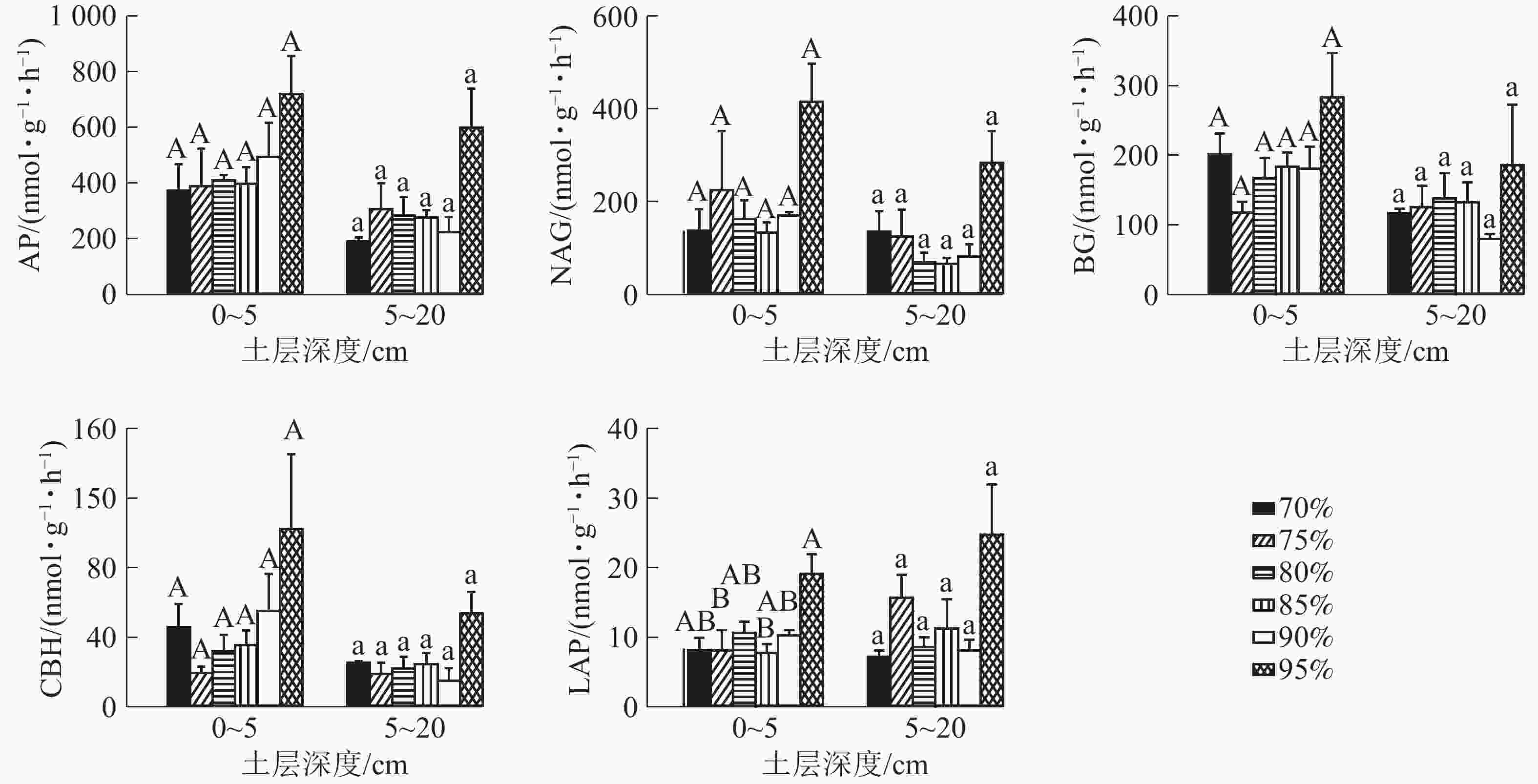
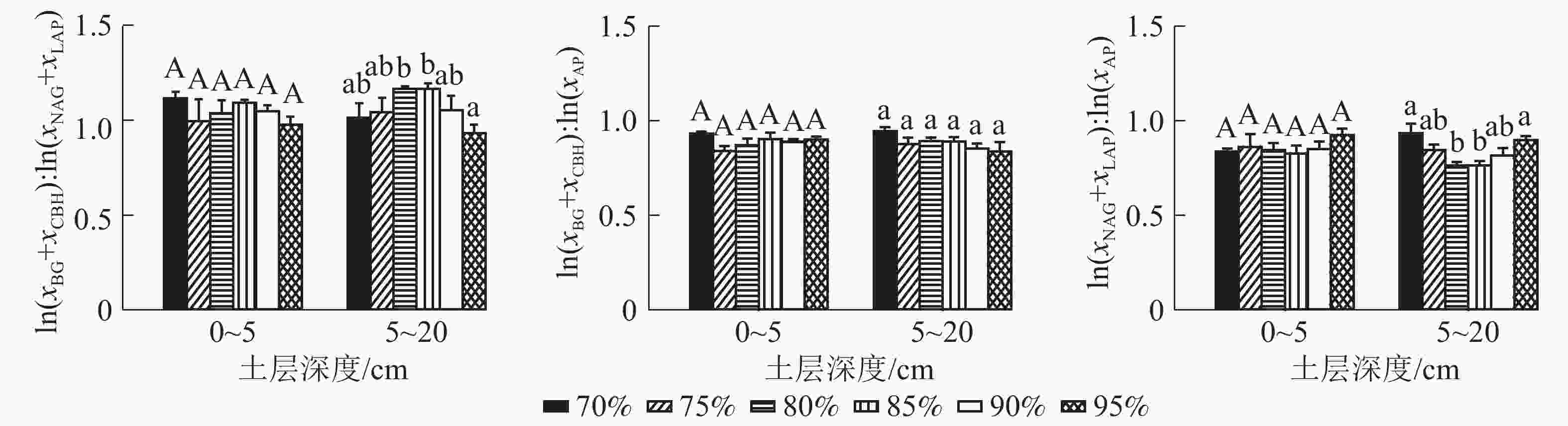
0~5 cm土层各梯度间AP、NAG、BG、CBH活性均无显著差异,兴安落叶松比例为95%的群落LAP活性比兴安落叶松比例为75%和85%的群落显著提高57.44%和59.40%。5~20 cm土层各梯度间AP、NAG、BG、CBH、LAP活性均无显著差异。5种酶活性中AP酶活性最高,0~5与5~20 cm土层均值分别为463.74和312.91 nmol·g−1·h−1(图1)。
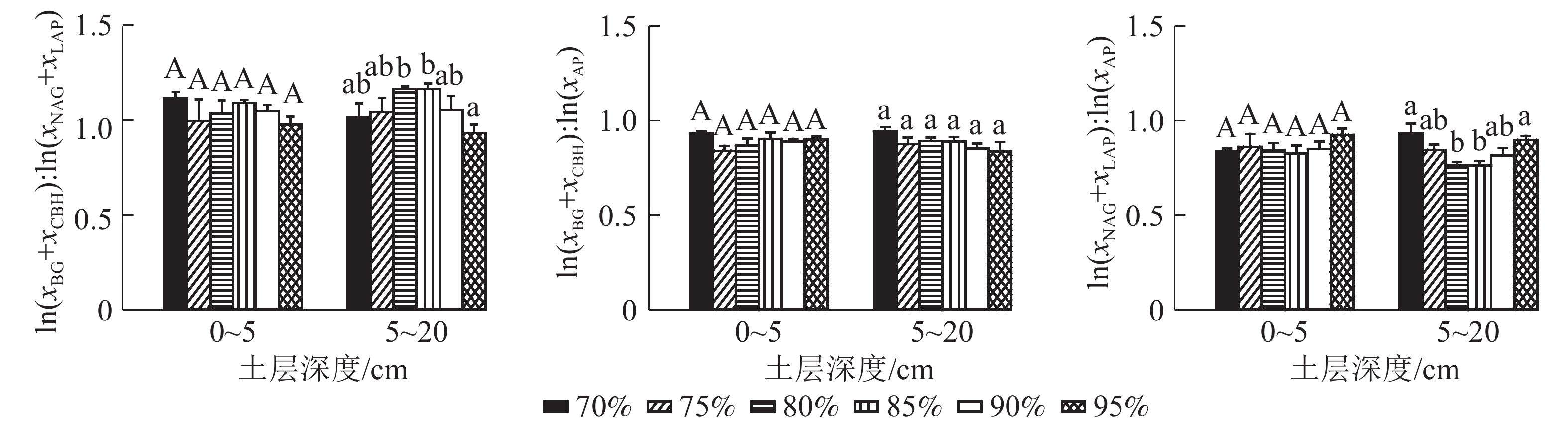
0~5 cm土层土壤酶化学计量比C∶N、土壤酶化学计量比C∶P、土壤酶化学计量比N∶P均无显著变化。5~20 cm土层土壤酶化学计量比C∶P无显著差异,土壤酶化学计量比C∶N随兴安落叶松所占比例的增加先增加后降低,且兴安落叶松比例为95%的群落显著低于兴安落叶松比例为80%和85%的群落(P95%-80%=0.030, P95%-85%=0.030)。土壤酶化学计量比N∶P随兴安落叶松所占比例的增加先降低后增加,且兴安落叶松比例为70%和95%的群落显著高于兴安落叶松比例为80%、85%的群落(P70%-80%=0.020, P70%-85%=0.020, P95%-80%=0.020, P95%-85%=0.020) (图2)。
-
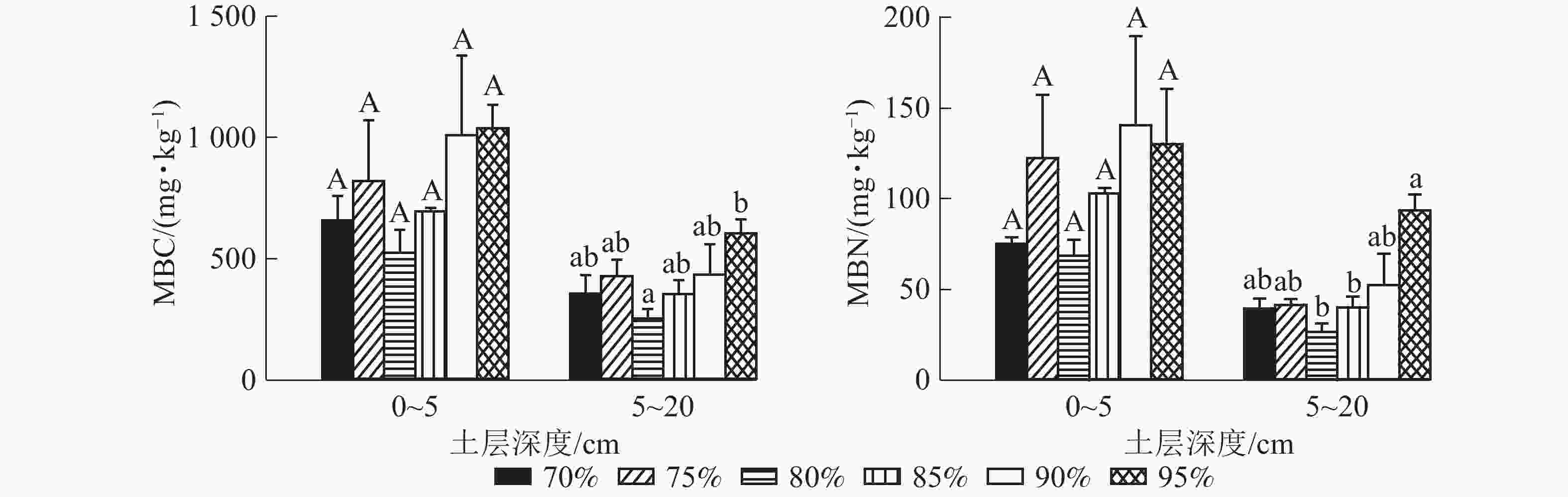
0~5 cm土层各梯度之间土壤微生物量碳(MBC)无显著差异,兴安落叶松比例为80%的群落MBC质量分数最低,最低值为525.10 mg·kg−1;兴安落叶松比例为95%的群落MBC质量分数最高,最大值为1 035.80 mg·kg−1。5~20 cm土层兴安落叶松比例为95%的群落MBC质量分数显著高于兴安落叶松比例为80%的群落(P95%-80%=0.040)。0~5 cm土层各梯度之间微生物量氮(MBN)无显著差异,兴安落叶松比例为80%的群落MBN质量分数最低,最低值为68.73 mg·kg−1;兴安落叶松比例为90%的群落MBN质量分数最高,最大值为140.72 mg·kg−1。5~20 cm土层兴安落叶松比例为95%的群落MBN显著高于兴安落叶松比例为80%和85% 的群落(P95%-80%=0.002, P95%-85%=0.040) (图3)。总体上看,土壤微生物量随兴安落叶松所占比例的增加呈现先增加后降低再增加的趋势。
-
表1和表2显示:0~5与5~20 cm土层各梯度之间土壤pH无显著差异。5~20 cm 土层,兴安落叶松比例为95%的群落土壤有机碳(SOC)质量分数显著高于兴安落叶松比例为85%的群落(P95%-85%=0.030)。5~20 cm土层兴安落叶松比例为95%的群落全氮(TN)质量分数显著高于其他兴安落叶松群落(P95%-70%=0.001, P95%-75%=0.007, P95%-80%=9×10−4, P95%-85%=0.001, P95%-90%=0.001)。0~5 cm土层兴安落叶松比例为95%的群落土壤全磷(TP)质量分数显著高于兴安落叶松比例为70%、80%、85%、90%的群落(P95%-70%=0.050, P95%-80%=0.001, P95%-85%=0.030, P95%-90%=0.040),兴安落叶松比例为75%的群落土壤TP质量分数显著高于兴安落叶松比例为80%的群落(P75%-80%=0.050)。5~20 cm土层兴安落叶松比例为95%的群落TP质量分数显著低于兴安落叶松比例为80%的群落(P95%-80%=0.010)。5~20 cm土层兴安落叶松比例为95%的群落易氧化碳(EOOC)质量分数显著高于兴安落叶松比例为70%的群落(P95%-70%=0.010)。0~5与5~20 cm土层各梯度之间碱解氮(AHN)和土壤C∶N均无显著差异。0~5 cm土壤N∶P随兴安落叶松所占比例的变化呈现先降低后增加再降低的趋势,兴安落叶松比例为80%的群落显著高于兴安落叶松比例为95%、75%的群落(P80%-95%=0.010, P80%-75%=0.030),5~20 cm土层土壤N∶P与5~20 cm土层 TP变化规律相反,兴安落叶松比例为95%的群落土壤N∶P显著高于兴安落叶松比例为70%、80%、85%的群落(P95%-70%=0.020, P95%-80%=0.003, P95%-85%=0.003)。5~20 cm土壤C∶P呈现先增加后降低再增加的趋势,兴安落叶松比例为95%的群落显著高于兴安落叶松比例为70%、80%、85%的群落(P95%-70%=0.030, P95%-80%=0.006, P95%-85%=0.005)。
兴安落叶松比例/% pH SOC/(g·kg−1) TN/(g·kg−1) TP/(g·kg−1) EOOC/(g·kg−1) AHN/(g·kg−1) C∶N N∶P C∶P 70 4.69 a 107.96 a 3.66 a 0.65 bcd 49.65 a 0.21 a 29.18 a 5.55 ab 163.20 a 75 4.95 a 109.71 a 3.85 a 0.90 ac 33.27 a 0.26 a 28.68 a 4.23 b 119.92 a 80 5.15 a 85.06 a 3.43 a 0.52 d 33.16 a 0.29 a 24.98 a 6.75 a 174.83 a 85 5.08 a 91.11 a 3.68 a 0.72 bcd 42.26 a 0.66 a 25.43 a 5.10 ab 127.20 a 90 4.80 a 87.56 a 3.53 a 0.69 bcd 32.70 a 0.32 a 23.67 a 5.23 ab 122.32 a 95 4.70 a 126.63 a 4.32 a 1.09 a 42.34 a 0.32 a 29.82 a 3.98 b 115.48 a 说明:不同小写字母表示差异显著(P<0.05) Table 1. Soil chemical properties in the depth of 0−5 cm of in different L. gmelinii stands
兴安落叶松比例/% pH SOC/(g·kg−1) TN/(g·kg−1) TP/(g·kg−1) EOOC/(g·kg−1) AHN/(g·kg−1) C∶N N∶P C∶P 70 5.15 a 47.21 ab 1.41 b 0.50 ab 9.16 a 0.14 a 33.45 a 2.80 b 94.38 b 75 5.06 a 45.44 ab 1.70 b 0.26 ab 15.40 ab 0.13 a 26.76 a 15.07 ab 369.03 ab 80 5.45 a 35.33 ab 1.57 b 0.47 b 14.33 ab 0.16 a 22.36 a 3.42 b 76.97 b 85 5.21 a 29.40 b 1.59 b 0.57 ab 14.15 ab 0.21 a 18.86 a 2.82 b 51.29 b 90 4.88 a 38.16 ab 1.58 b 0.14 ab 13.97 ab 0.16 a 24.73 a 26.18 ab 294.67 ab 95 4.93 a 55.37 a 2.74 a 0.08 a 25.23 b 0.21 a 20.18 a 39.06 a 779.56 a 说明:不同小写字母表示差异显著(P<0.05) Table 2. Soil chemical properties in the depth of 0−5 cm of in different L. gmelinii stands
-
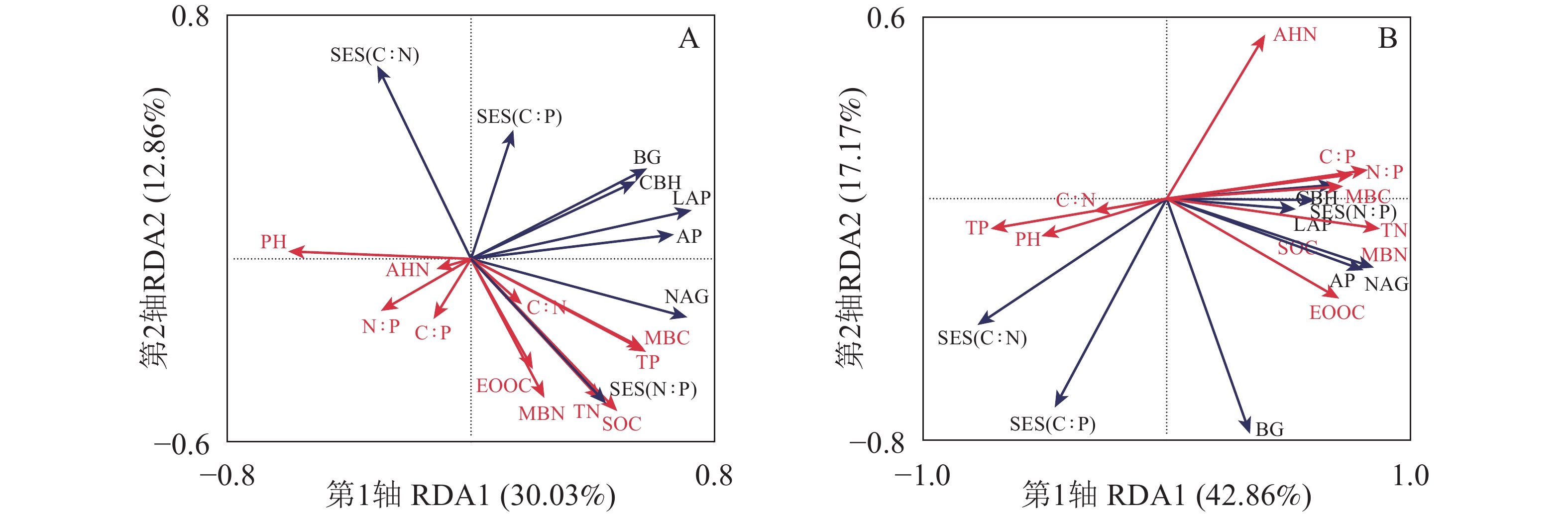
RDA排序图结果(图4)显示:0~5 cm土层第1轴和第2轴的解释变量分别为30.03%和12.86%(图4A),土壤pH(F=2.7,P=0.040)是土壤酶活性和酶化学计量比的显著影响因子。5~20 cm土层第1轴和第2轴的解释变量分别为42.86%和17.17%(图4B),土壤TN(F=8.9,P=0.002)和AHN(F=10.1,P=0.034)是土壤酶活性和酶化学计量比的显著影响因子。表3和表4中土壤微生物量和酶活性与土壤理化性质之间相关性分析表明:在0~5 cm土层,土壤BG、CBH与AP,土壤NAG、LAP与AP呈显著正相关(PBG-AP=0.001, PCBH-AP=3×10−4, PNAG-AP=8×10−4, PLAP-AP=1×10−5) (表3)。5~20 cm土层土壤MBC、MBN与SOC、TN、EOOC、CBH、NAG、AP、LAP显著正相关(PMBC-SOC=0.020, PMBC-TN=2×10−4, PMBC-EOOC=2×10−4, PMBC-CBH=0.050, PMBC-NAG=0.020, PMBC-AP=0.050, PMBC-LAP=0.010, PMBN-SOC=0.010, PMBN-TN=4×10−7, PMBN-EOOC=3×10−6, PMBN-CBH=0.020, PMBN-NAG=3×10−4, PMBN-AP=0.003, PMBN-LAP=0.030) (表4)。0~5 cm土层BG、NAG与pH呈显著负相关(PpH-BG=−0.010, PpH-NAG=−0.030)。5~20 cm土层 LAP、NAG与TN呈显著正相关(PLAP-TN=0.020, PNAG-TN=2×10−4)。AP与TP呈显著负相关(PAP-TP=−0.020)。5~20 cm土层土壤酶化学计量比C∶N与土壤N∶P、C∶P呈显著负相关(PSES(C∶N)-N∶P=−2×10−4, PSES(C∶N)-C∶P=−4×10−4),土壤酶化学计量比N∶P与土壤N∶P、土壤C∶P呈显著正相关(PSES(N∶P)-N∶P=0.007, PSES(N∶P)-C∶P=0.005)。
指标 MBC∶MBN C∶P N∶P C∶N SES(N∶P) SES(C∶P) SES(C∶N) AHN EOOC LAP SOC 0.11 0.36 −0.09 0.64** 0.37 −0.25 −0.44* 0.06 0.60** 0.21 pH −0.53* −0.23 0.14 −0.43 −0.41 −0.29 0.21 0.09 −0.22 −0.29 MBC 0.11 −0.05 −0.30 0.30 0.33 −0.13 −0.36 −0.02 0.15 0.30 MBN −0.35 −0.07 −0.36 0.36 0.22 −0.31 −0.36 0.03 0.11 0.05 TN 0.27 0.09 0.10 0.03 0.30 −0.28 −0.41 0.25 0.75*** 0.15 TP 0.03 −0.37 −0.68** 0.34 0.29 −0.20 −0.33 0.11 0.41 0.36 BG 0.64** −0.05 −0.09 0.01 0.24 0.49* 0.04 0.11 0.19 0.67** CBH 0.21 −0.18 −0.30 0.07 0.03 0.26 0.09 −0.17 −0.07 0.72*** NAG 0.44 −0.07 −0.22 0.18 0.73*** −0.12 −0.69*** −0.04 0.26 0.63** AP 0.28 −0.10 −0.21 0.09 0.13 −0.15 −0.20 −0.03 0.23 0.81*** LAP 0.49* −0.12 −0.27 0.13 0.16 0.12 −0.08 −0.28 −0.10 EOOC 0.08 0.07 0.10 0.04 0.21 −0.19 −0.28 0.45* AHN −0.11 −0.13 0.03 −0.17 0.02 0.05 0.00 SES(C∶N) −0.04 −0.11 −0.05 −0.13 −0.86*** 0.49* SES(C∶P) 0.46* −0.10 −0.06 −0.09 0.02 SES(N∶P) 0.32 0.03 −0.02 0.10 C∶N −0.19 0.51* −0.23 N∶P 0.15 0.71*** C∶P 0.01 指标 AP NAG CBH BG TP TN MBN MBC pH SOC 0.35 0.48* 0.04 0.23 0.69 *** 0.78 *** 0.49* 0.59** −0.64** pH −0.30 −0.50* −0.15 −0.54* −0.40 −0.44 −0.21 -0.50* MBC 0.46* 0.46* 0.23 0.27 0.64** 0.51* 0.88*** MBN 0.27 0.24 0.10 −0.05 0.58** 0.33 TN 0.38 0.43 −0.02 0.27 0.62** TP 0.44 0.51* 0.24 0.23 BG 0.66** 0.62** 0.49* CBH 0.73*** 0.33 NAG 0.69*** 说明:土壤酶化学计量比用SES表示,*表示P<0.05,**表示P<0.01,***表示P<0.001 Table 3. Peaeson correlation between soil enzymes, ecoenzymate stoichiometry and physicochemical properties in the depth of 0−5 cm of in different L. gmelinii stands
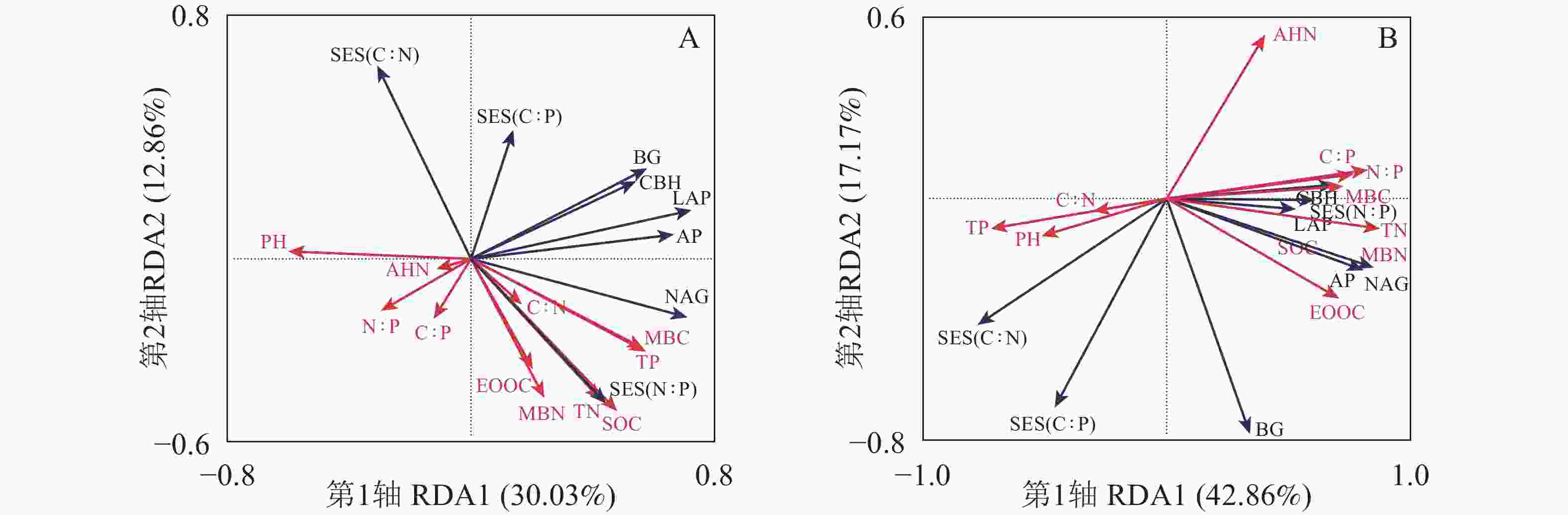
Figure 4. 0−5 (A) and 5−20 cm(B) redundancy analysis of soil enzyme activities and ecoenzymatic stoichiometry
指标 MBC∶MBN C∶P N∶P C∶N SES(N∶P) SES(C∶P) SES(C∶N) AHN EOOC LAP SOC −0.33 0.53* 0.48* 0.58** 0.54* 0.10 −0.39 −0.02 0.55* 0.44* pH 0.34 −0.65** −0.59** −0.02 −0.53* 0.23 0.58** 0.15 −0.37 −0.46* MBC −0.13 0.77*** 0.76*** −0.15 0.59** −0.26 −0.66** 0.26 0.74*** 0.55* MBN −0.56* 0.83*** 0.85*** −0.20 0.58** −0.20 −0.60** 0.16 0.85*** 0.50* TN −0.42 0.77*** 0.81*** −0.30 0.52* −0.30 −0.61** 0.41 0.91*** 0.53* TP 0.14 −0.90*** −0.88*** 0.15 −0.50* 0.40 0.66** −0.24 −0.59** −0.32 BG −0.15 0.21 0.23 −0.17 0.24 0.46* 0.08 −0.31 0.48* 0.08 CBH −0.14 0.44 0.50* −0.28 0.42 −0.23 −0.49* 0.64** 0.59** 0.15 NAG −0.32 0.70*** 0.75*** −0.26 0.71*** −0.17 −0.67** 0.19 0.64** 0.28 AP −0.25 0.53* 0.60** −0.40 0.36 −0.29 −0.48* 0.30 0.59** 0.30 LAP −0.15 0.31 0.31 −0.05 0.33 −0.26 −0.43 0.13 0.45* EOOC −0.37 0.73*** 0.74*** −0.28 0.43 −0.05 −0.39 0.18 AHN 0.10 0.17 0.25 −0.37 0.01 −0.63** −0.38 SES(C∶N) 0.15 −0.71*** −0.74*** 0.13 −0.79*** 0.59** SES(C∶P) 0.07 −0.37 −0.44 0.40 0.02 SES(N∶P) −0.15 0.60** 0.58** 0.11 C∶N 0.03 −0.14 −0.23 N∶P −0.37 0.99*** C∶P −0.33 指标 AP NAG CBH BG TP TN MBN MBC pH SOC 0.21 0.38 0.30 0.15 −0.42 0.59** 0.56** 0.51* −0.4 pH −0.27 −0.35 −0.14 −0.10 0.62** −0.39 −0.62** −0.68** MBC 0.45* 0.53* 0.45* 0.21 −0.73*** 0.74*** 0.85*** MBN 0.63** 0.73*** 0.51* 0.42 −0.68*** 0.88*** TN 0.69*** 0.75*** 0.69*** 0.35 −0.64** TP −0.53* −0.62** −0.41 −0.19 BG 0.67** 0.61** 0.36 CBH 0.75*** 0.70*** NAG 0.86*** 说明:土壤酶化学计量比用SES表示,*表示P<0.05,**表示P<0.01,***表示P<0.001 Table 4. Peaeson correlation between soil enzymes, ecoenzymate stoichiometry and physicochemical properties in the depth of 5−20 cm of in different L. gmelinii stands
2.1. 生态酶活性及其化学计量比随兴安落叶松不同比例的变化特征
2.2. 兴安落叶松所占不同比例的针阔混交林土壤微生物量的变化
2.3. 兴安落叶松所占比例不同的针阔混交林土壤的理化性质
2.4. 土壤微生物量、酶活性及土壤酶化学计量比影响因子分析
-
土壤SOC、TN、微生物量、酶活性均随兴安落叶松所占比例的变化而发生改变,这是因为兴安落叶松所占比例的变化改变了林分环境,进而影响了凋落物的输入、土壤微生物量以及土壤理化性质,从而改变土壤酶的活性[28]。植物凋落物作为土壤主要的有机碳源,通过微生物转化为腐殖质[29]。随着兴安落叶松所占比例的改变,兴安落叶松比例为80%、85%的群落SOC质量分数较低而pH较高,这是因为土壤pH的变化与有机质分解过程中产生的H+多少有关[30],改变了微生物酶活性,进而影响凋落物的分解。有机物中的磷需要在土壤微生物和磷酸酶作用下转化为无机磷才可被植物吸收利用[31],但本研究发现:0~5 cm土层AP活性与TP质量分数无关,5~20 cm土层AP酶活性随TP质量分数增加而降低,且AP活性在5种酶中最高。由于AP酶活性与有效磷呈显著负相关[32],说明研究地区土壤可能缺乏有效磷。前人研究表明:当全磷为0.8~1.0 g·kg−1时,土壤可能会出现供磷不足[33],且由于研究地区土壤呈酸性,磷会形成难溶的磷酸铁(FePO4)和磷酸铝(AlPO4), 从而降低有效磷含量[28]。虽然研究地区土壤TP质量分数普遍低于0.8 g·kg−1,但研究地区是否缺磷还需要结合土壤化学计量比进一步探讨。
土壤微生物量的多少与土壤养分以及有机质密切相关[34-35],有机物分解也受到土壤酶活性与土壤微生物量等的影响[36]。在兴安落叶松所占比例不同的针阔混交林中,0~5 cm土层SOC、TN、EOOC和AHN质量分数均无显著变化,0~5 cm土层由于各梯度之间EOOC和AHN无显著变化,微生物量随兴安落叶松所占比例的改变无显著变化。5~20 cm土层兴安落叶松比例为95%的群落,土壤SOC、TN、EOOC和AHN质量分数达最大值,此时土壤微生物量也达最大值。前人发现:土壤酶活性与土壤微生物和土壤环境密切相关[37],NAG酶活性随微生物量增加而增大[38]。本研究发现:在5~20 cm土层土壤微生物量与CBH、NAG、LAP呈显著正相关,说明在5~20 cm土层,随落兴安叶松所占比例的变化,土壤微生物量与土壤碳氮养分以及土壤微生物量与土壤碳氮酶活性变化具有趋同性。
-
土壤酶化学计量可以衡量微生物对养分的需求情况[14]。本研究结果表明:5~20 cm土层TN、AHN是影响土壤酶活性的显著因子,相关性分析也证明了5~20 cm土层土壤酶化学计量比N∶P和土壤酶化学计量比C∶P与TN呈显著正相关。研究发现:5~20 cm土层土壤酶化学计量比C∶N与土壤酶化学计量比N∶P变化规律相反,表明随兴安落叶松所占比例的变化,氮元素成为土壤微生物的限制因素。相关性分析显示:0~5 cm土层土壤酶化学计量比与土壤化学计量比均无显著相关性,表明0~5 cm土层土壤酶化学计量关系比较复杂,与多种因素有关。
本研究区域中,仅有5~20 cm土层土壤酶化学计量比N∶P与土壤N∶P呈显著正相关,土壤酶化学计量比C∶N与土壤N∶P和土壤C∶P显著负相关,表明土壤酶化学计量和土壤化学计量比之间存在差异,进一步证实了土壤酶化学计量和土壤化学计量比结果不一致的结论[14]。这是因为土壤化学计量反映的是土壤养分状况而非微生物可利用养分的状况,而土壤酶化学计量比既受到土壤微生物和土壤养分元素的影响,还受到有效性碳氮磷的调控[39]。RDA分析也表明:5~20 cm土层土壤酶化学计量比受到TN、AHN的影响,进一步证实了上述观点。
-
全球尺度上,土壤ln(xCBH+xBG)∶ln(xNAG+xLAP)∶ln(xAP) = 1∶1∶1[12]( x为酶活性)。兴安落叶松比例为95%的群落上下土层土壤酶化学计量比C∶N均小于1,这表明林地受到氮元素的限制。0~5和5~20 cm土层土壤酶化学计量比C∶P、土壤酶化学计量比N∶P均小于1,这表明研究地区普遍缺乏微生物可利用的有效磷。5~20 cm土层兴安落叶松比例为95%、70% 时土壤酶化学计量比N∶P显著高于80%与85%,这表明兴安落叶松比例为80%与85%的群落AP酶活性较高,有效磷元素相对缺乏。因为当土壤养分利用率较低时,土壤微生物增加了相应酶的活性,以提高有效氮和有效磷等养分的供应,这与BLOOM等[40]认为微生物会将其资源最优地分配给获取最有限的资源观点相一致。
0~5 cm土层兴安落叶松比例为70%、80%群落的土壤C∶P大于中国土壤C∶P(136),土壤N∶P低于中国土壤N∶P(9.3)[41],这说明兴安落叶松比例为70%、80%的群落缺乏磷元素,5~20 cm土层兴安落叶松比例为75%、90%、95%的群落土壤N∶P、C∶P高于中国土壤N∶P(9.3)、土壤C∶P(136)[41],表明兴安落叶松比例为75%、90%、95%的群落普遍存在磷元素的限制。
3.1. 兴安落叶松所占比例的变化对土壤养分和酶活性的影响
3.2. 兴安落叶松林土壤酶化学计量比与土壤理化性质的关系
3.3. 兴安落叶松林土壤养分限制因子
-
在兴安落叶松所占比例不同的针阔混交林中5种酶中AP酶活性最高。兴安落叶松比例不同的群落所受的限制因子存在差异,0~5 cm土层兴安落叶松比例为70%、90%的群落、5~20 cm土层兴安落叶松比例为75%、90%、95%的群落受到TP限制。5~20 cm土层兴安落叶松比例为80%、85%的群落可能受到土壤有效磷限制。兴安落叶松比例为95%的群落上下层均受到土壤有效氮的限制。0~5和5~20 cm土壤酶化学计量比与全球土壤酶化学计量比标准值1∶1∶1有所偏离,0~5 cm土层土壤酸碱度是影响土壤酶化学计量比的关键因子,而在5~20 cm土层,则主要受到土壤全氮和有效氮质量分数的影响。由此可见,暖温带针阔混交林中兴安落叶松所占比例是调控土壤养分动态的一个重要生物因子,而其调控作用的发挥则主要依赖于土壤中酶的活性及其化学计量特征。
-
感谢内蒙古农业大学张秋良教授、内蒙古大兴安岭森林生态系统国家级野外研究站张广亮技术员、根河林业局于海俊先生,以及张欢、朱雍、曹雨松、郭金粲等同志的帮助。










 DownLoad:
DownLoad: