-
抚育间伐是常用的森林管理措施[1],因伐除林冠相对密集的部分树木,增加了太阳辐射,改变了森林小气候和土壤微生境,必然影响森林生态系统的养分和生物地球化学循环过程,以及该循环过程的核心环节——土壤微生物活动和酶活性。目前,土壤胞外酶研究更多关注于碳、氮和磷循环相关的降解酶,如碳酶[β-葡糖苷酶(BG)、纤维二糖水解酶(CBH)、β-木糖苷酶(BX)],氮酶[β-1,4-N-乙酰氨基葡萄糖苷酶(NAG)、亮氨酸氨基肽酶(LAP)]和磷酶[酸性或碱性磷酸酶(AcP)],其活性可作为微生物资源分配的代理指标[2]。在养分循环期间酶活性的相对丰度变化可反映微生物群落的代谢水平。SINSABAUGH等[3]最先通过整合分析发现:在全球尺度上碳、氮和磷循环相关酶计量比接近1∶1∶1,表明土壤酶化学计量比呈稳态性。但也有研究发现:土壤酶化学计量比呈非稳态性[4−6],说明微生物可能受到能量或关键营养物质(即碳、氮和磷)的限制[7]。
间伐措施对土壤胞外酶活性和酶化学计量的影响仍不确定。如土壤酶活性在森林间伐后会增加[8]、减少[9]或保持不变[10]。大多数研究主要围绕不同间伐强度对酶活性的影响[11]。间伐措施的影响效果还会随森林恢复过程而发生改变。如QIU等[12]对塞罕坝林场内华北落叶松Larix principis-rupprechtii人工林进行间伐恢复9 a后的结果显示:间伐措施显著增加了土壤BG、NAG+LAP和AcP活性。而LULL等[13]对地中海栎Quercus ilex林间伐后5个月至7 a内,氮和磷循环酶的活性并未发生显著改变。间伐处理和林下移除可在短时间内减少微生物对土壤资源的竞争,进而改变酶的活性[14]。但随树木生长速度和土壤养分含量的变化,微生物资源利用策略也发生改变,可能造成微生物受到不同养分的限制[15]。
目前,关于间伐处理对土壤胞外酶活性的研究大多侧重于间伐强度和人工林生态系统的研究,而对天然林生态系统间伐后不同恢复阶段土壤酶活性的研究较少。鉴于此,本研究采用空间代替时间的方法,探讨北亚热带秦岭松栎混交林在抚育间伐后不同恢复时间内林地表层土壤酶活性、酶化学计量比的变化规律,为制定森林可持续经营方案及合理的生态恢复措施提供理论依据。
-
研究区位于陕西省安康市宁东林业局新矿林场(33°20′~33°26′N,108°32′~108°34′E),地处秦岭山脉,海拔为1 400.0~1 800.0 m。该区属于北亚热带与温带过渡区,年均气温为8.5 ℃,年平均降水量为908.0 mm,土壤为山地棕壤。研究区域为20世纪70年代末采伐后天然更新形成的次生针阔混交林[16],采取的是低强度间伐和林冠下补植等保护经营作业法。林内主要以油松Pinus tabuliformis、锐齿槲栎Quercus aliena var. acutiserrata、华山松Pinus armandii为主要建群种,伴生有漆树Toxicodendron vernicifluum、小叶女贞Ligustrum quihoui、青榨槭Acer davidii等树种。林下植被以卫矛Euonymus alatus、木姜子Litsea pungens、披针叶薹草Carex lanceolata、龙牙草Agrimonia pilosa、茜草Rubia cordifolia为主。
2021年10月,根据研究区内实际间伐处理、林木生长和分布状况,选择立地条件基本一致的林分,设置3种间伐处理,即未间伐(ck)、间伐恢复5 a (5 a,2018年间伐)和间伐恢复13 a (13 a,2010年间伐)。每个间伐处理设置4块面积为20 m×30 m的样地,共计12块样地。为防止样地之间相互干扰,样方之间的间隔不小于100 m。进行间伐处理后林下物种数量增加,更新了枫杨Pterocarya stenoptera、栗Castanea mollissima、桤木Alnus cremastogyne、灯台树Cornus controversa和胡桃楸Juglans mandshurica等树种。其中各样地内物种丰富度和Shannon-Wiener指数参照刘思泽等[17]的方法计算。样地调查基本概况见表1。
表 1 试验样地基本概况
Table 1. Basic survey of test plots
间伐后恢
复时间/a海拔/
m株数密度/
(株·hm−2)胸径/
cm郁闭度 物种
丰富度Shannon-Wiener
指数凋落物量/
(t·hm−2·a−1)林内主要树种 ck 1 585.00±61.85 1 420±88 14.60±0.49 0.7 25 2.48 7.01±0.37 油松、锐齿槲栎、华山松、毛樱桃、垂柳、
木姜子、三桠乌药5 1 457.32±13.14 1 208±355 13.80±0.84 0.5 32 2.78 5.69±0.26 锐齿槲栎、栗、油松、白桦、垂柳、
榆树、桤木13 1 757.57±20.17 1 254±207 13.80±1.19 0.6 29 2.68 6.55±0.29 毛樱桃、油松、锐齿槲栎、漆树、水蜡树、
木姜子、灯台树说明:毛樱桃Prunus tomentosa,垂柳Salix babylonica,三桠乌药Lindera obtusiloba,白桦Betula platyphylla,榆树Ulmus pumila,水蜡树Ligustrum obtusifolium。 -
2023年7月,根据S型取样方法,在ck、5 a、13 a间伐样地内,用直径为3.6 cm的土钻采集0~10 cm的表层土样,为避免样品受到污染,将土壤混合储存于灭菌自封袋中,再用便携冷藏箱带回实验室。在室内充分混匀后过2 mm筛。一份新鲜土样于4 ℃冰箱保存,用于有效氮、土壤酶活性和土壤微生物生物量的测定;另一份土壤样品自然风干,用于其他土壤理化性质的测定。
-
土壤含水率采用105 ℃烘干法;土壤pH采用电位法(土水体积质量比为1.0∶2.5);土壤总氮采用元素分析仪测定;土壤有机碳采用重铬酸钾氧化-外加热法;土壤有效氮指铵态氮和硝态氮的总和,分别采用2 mol·L−1氯化钾浸提-靛酚蓝比色法、氯化钾提取-双波长紫外分光光度法测定;土壤总磷采用硫酸-高氯酸-钼锑抗比色法[18]。微生物生物量碳、氮采用氯仿熏蒸法,使用岛津总有机碳分析仪测定。
-
参照SAIYA-CORK等[19]的方法,测定与碳、氮、磷循环密切相关的酶活性,各种土壤酶的名称、简称及底物见表2。其中,水解酶(BG、BX、CBH、NAG、LAP、AcP)活性采用微孔板荧光法,用多功能酶标仪在365 nm波长处激发,450 nm波长处荧光测定;氧化酶(POX、PER)活性采用DOPA-紫外分光光度法,用多功能酶标仪在450 nm波长处测定。
表 2 土壤胞外酶的简称及所用底物
Table 2. Soil enzyme along with their enzyme abbreviation and substrate of soil enzyme
酶名称 简称 底物 β-葡糖苷酶β-glucosidase BG 4-MUB-β-D-glucoside β-木糖苷酶β-xalosidase BX 4-MUB-β-D-xylopyranoside 纤维二糖水解酶Cellobiohydrolase CBH 4-MUB-β-D-cellobioside β-N-乙酰氨基葡萄糖苷酶β-N-acetylglucosaminidase NAG 4-MUB-N-acetyl-β-D-glucosaminde 亮氨酸氨基肽酶Leucine aminopeptidase LAP L-leucine-7-amido-4 methylcounarin 酸性磷酸酶Acid phosphatase AcP 4-MUB-phosphatase 酚氧化物酶Phenol oxidase POX L-dihydroxyphenylalanine(L-DOPA) 过氧化物酶Peroxidase PER L-dihydroxyphenylalanine(L-DOPA) and H2O2 说明:MUB为甲基伞形酮酰Methylumbelliferyl。 通过计算碳、氮和磷酶活性的比值研究土壤胞外酶化学计量[20],同时,采用酶计量的载体分析,即用矢量长度(VL)及矢量角(VA)分析间伐处理对微生物能量和营养的相对限制状况[21],计算公式如下。
$$ {E}_\text{C/N}\text{}\text=\text{}\text{ln}{H}_{\mathrm{B}\mathrm{G}}\text{/ln}\text{(}{H}_{\text{NAG}}\text+{H}_{\text{LAP}}\text{)}\text{;}\text{}\text{}\text{} $$ (1) $$ {E}_\text{C/P}\text{}\text=\text{}\text{ln}{H}_{\text{BG}}\text{/ln}{H}_{{\mathrm{Ac}}\mathrm{P}};\text{}\text{}\text{}\text{}\text{}$$ (2) $$ {E}_\text{N/P}\text{= ln}\text{(}{H}_{\text{NAG}}\text+{H}_{\text{LAP}}\text{)}\text{/ln}{H}_{{\mathrm{Ac}}\mathrm{P}}; $$ (3) $$ {V}_{\text{L}}\text=\text{SQRT}\text{[}\text{(}{E}_\text{C/N}\text{)}^2\text+\text{(}{E}_\text{C/P}\text{)}^2\text{]}\text{;} $$ (4) $$ {V}_{\text{A}}\text=\text{Degrees}\text{[}\text{ATAN2}\text{(}{E}_\text{C/P}\text{,}\text{}{E}_\text{C/N}\text{)}\text{]}\text{。}$$ (5) 式(1)~(5)中:$ {E}_\text{C/N} $、$ {E}_\text{C/P} $、$ {E}_\text{N/P} $分别为土壤碳获取酶/氮获取酶比值、土壤碳获取酶/磷获取酶比值、土壤氮获取酶/磷获取酶比值;$ {H}_{\mathrm{B}\mathrm{G}}\mathrm{、}{H}_{\text{NAG}}\mathrm{、}{H}_{\text{LAP}}\mathrm{、}{H}_{{\mathrm{Ac}}\mathrm{P}} $分别为BG、NAG、LAP、AcP的酶活性;SQRT为平方根函数,Degrees为角度转换函数,ATAN2为反正切函数。VL越大,表明碳限制越严重。VA以45°为分界线,>45°为磷限制,<45°为氮限制。偏离程度越大,限制程度越强。
-
使用SPSS 25.0对不同间伐恢复时间下的土壤理化性质、胞外酶活性、酶化学计量比、酶矢量长度和角度的差异进行单因素方差分析(one-way ANOVA)和最小显著性差异法(LSD)(P<0.05);利用Sperman检验分析与土壤酶活性和酶矢量变化显著相关的土壤因子,利用Origin 2021绘图。以酶活性及其矢量作为物种因子,土壤理化性质作为环境因子,利用Canoco 5.0进行冗余分析。通过方差膨胀因子(VIF)判断解释变量之间的线性关系,剔除共线性较强(VIF>5)的变量,对剩余的pH、有效氮、有机碳和全磷共4个变量进行研究。
-
从表3可见:间伐恢复对土壤pH、有效氮、全磷、碳氮比、氮磷比、有机碳、微生物量碳、微生物量氮和微生物量碳氮比均有显著影响(P<0.05)。恢复5 a的土壤pH显著高于ck (P<0.05)。恢复13 a的土壤全磷、微生物量碳和微生物量氮均显著高于ck (P<0.05),分别是ck的1.28、1.19和1.15倍。土壤有效氮、碳氮比和氮磷比均显著低于ck (P<0.05)。恢复5 a的土壤有机碳显著降低了25.93% (P<0.05),但恢复13 a的土壤有机碳质量分数逐渐恢复至未间伐前水平。间伐恢复对土壤含水率和全氮无显著影响。
表 3 不同间伐恢复时间下土壤理化特性状况
Table 3. Soil physical and chemical properties under different thinning treatments
间伐后恢复时间/a pH 含水率/% 有效氮/(mg·kg−1) 全氮/(g·kg−1) 全磷/(g·kg−1) 碳氮比 ck 5.48±0.10 b 37.28±4.01 a 21.34±1.96 a 4.58±0.86 a 0.60±0.08 b 10.02±1.16 a 5 5.98±0.13 a 35.10±6.81 a 17.19±0.48 ab 3.28±0.68 a 0.52±0.10 b 9.34±1.41 ab 13 5.76±0.17 ab 40.37±1.67 a 16.56±0.58 b 3.93±0.44 a 0.77±0.07 a 8.55±1.32 b 间伐后恢复时间/a 氮磷比 有机碳/(g·kg−1) 微生物量碳/(g·kg−1) 微生物量氮/(g·kg−1) 微生物量碳氮比 ck 7.49±0.71 a 35.94±3.84 a 1.14±0.04 b 0.20±0.01 b 5.97±0.37 ab 5 6.45±0.95 ab 26.62±2.79 b 1.14±0.09 b 0.22±0.01 ab 5.09±0.13 b 13 5.04±0.34 b 33.33±2.27 ab 1.36±0.02 a 0.23±0.01 a 6.11±0.33 a 说明:数据均为平均值±标准误。不同小写字母表示不同处理间差异显著 (P<0.05)。 -
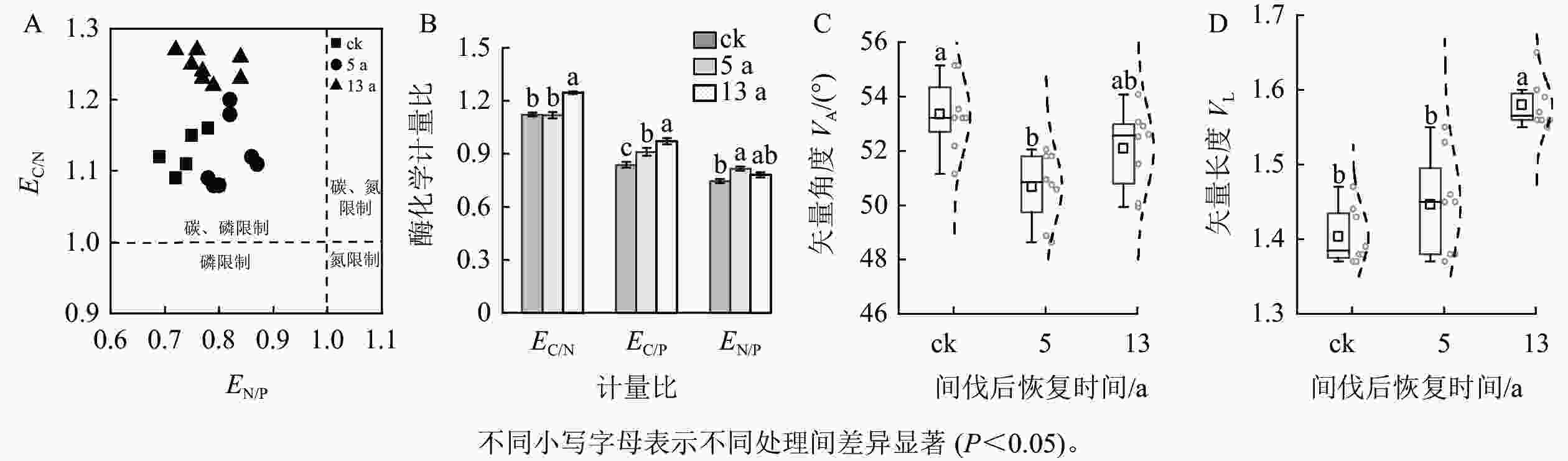
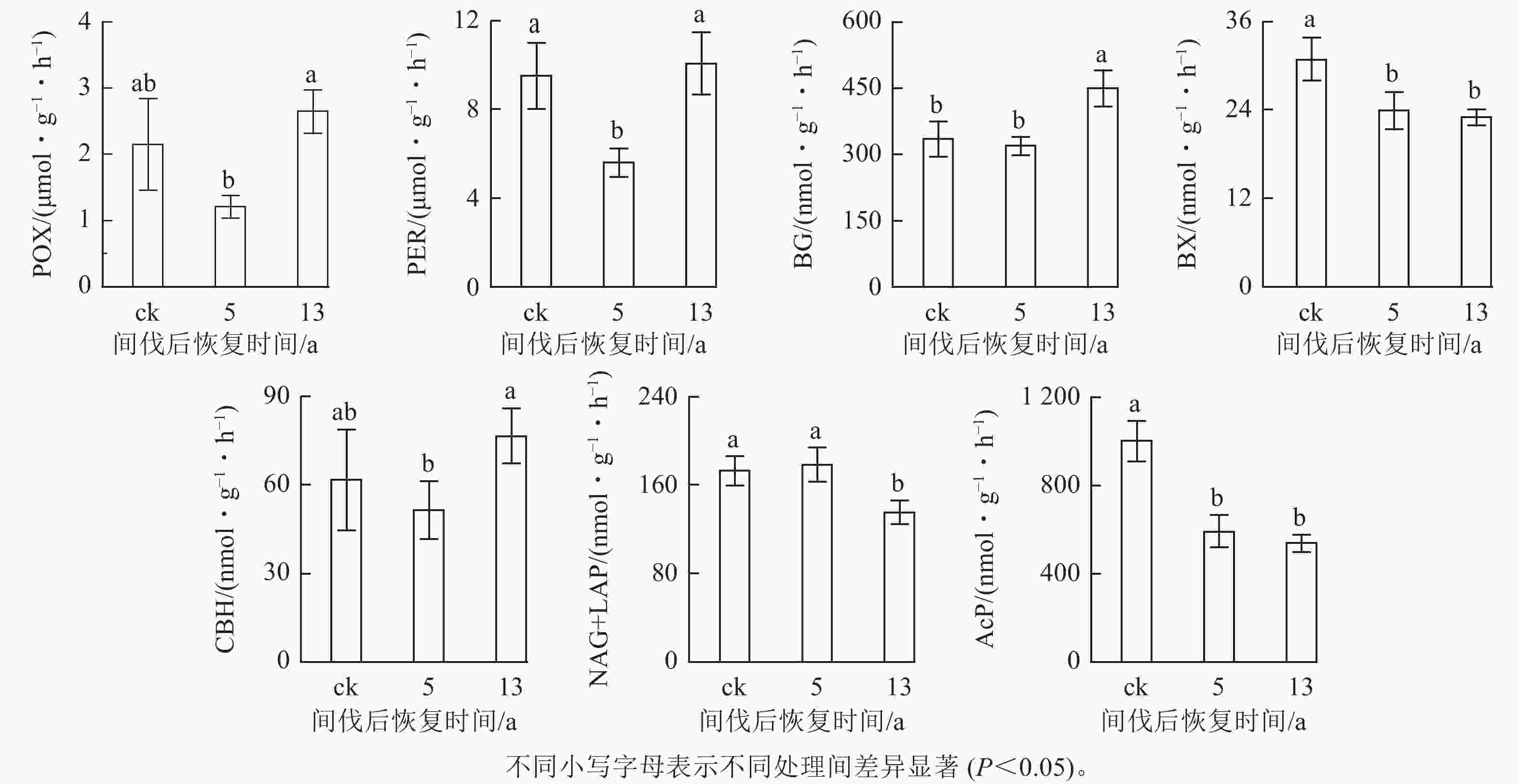
从图1可见:间伐恢复对不同土壤酶活性的影响并不一致。恢复13 a时土壤BX、AcP和NAG+LAP活性显著下降(P<0.05),较ck分别降低了25.39%、22.92%和46.25%,同时土壤BG活性还显著提高(P<0.05),是ck的1.34倍(P<0.05)。土壤氧化酶(POX、PER)和CBH活性变化趋势与前4种酶不同,在恢复5 a时活性最低,在恢复13 a时活性最高。
通过矢量分析发现:VA>45°,且EN/P<1、EC/N>1 (图2A),表明研究区土壤微生物生长代谢主要受碳和磷共同限制。森林土壤EC/P和EN/P显著偏离1,且随间伐后时间的持续而逐渐恢复或显著增大(P<0.05,图2B)。VA和VL在3个间伐恢复间均有明显差异(图2C~D)。与ck相比,间伐恢复5 a的VA显著降低了4.42%,13 a的VL是ck的1.13倍(P<0.05)。表明间伐措施在恢复初期能够缓解土壤微生物受磷限制的状况,而后随恢复时间的持续,微生物受碳限制程度显著增加(P<0.05)。
-
相关性分析(表4)表明:水解酶活性与有效氮、有机碳和微生物量碳氮比均呈正相关关系。其中土壤碳获取酶(BG、CBH)与土壤全磷、有机碳、微生物量碳呈显著(P<0.05)或极显著(P<0.01)正相关,BX活性与土壤有效氮、微生物量碳氮比呈显著正相关(P<0.05)。土壤氮获取酶(NAG+LAP)和磷获取酶(AcP)均与土壤有效氮呈极显著正相关(P<0.01)。酚氧化物酶(PER)除与pH呈显著负相关外(P<0.05),还与有机碳、微生物量碳氮比呈极显著正相关(P<0.01)。VA仅与pH呈极显著负相关(P<0.01)。VL与全磷和微生物量碳呈显著正相关外(P<0.05),还与氮磷比呈极显著负相关(P<0.01)。
表 4 土壤酶变化与土壤理化性质的相关性分析
Table 4. Correlation analysis between soil enzyme changes and soil physical and chemical properties
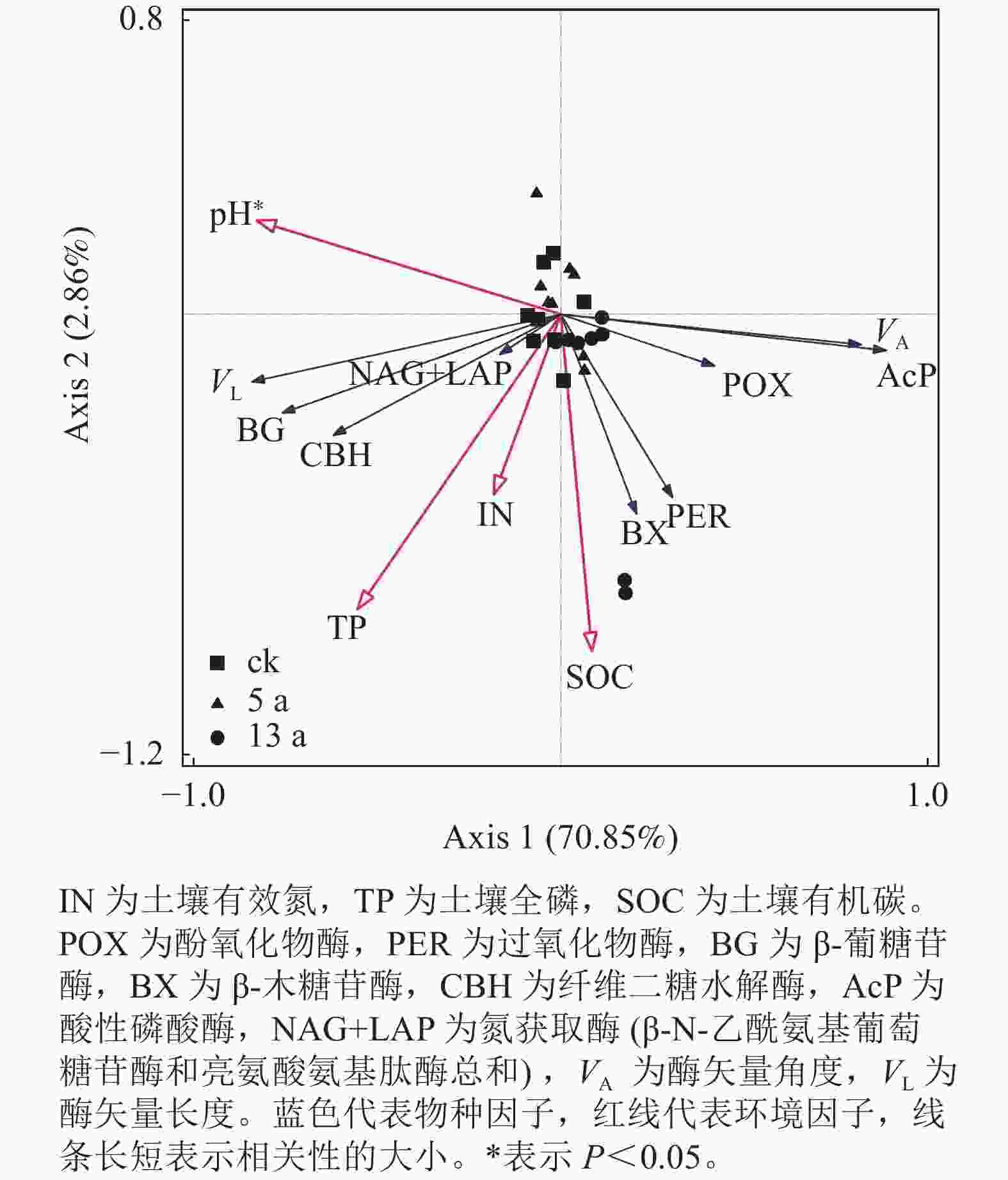
指标 pH IN TP SOC MBC MBC/MBN N/P POX −0.54 −0.29 −0.04 −0.07 0.26 0.30 −0.04 PER −0.65* 0.19 0.32 0.45* 0.22 0.52** 0.21 BG 0.28 0.35 0.73** 0.55** 0.63** 0.38 −0.25 BX −0.53 0.54** −0.01 0.27 0.10 0.45* 0.56 CBH −0.01 0.24 0.46* 0.43* 0.53** 0.65** 0.17 AcP −0.72* 0.57** −0.38 0.06 −0.13 0.22 0.85** NAG+LAP 0.17 0.66** −0.08 0.14 −0.01 0.00 0.60 VA −0.95** 0.01 −0.30 −0.06 −0.04 0.35 0.43 VL 0.45 −0.28 0.70** 0.31 0.48* 0.15 −0.63* 说明:IN为土壤有效氮,TP为土壤全磷,SOC为土壤有机碳,MBC为微生物量碳,MBN为微生物量氮,N/P为氮磷比。POX为酚氧化物酶,PER为过氧化物酶,BG为β-葡糖苷酶,BX为β-木糖苷酶,CBH为纤维二糖水解酶,AcP为酸性磷酸酶,NAG+LAP为氮获取酶(β-N-乙酰氨基葡萄糖苷酶和亮氨酸氨基肽酶总和),VA为酶矢量角度,VL为酶矢量长度。*表示显著相关(P<0.05),**表示极显著相关(P<0.01)。 冗余分析(图3)表明:剔除存在共线性关系的变量后,pH、有效氮、有机碳和全磷共解释了酶活性和酶矢量变异的73.71%。其中pH和有机碳是对土壤酶整体变化解释度最高的因子,分别解释了变量的48.80%和13.10%,且pH与酶指标变化显著相关(P<0.05)。
-
间伐改变了秦岭松栎混交林表层土壤pH和养分质量分数,但在不同恢复阶段规律不一致。在本研究中,间伐导致pH提高,尤其是间伐恢复5 a后,这与许多学者的研究结果一致。如对云杉Picea crassifolia[22]林和火炬松Pinus taeda[23]林研究表明:间伐减少了针叶凋落物作为有机酸主要输入组分的产生,从而显著提高土壤表层pH。本研究中针叶树种的胸高断面积占比在间伐后有所降低,这在一定程度上能缓解土壤酸化。同时,间伐后土壤含水率、全氮、全磷和有机碳质量分数均呈先减少后逐步恢复的趋势。这可能是因为间伐短期内树冠层郁闭度减小,导致土壤蒸发增强的同时,也促进林下植被的快速生长,加快了土壤水分的消耗[24]。凋落物作为土壤最主要的有机碳源,通过微生物转化为腐殖质的同时也改变了土壤pH,影响凋落物的分解,改变土壤养分水平[25]。相较于ck,间伐恢复5、13 a后,凋落物量分别恢复至81.16%和93.41%,间伐恢复13 a的土壤全氮、全磷和有机碳质量分数有所提高,表明随时间的持续,林分结构及相关生态过程在一定程度上得到恢复。此外,本研究中微生物量碳、氮和土壤有效氮在间伐恢复13 a后的变化趋势不一致,可能因为间伐后林地内出现了栗、桤木和水蜡树等阳性植物,以及毛樱桃、白桦和漆树等阔叶树种,林地内相对多度增加,根系密度和根系分泌物增多,有利于土壤微生物生物量的积累[26]。而林下喜光物种的快速生长[27],对土壤游离态氮的需求增大,导致土壤有效氮质量分数有所降低。这与周璇等[28]对8年生柳杉Cryptomeria japonica人工林进行间伐后的研究结果一致。
-
在本研究中,间伐恢复年限导致土壤BX、AcP和NAG+LAP活性显著降低,但对其他土壤酶活性影响趋势不同,如POX、PER、BG和CBH通常在间伐恢复5 a时活性最低,在13 a时恢复到间伐前水平或高于未间伐处理(如BG)。这与其他研究结果相似,但并不完全一致[29−30]。这种结果可能是由于不同的林分环境以及微生物利用资源多寡的差异,导致土壤酶活性对同一干扰方式的不同改变[31]。随着间伐恢复时间的持续,易分解有机物质减少而难降解的碳相对较多[32],POX、PER和BG、CBH作为土壤中主要的木质素降解酶和纤维素降解酶,其活性得到显著提高,以增强微生物利用顽固性有机碳的能力。这与MEISAM等[33]的研究结果一致。而以分解几丁质和蛋白质、半纤维素等易分解物质为主的NAG+LAP、BX活性的显著降低也映证了SINSABAUGH等[34]的资源分配理论。
土壤胞外酶与土壤养分输入和微生物量等密切相关[35]。通过相关分析发现:BG和CBH活性与微生物量碳、全磷显著正相关,表明土壤微生物数量的变化与碳循环土壤酶活性的变化关系最为密切,而全磷则是磷素限制环境中影响微生物生长的主要因素[7, 16]。有效氮质量分数的减少虽然在一定程度上能促使氮获取酶的产生,但同样也会降低土壤微生物的活性和限制酶促反应底物供应,从而减少部分酶的释放[36],这与孙鹏跃等[37]的研究结果一致。冗余分析发现:土壤pH也是影响土壤酶活性的主要因素,并与部分酶变化表现出负相关关系,这与多数研究结果是一致的[3]。有研究表明:大多数土壤酶在特定的pH范围(最适值在4.0~5.5)内表现出最大的活性和稳定性,当pH超过这个范围时,酶活性会降低[38]。
-
本研究中所有处理的土壤酶矢量角度均>45°,符合亚热带地区森林土壤微生物更受磷素限制的理论[39]。同时参与土壤碳、氮和磷循环相关酶计量比偏离了表层土壤中接近1∶1∶1的平均水平[3],也在一定程度上反映了秦岭区域松栎混交林间伐恢复过程中微生物受碳和磷的共同限制,这与薛悦等[40]对安康市火池塘林区撂荒地恢复过程的研究结果相一致。与未间伐样地相比,间伐后恢复5 a时显著降低的酶矢量角度表征了微生物受到的磷限制减弱,随时间进程减弱效应逐渐消失,林内物种丰富度的提高和凋落物量的增加,促使土壤微生物分泌更多碳获取酶(如BG)来降解有机质,释放磷以供给微生物活动,以缓解磷限制,这些过程都会导致微生物碳限制的进一步增加。相关性分析结果中,酶矢量长度与微生物量碳呈显著正相关,证实了微生物需要更多的碳源来满足代谢活动所耗的能量,这与CUI等[41]的研究结果相似。
-
间伐改变了松栎混交林区域内的年凋落物总量及针叶与阔叶的凋落量比例,同时改变了林内物种丰富度和林分郁闭度,从而影响了土壤基本理化性质。抚育间伐在一定程度上能够缓解土壤微生物受磷限制的状况,但随恢复时间持续,林内凋落物量逐渐增加使土壤微生物受碳限制更为严重。
Effect of thinning restoration on enzyme activity and enzyme stoichiometry in the topsoil of oak-pine mixed forest
-
摘要:
目的 探讨间伐后不同恢复时间下秦岭松栎混交林土壤理化性质和土壤胞外酶活性的变化,研究该地区抚育间伐措施对森林生态系统养分循环过程,为制定森林可持续经营方案及合理的生态恢复措施提供理论依据。 方法 采用空间代替时间的方法,对松栎混交林间伐后不同恢复时间(5、13 a)的表层土壤(0~10 cm)理化性质和胞外酶活性进行测定,并计算各处理样地酶化学计量比和酶矢量,以未间伐为对照。 结果 ①间伐后土壤pH提高,恢复13 a时土壤全磷、微生物量碳和微生物量氮质量分数显著增加(P<0.05),土壤有效氮质量分数降低。土壤有机碳质量分数在恢复5 a时显著下降(P<0.05),在13 a时逐渐恢复至间伐前水平。②间伐恢复显著降低了β-木糖苷酶、氮获取酶和酸性磷酸酶的活性(P<0.05),提高了β-葡糖苷酶活性;酚氧化物酶和过氧化物酶活性在间伐恢复初期(5 a)表现为降低趋势, 13 a时恢复到间伐前水平。③间伐恢复13 a时土壤碳获取酶/氮获取酶比值(EC/N)、土壤碳获取酶/磷获取酶比值(EC/P)和酶矢量长度显著提高(P<0.05),间伐恢复5 a时EC/P和土壤氮获取酶/磷获取酶比值(EN/P)显著提高,同时酶矢量角度也显著降低(P<0.05)。 结论 随间伐恢复时间延长,土壤养分、有机碳和氧化酶活性呈现逐渐恢复的趋势;pH是影响土壤酶活性及酶矢量变化的关键因子。间伐导致土壤微生物在初期恢复阶段的磷限制有所缓解,后期并未改变受碳、磷共同限制的状况。图3表4参41 Abstract:Objective This study, with an investigation of the effects of different recovery periods after thinning on soil physical-chemical proprieties and extracellular enzyme activities in oak-pine mixed forests in the Qinling Mountains, is aimed to better understand the nutrient cycling processes under thinning treatments, providing basis for developing programs of sustainable forest management and exploring better ecological restoration measures. Method First, pace-for-time substitution was employed to explore the effects of thinning restoration process (5 and 13 years, no thinning as the control) on soil physical-chemical proprieties and enzyme activity changes in the surface layer at 0−10 cm depth. Then the enzyme stoichiometric ratios and enzyme vectors were calculated for each treatment. Result (1) The total phosphorus, microbial biomass carbon, and microbial biomass nitrogen contents in the soil increased significantly, whereas the inorganic nitrogen content decreased in the 13-year restoration (P<0.05) and the soil pH increased in the 5-year and 13-year restorations; following a 13-year restoration, the soil organic carbon content progressively recovered to pre-thinning levels after declining dramatically in the 5-year restoration (P<0.05). (2) Thinning significantly increased the activity of β-glucosidase (BG), while decreasing the activities of β-xylosidase (BX), nitrogen acquiring enzyme (NAG+LAP), and acid phosphatase (AcP) (P<0.05); after the 13-year restoration, the activities of phenol oxidase (POX) and peroxidase (PER) showed a decreasing tendency during the initial stage of thinning (5 years treatment) and then reverted to pre-thinning values. (3) In the 13-year restoration, thinning significantly increased the soil enzyme carbon-nitrogen ratio (EC/N), soil enzyme carbon-phosphorus ratio (EC/P) and vector length value (P<0.05) whereas the EC/P and soil enzyme nitrogen-phosphorus ratio (EN/P) increased significantly and the vector angle value decreased in the 5-year restoration (P<0.05). Conclusion Soil nutrients, organic carbon, and oxidase activities showed a recovery trend with the thinning recovery stage with the soil pH being a key factor affecting soil enzyme activity and the change of enzyme vectors. Thinning decreases the phosphorus limitations of soil microorganisms during the initial stage of recovery, but it has little effect on the phosphorus and carbon limitation in the later stage of recovery. [Ch, 3 fig. 4 tab. 41 ref.] -
Key words:
- forest tending /
- soil extracellular enzyme /
- enzyme stoichiometry /
- nutrient limitation
-
表 1 试验样地基本概况
Table 1. Basic survey of test plots
间伐后恢
复时间/a海拔/
m株数密度/
(株·hm−2)胸径/
cm郁闭度 物种
丰富度Shannon-Wiener
指数凋落物量/
(t·hm−2·a−1)林内主要树种 ck 1 585.00±61.85 1 420±88 14.60±0.49 0.7 25 2.48 7.01±0.37 油松、锐齿槲栎、华山松、毛樱桃、垂柳、
木姜子、三桠乌药5 1 457.32±13.14 1 208±355 13.80±0.84 0.5 32 2.78 5.69±0.26 锐齿槲栎、栗、油松、白桦、垂柳、
榆树、桤木13 1 757.57±20.17 1 254±207 13.80±1.19 0.6 29 2.68 6.55±0.29 毛樱桃、油松、锐齿槲栎、漆树、水蜡树、
木姜子、灯台树说明:毛樱桃Prunus tomentosa,垂柳Salix babylonica,三桠乌药Lindera obtusiloba,白桦Betula platyphylla,榆树Ulmus pumila,水蜡树Ligustrum obtusifolium。 表 2 土壤胞外酶的简称及所用底物
Table 2. Soil enzyme along with their enzyme abbreviation and substrate of soil enzyme
酶名称 简称 底物 β-葡糖苷酶β-glucosidase BG 4-MUB-β-D-glucoside β-木糖苷酶β-xalosidase BX 4-MUB-β-D-xylopyranoside 纤维二糖水解酶Cellobiohydrolase CBH 4-MUB-β-D-cellobioside β-N-乙酰氨基葡萄糖苷酶β-N-acetylglucosaminidase NAG 4-MUB-N-acetyl-β-D-glucosaminde 亮氨酸氨基肽酶Leucine aminopeptidase LAP L-leucine-7-amido-4 methylcounarin 酸性磷酸酶Acid phosphatase AcP 4-MUB-phosphatase 酚氧化物酶Phenol oxidase POX L-dihydroxyphenylalanine(L-DOPA) 过氧化物酶Peroxidase PER L-dihydroxyphenylalanine(L-DOPA) and H2O2 说明:MUB为甲基伞形酮酰Methylumbelliferyl。 表 3 不同间伐恢复时间下土壤理化特性状况
Table 3. Soil physical and chemical properties under different thinning treatments
间伐后恢复时间/a pH 含水率/% 有效氮/(mg·kg−1) 全氮/(g·kg−1) 全磷/(g·kg−1) 碳氮比 ck 5.48±0.10 b 37.28±4.01 a 21.34±1.96 a 4.58±0.86 a 0.60±0.08 b 10.02±1.16 a 5 5.98±0.13 a 35.10±6.81 a 17.19±0.48 ab 3.28±0.68 a 0.52±0.10 b 9.34±1.41 ab 13 5.76±0.17 ab 40.37±1.67 a 16.56±0.58 b 3.93±0.44 a 0.77±0.07 a 8.55±1.32 b 间伐后恢复时间/a 氮磷比 有机碳/(g·kg−1) 微生物量碳/(g·kg−1) 微生物量氮/(g·kg−1) 微生物量碳氮比 ck 7.49±0.71 a 35.94±3.84 a 1.14±0.04 b 0.20±0.01 b 5.97±0.37 ab 5 6.45±0.95 ab 26.62±2.79 b 1.14±0.09 b 0.22±0.01 ab 5.09±0.13 b 13 5.04±0.34 b 33.33±2.27 ab 1.36±0.02 a 0.23±0.01 a 6.11±0.33 a 说明:数据均为平均值±标准误。不同小写字母表示不同处理间差异显著 (P<0.05)。 表 4 土壤酶变化与土壤理化性质的相关性分析
Table 4. Correlation analysis between soil enzyme changes and soil physical and chemical properties
指标 pH IN TP SOC MBC MBC/MBN N/P POX −0.54 −0.29 −0.04 −0.07 0.26 0.30 −0.04 PER −0.65* 0.19 0.32 0.45* 0.22 0.52** 0.21 BG 0.28 0.35 0.73** 0.55** 0.63** 0.38 −0.25 BX −0.53 0.54** −0.01 0.27 0.10 0.45* 0.56 CBH −0.01 0.24 0.46* 0.43* 0.53** 0.65** 0.17 AcP −0.72* 0.57** −0.38 0.06 −0.13 0.22 0.85** NAG+LAP 0.17 0.66** −0.08 0.14 −0.01 0.00 0.60 VA −0.95** 0.01 −0.30 −0.06 −0.04 0.35 0.43 VL 0.45 −0.28 0.70** 0.31 0.48* 0.15 −0.63* 说明:IN为土壤有效氮,TP为土壤全磷,SOC为土壤有机碳,MBC为微生物量碳,MBN为微生物量氮,N/P为氮磷比。POX为酚氧化物酶,PER为过氧化物酶,BG为β-葡糖苷酶,BX为β-木糖苷酶,CBH为纤维二糖水解酶,AcP为酸性磷酸酶,NAG+LAP为氮获取酶(β-N-乙酰氨基葡萄糖苷酶和亮氨酸氨基肽酶总和),VA为酶矢量角度,VL为酶矢量长度。*表示显著相关(P<0.05),**表示极显著相关(P<0.01)。 -
[1] ZHANG Xinzhong, GUAN Dexin, LI Weibin, et al. The effects of forest thinning on soil carbon stocks and dynamics: a meta-analysis [J]. Forest Ecology and Management, 2018, 429: 36 − 43. [2] FANIN N, MOORHEAD D, BERTRAND I. Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient [J]. Biogeochemistry, 2016, 129: 21 − 36. [3] SINSABAUGH R L, LAUBER C L, WEINTRAUB M N, et al. Stoichiometry of soil enzyme activity at global scale [J]. Ecology Letters, 2008, 11(11): 1252 − 1264. [4] ZHOU Luhong, LIU Shangshi, SHEN Haihua, et al. Soil extracellular enzyme activity and stoichiometry in China’s forests [J]. Functional Ecology, 2020, 34(7): 1461 − 1471. [5] PENG Xiaoqian, WANG Wei. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China [J]. Soil Biology and Biochemistry, 2016, 98: 74 − 84. [6] WU Yang, CHEN Wenjing, LI Qiang, et al. Ecoenzymatic stoichiometry and nutrient limitation under a natural secondary succession of vegetation on the Loess Plateau, China [J]. Land Degradation & Development, 2021, 32(1): 399 − 409. [7] XU Miaoping, LI Wenjie, WANG Jiayi, et al. Soil ecoenzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the Loess Plateau, China [J/OL]. Science of the Total Environment, 2022, 815 : 152918[2024-02-01]. doi: 10.1016/j.scitotenv.2022.152918. [8] QIU Xincai, WANG Haibin, PENG Daoli, et al. Thinning drives C∶N∶P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in north China [J/OL]. Forest Ecology and Management, 2020, 462 (8): 117984[2024-02-01]. doi: 10.1016/j.foreco.2020.117984. [9] GENG Yuqing, DIGHTON J, GRAY D. The effects of thinning and soil disturbance on enzyme activities under pitch pine soil in New Jersey Pinelands [J]. Applied Soil Ecology, 2012, 62: 1 − 7. [10] KIM S, LI Guanlin, HAN S H, et al. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora Sieb. et Zucc. forests after 7 years [J/OL]. Annals of Forest Science, 2018, 75 (1): 13[2024-02-01]. doi: 10.1007/s13595-018-0690-1. [11] 于立忠, 张景普, 刘利芳, 等. 间伐对不同肥力日本落叶松人工林土壤酶活性的影响 [J]. 生态学杂志, 2017, 36(11): 3017 − 3027. YU Lizhong, ZHANG Jingpu, LIU Lifang, et al. The effects of thinning on soil enzyme activities in Larix kaempferi plantations with different site conditions [J]. Chinese Journal of Ecology, 2017, 36(11): 3017 − 3027. [12] QIU Xincai, PENG Daoli, TIAN Huixia, et al. Soil ecoenzymatic stoichiometry and microbial resource limitation driven by thinning practices and season types in Larix principis-rupprechtii plantations in North China [J/OL]. Forest Ecology and Management, 2021, 482 : 118880[2024-02-01]. doi: 10.1016/j.foreco.2020.118880. [13] LULL C, BAUTISTA I, LIDON A, et al. Temporal effects of thinning on soil organic carbon pools, basal respiration and enzyme activities in a Mediterranean Holm oak forest [J/OL]. Forest Ecology and Management, 2020, 464 : 118088[2024-02-01]. doi: 10.1016/j.foreco.2020.118088. [14] LEI Lei, XIAO Wenfa, ZENG Lixiong, et al. Effects of thinning intensity and understory removal on soil microbial community in Pinus massoniana plantations of subtropical China [J/OL]. Applied Soil Ecology, 2021, 167 : 104055[2024-02-01]. doi: 10.1016/j.apsoil.2021.104055. [15] ZHOU Zhenghu, WANG Chuankuan, JIN Ying, et al. Impacts of thinning on soil carbon and nutrients and related extracellular enzymes in a larch plantation [J/OL]. Forest Ecology and Management, 2019, 450 : 117523[2024-02-01]. doi: 10.1016/j.foreco.2019.117523. [16] LI Yu, AJLOON F H, WANG Xiangfu, et al. Temporal effects of thinning on soil organic carbon and carbon cycling-related enzyme activities in oak-pine mixed forests [J/OL]. Forest Ecology and Management, 2023, 545 : 121293[2024-02-01]. doi: 10.1016/j.foreco.2023.121293. [17] 刘思泽, 尹海锋, 沈逸, 等. 间伐强度对马尾松人工林间伐初期林下植被群落物种组成和多样性的影响 [J]. 应用生态学报, 2020, 31(9): 2866 − 2874. LIU Size, YIN Haifeng, SHEN Yi, et al. Effects of thinning intensity on species composition and diversity of undergrowth vegetation community in Pinus massoniana plantation at initial stage of thinning [J]. Chinese Journal of Applied Ecology, 2020, 31(9): 2866 − 2874. [18] 鲍士旦. 土壤农化分析[M]. 3版. 北京: 中国农业出版社, 2000. BAO Shidan. Soil Agrochemical Analysis [M]. 3rd ed. Beijing: China Agriculture Press, 2000. [19] SAIYA-CORK K R, SINSABAUGH R L, ZAK D R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil [J]. Soil Biology & Biochemistry, 2002, 34(9): 1309 − 1315. [20] 林惠瑛, 周嘉聪, 曾泉鑫, 等. 土壤酶计量揭示了武夷山黄山松林土壤微生物沿海拔梯度的碳磷限制变化 [J]. 应用生态学报, 2022, 33(1): 33 − 41. LIN Huiying, ZHOU Jiacong, ZENG Quanxin, et al. Soil enzyme stoichiometry revealed the changes of soil microbial carbon and phosphorus limitation along an elevational gradient in a Pinus taiwanensis forest of Wuyi Mountains, southeast China [J]. Chinese Journal of Applied Ecology, 2022, 33(1): 33 − 41. [21] MOORHEAD D L, SINSABAUGH R L, HILL B H, et al. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics [J]. Soil Biology and Biochemistry, 2016, 93: 1 − 7. [22] HE Zhibin, CHEN Longfei, DU Jun, et al. Responses of soil organic carbon, soil respiration, and associated soil properties to long-term thinning in a semiarid spruce plantation in northwestern China [J]. Land Degradation & Development, 2018, 29(12): 4387 − 4396. [23] TRENTINI C P, CAMPANELLO P I, FERRERAS J, et al. Thinning partially mitigates the impact of atlantic forest replacement by pine monocultures on the soil microbiome [J/OL]. Frontiers in Microbiology, 2020, 11 : 1491[2024-02-01]. doi:10.3389/fmicb.2020.01491. [24] HE Lingli, IVANOV V Y, BOHRER G, et al. Temporal dynamics of soil moisture in a northern temperate mixed successional forest after a prescribed intermediate disturbance [J]. Agricultural and Forest Meteorology, 2013, 180: 22 − 33. [25] 王博, 周志勇, 张欢, 等. 针阔混交林中兴安落叶松比例对土壤化学性质和酶化学计量比的影响 [J]. 浙江农林大学学报, 2020, 37(4): 611 − 622. WANG Bo, ZHOU Zhiyong, ZHANG Huan, et al. Effect of Larix gmelinii proportion on soil chemical properties and enzymatic stoichiometry in mixed coniferous and broad-leaved forest [J]. Journal of Zhejiang A&F University, 2020, 37(4): 611 − 622. [26] 田地, 陈义堂, 史月冬, 等. 间伐和施肥对杉木近熟林土壤微生物特征的影响 [J]. 森林与环境学报, 2023, 43(6): 569 − 578. TIAN Di, CHEN Yitang, SHI Yuedong, et al. Effects of thinning and fertilization on soil microbial characteristics in a near-mature Chinese fir plantation [J]. Journal of Forest and Environment, 2023, 43(6): 569 − 578. [27] LI Pingwei, FREDERIC A, FLORIAN H, et al. Stand attributes or soil micro-environment exert greater influence than management type on understory plant diversity in even-aged oak high forests [J/OL]. Forest Ecology and Management, 2020, 460 : 117897[2024-02-01]. doi: 10.1016/j.foreco.2020.117897. [28] 周璇, 刘凯利, 胡佳怡, 等. 川西柳杉幼林间伐后林下植被和土壤特征变化 [J]. 西北林学院学报, 2024, 39(1): 44 − 51. ZHOU Xuan, LIU Kaili, HU Jiayi, et al. Understory vegetation and soil characteristic variations of young Cryptomeria japonica var. sinensis plantations after thinning in western Sichuan [J]. Journal of Northwest Forestry University, 2024, 39(1): 44 − 51. [29] KIM S, LI Guanlin, HAN S H , et al. Microbial biomass and enzymatic responses to temperate oak and larch forest thinning: influential factors for the site-specific changes [J]. Science of the Total Environment, 2019, 651: 2068 − 2079. [30] WU Ran, CHENG Xiaoqin, HAN Hairong. The effect of forest thinning on soil microbial community structure and function [J/OL]. Forests, 2019, 10 (4): 352[2024-02-01]. doi: 10.3390/f10040352. [31] 刘捷豹, 陈光水, 郭剑芬, 等. 森林土壤酶对环境变化的响应研究进展 [J]. 生态学报, 2017, 37(1): 110 − 117. LIU Jiebao, CHEN Guangshui, GUO Jianfen, et al. Advances in research on the responses of forest soil enzymes to environmental change [J]. Acta Ecologica Sinica, 2017, 37(1): 110 − 117. [32] GIAI C, BOERNER R E J. Effects of ecological restoration on microbial activity, microbial functional diversity, and soil organic matter in mixed-oak forests of southern Ohio, USA [J]. Applied Soil Ecology, 2007, 35(2): 281 − 290. [33] MEISAM N, JOHANNA P, SAMUEL B, et al. Keeping thinning-derived deadwood logs on forest floor improves soil organic carbon, microbial biomass, and enzyme activity in a temperate spruce forest [J]. European Journal of Forest Research, 2022, 142(2): 287 − 300. [34] SINSABAUGH R L, MOORHEAD D L. Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition [J]. Soil Biology and Biochemistry, 1994, 26(10): 1305 − 1311. [35] 张孝良, 车荣晓, 段兴武, 等. 土壤胞外酶活性对气候变化响应的研究进展 [J]. 浙江农林大学学报, 2023, 40(4): 910 − 920. ZHANG Xiaoliang, CHE Rongxiao, DUAN Xingwu, et al. Research progress in the response of soil extracellular enzymes activity to climate changes [J]. Journal of Zhejiang A&F University, 2023, 40(4): 910 − 920. [36] 刘仁, 陈伏生, 方向民, 等. 凋落物添加和移除对杉木人工林土壤水解酶活性及其化学计量比的影响 [J]. 生态学报, 2020, 40(16): 5739 − 5750. LIU Ren, CHEN Fusheng, FANG Xiangmin, et al. Effects of litter addition and removal on soil hydrolytic enzyme activities and ecoenzymatic stoichiometry in Chinese fir plantation [J]. Acta Ecologica Sinica, 2020, 40(16): 5739 − 5750. [37] 孙鹏跃, 徐福利, 王渭玲, 等. 华北落叶松人工林地土壤养分与土壤酶的季节变化及关系 [J]. 浙江农林大学学报, 2016, 33(6): 944 − 952. SUN Pengyue, XU Fuli, WANG Weiling, et al. Seasonal dynamics of soil nutrients and soil enzyme activities in Larix principis-rupprechtii plantations [J]. Journal of Zhejiang A&F University, 2016, 33(6): 944 − 952. [38] KIVLIN S N, TRESEDER K K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition [J]. Biogeochemistry, 2014, 117(1): 23 − 37. [39] WARING B G, ADAMS R, BRANCO S, et al. Scale-dependent variation in nitrogen cycling and soil fungal communities along gradients of forest composition and age in regenerating tropical dry forests [J]. New Phytologist, 2016, 209(2): 845 − 854. [40] 薛悦, 康海斌, 杨航, 等. 秦岭中段撂荒地植被恢复过程中土壤微生物代谢特征 [J]. 环境科学, 2022, 43(1): 550 − 559. XUE Yue, KANG Haibin, YANG Hang, et al. Extracellular enzyme stoichiometry and microbial metabolism limitation during vegetation restoration process in the middle of the Qinling Mountains, China [J]. Environmental Science, 2022, 43(1): 550 − 559. [41] CUI Yongxing, FANG Linchuan, GUO Xiaobin, et al. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: evidence from nutrient limitation of soil microbes [J]. Science of the Total Environment, 2019, 648: 388 − 397. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240185






 下载:
下载: