-
石榴Punica granatum是千屈菜科Lythraceae石榴属Punica落叶小乔木或灌木,在中国约2 000 a的栽培史,200余个品种[1-2]。常分为食用石榴和观赏石榴,观赏石榴株型优美,花期长,花色艳,春叶红、秋叶黄,果实奇特,可观性果实宿存树体时间长,是春、夏、秋3季可赏的重要花木,广泛应用于园林绿化、盆栽、盆景等[2-3]。目前国内外的研究主要集中于利用分子标记对石榴种质资源遗传多样性的分析和评价[4-11],以及对食用石榴果实形态及生化指标的测定分析[12-17],对观赏资源的遗传多样性研究较少。MARTINEZ-NICOLAS等[18]对53个西班牙品种的果实、种子、叶、花等性状进行过表型研究,但没有针对树型、枝型、花型、瓣化雄蕊数等观赏性状的综合分析。陈俊愉等[19]根据株型、花、果、叶片大小将国内观赏石榴分为花石榴和果石榴,其中花石榴分为一般种与矮生种,再依据花色和单、重瓣分类。汪小飞[20]将包括观赏石榴在内的全国87个石榴品种分为单瓣、复瓣、重瓣、台阁4个品种群。王庆军等[21]对35份种质资源进行了表型性状的初步调查,但未对表型性状多样性及亲缘关系进行综合评价。表型多样性是遗传多样性与观赏植物评价的重要基础,全面了解石榴种质的优异性状,可为石榴种质资源的鉴定、保存和推广提供参考。C值指生物体单倍体细胞核的DNA总质量,C值的变异对研究物种的亲缘关系以及系统发育具有重要意义,结合倍性水平分析也可用来鉴定杂交物种[22]。目前中国石榴品种‘泰山红’‘Taishanhong’大笨籽’‘Dabenzi’和‘大籽’‘Dazi’被鉴定为二倍体,美国品种‘Nana’为四倍体[23-25]。多倍体植物具有花大、果实大、植株健壮、抗逆性强等优点,是育种的好材料。流式细胞术作为快速准确测定倍性与基因组含量的先进技术,可为石榴种质资源的创新与遗传育种提供理论基础。本研究对24个观赏石榴品种的株型、枝型、花型、1年生小枝颜色、花瓣色、花萼色、花长、花宽、花萼长、花萼宽、花萼瓣数、花瓣数、瓣化雄蕊数等13个观赏性状进行了表型多样性分析、主成分分析与聚类分析,并利用流式细胞仪对24个品种进行了倍性及C值测定,为观赏石榴种质资源的评价、创新与遗传育种提供理论基础。
-
供试材料为生长良好且无病虫害的24个观赏石榴品种(表1),其中单瓣品种群(single-flower group)8个、复瓣品种群(semi-double-flower group)4个、重瓣品种群(double-flower group)11个、台阁品种群(proliferation-flower group)1个。
序号 品种名 类型 引种地 1 ‘泰安红牡丹’‘Taianhongmudan’ 重瓣 中国山东 2 ‘榴缘白’‘Double red-white ’ 台阁 美国 3 ‘青皮月季’‘Qingpiyueji’ 重瓣 中国山东 4 ‘峄城重瓣红皮酸’‘Yichengchongbanhongpisuan’ 重瓣 中国山东 5 ‘峄城粉红牡丹’‘Yichengfenhongmudan’ 复瓣 中国山东 6 ‘峄城红花重瓣紫皮酸’‘Yichenghonghuachongbanzipisuan’ 复瓣 中国山东 7 ‘榴花雪’‘Hakubatan’ 重瓣 美国 8 ‘峄城粉红重瓣白皮甜’‘Yichengfenhongchongbanbaipitian’ 重瓣 中国山东 9 ‘峄城重瓣玛瑙’‘Yichengchongbanmanao’ 重瓣 中国山东 10 ‘洛阳白马寺’‘Luoyangbaimasi’ 重瓣 中国河南 11 ‘礼泉重瓣红花青皮酸’‘Liquanchongbanhonghuaqingpisuan’ 重瓣 中国陕西 12 ‘峄城重瓣白花酸’‘Yichengchongbanbaihuasuan’ 复瓣 中国山东 13 ‘峄城单瓣粉红酸’‘Yichengdanbanfenhongsuan’ 单瓣 中国山东 14 ‘榴花红’‘Nochi-shibori’ 重瓣 美国 15 ‘紫皮甜’‘Zipitian’ 单瓣 中国山东 16 ‘峄城单瓣粉红甜’‘Yichengdanbanfenhongtian’ 单瓣 中国山东 17 ‘突尼斯软籽软枝’‘Tunisiruanziruanzhi’ 单瓣 突尼斯 18 ‘墨石榴’‘Moshiliu’ 单瓣 中国山东 19 ‘宫灯石榴’‘Gongdengshiliu’ 单瓣 中国山东 20 ‘泰山红’‘Taishanhong’ 单瓣 中国山东 21 ‘南林重瓣红’‘Nanlinchongbanhong’ 重瓣 中国江苏 22 ‘南林单瓣红’‘Nanlindanbanhong’ 单瓣 中国江苏 23 ‘南林重瓣白’‘Nanlinchongbanbai’ 复瓣 中国江苏 24 ‘南林重瓣玛瑙’‘Nanlinchongbanmanao’ 重瓣 中国江苏 Table 1. List of 24 ornamental pomegranate cultivars
-
试验于2018年5月在中国石榴种质资源圃(峄城)和南京林业大学进行,每品种随机选择3株生长健壮、无病虫害、长势一致的植株进行各表型性状的调查和统计。其中质量性状6个:株型(plant form,PF)、枝型(brach type,BT)、花型(flower pattern,FP)、1年生小枝颜色(color of branch,BC)、花瓣色(color of petal,PC)、花萼色(color of sepal,SC)。数量性状7个:花长(flower length,FL)、花宽(flower diameter,FD)、花萼长(sepal length,SL)、花萼宽(sepal diameter,SD)、花萼瓣数(number of sepals,NOS)、花瓣数(number of petals,NOP)、瓣化雄蕊数(number of petaloid stamens,NOPS)。用直尺或游标卡尺测量长度,小数点后保留2位有效数;用国际通用的英国皇家园艺学会比色卡(RHSCC)测量颜色,记录数字编码。调查取样标准根据《植物新品种特异性、一致性、稳定性测试指南 石榴属》的操作进行[26]。
-
以二倍体石榴‘大笨籽’为外标,选用LB01解离液。滤网、荧光染料、鞘液、培养皿、试剂均购自南京博巧生物技术有限公司。分别取各石榴品种嫩叶,用流式细胞仪(Influx,美国BD公司)测定倍性与C值,具体参照林峰等[27]方法进行。
-
质量性状均以1~6级进行分级和赋值(表2),数量性状根据平均值(X)和标准差(δ)分为10级,1级<X−2δ,10级≥X+2δ,中间每级相差0.5δ[28-29]。各性状的遗传多样性采用Shannon’s信息指数(H')进行评价:H' = −∑(Pi)(lnPi),其中Pi表示第i种变异类型出现的频率[30]。采用Excel进行性状数据(平均值、最大值、最小值、极差和变异系数等)的基本统计。使用SPSS 24.0软件计算表型数据的KMO检验值及Bartlett球形度检验的显著性。种质间距为平方欧式距离,聚类方法采用Ward法。
分级 株型 枝型 花型 1年生小枝颜色 花瓣色 花萼色 1 乔木状 直立 复瓣 粉红 白色 浅黄 2 矮生 开张 单瓣 玫红 橙黄 黄色 3 垂枝 重瓣 紫红 橙红 浅橙黄 4 台阁 浅棕 红色 橙黄 5 浅绿 粉红 橙红 6 复色 红色 Table 2. Description and grouping of qualitative characters of ornamental pomegranate cultivars
-
荧光染料PI的激发波长为488 nm,收集通道为FL2(670 nm±30 nm),检测PI的发射荧光强度。使用Influx自带软件FACSTM Sortware进行分析。‘大笨籽’基因组大小为328 Mbp[23],C值为0.33 pg,根据公式[31]:待测样本的细胞核DNA含量或倍性=对照样本细胞核DNA含量或倍性×(待测样本G0/G1峰荧光强度/对照样本G0/G1峰荧光强度)。变异系数(VC)控制在8%以内。
-
根据表2对观赏石榴品种的6个主要质量性状进行分级和赋值,统计各性状的频率分布和多样性指数,由表3可知:各性状多样性指数为0.512~1.683,平均为1.148。多样性指数最大的性状为花瓣色,其次为花萼色、花型和1年生小枝颜色,多样性指数均大于1.000;其中,花瓣色以橙黄(25.00%)、橙红(25.00%)为主,花萼色以橙黄(41.67%)为主,花型以重瓣为主(45.83%),1年生小枝颜色以粉红(62.50%)为主。多样性指数最小的性状是株型,其次是枝型;其中,株型主要为乔木型(79.17%),枝型主要为开张型(58.33%)。
性状 频率/% 多样性指数(H') 1级 2级 3级 4级 5级 6级 株型 79.17 20.83 0.512 枝型 37.50 58.33 4.17 0.815 花型 33.33 16.67 45.83 4.17 1.155 1年生小枝颜色 62.50 4.17 8.33 8.33 16.67 1.139 花瓣色 16.67 25.00 25.00 4.17 16.67 12.50 1.683 花萼色 8.33 16.67 8.33 41.67 8.33 16.67 1.583 Table 3. Frequency distribution and diversity of qualitative characteristics of ornamental pomegranate cultivars
-
24个观赏石榴的7个数量性状表现了广泛的变异,变异系数为11.76%~117.59%(表4),多样性指数为1.456~1.910,多样性指数平均为1.715,高于质量性状,说明观赏石榴数量性状多样性更丰富。7个性状中瓣化雄蕊数的变异系数最大,达117.59%,花瓣数次之,为78.86%,而花萼瓣数、花长、花萼长的变异系数较小。多样性指数最大的性状是花萼长(1.910),其次是花长(1.906)、花宽(1.802)、花萼宽(1.683)、花瓣数(1.630)、花萼瓣数(1.619)、瓣化雄蕊数(1.456),多样性指数均大于1.600。
性状 花长/cm 花宽/cm 花萼长/cm 花萼宽/cm 花萼瓣数/片 花瓣数/片 瓣化雄蕊数/片 平均值 4.43±0.61 4.70±1.25 3.09±0.57 3.60±0.92 6.48±0.76 41.94±33.08 57.11±67.15 最大值 5.42 6.43 4.11 4.87 8 134 276.67 最小值 3.03 2.33 1.63 2.00 5 6 0 变异系数/% 13.85 26.60 18.40 25.64 11.76 78.86 117.59 Table 4. Morphological diversity of statistics of quantitative of ornamental pomegranate cultivars
-
根据汪小飞[20]方法将观赏石榴品种划分为3个品种群,即单瓣品种群、复瓣品种群、重瓣品种群。由表5可知:3个品种群的表型性状多样性指数分别为0.813、0.600和1.157,其平均多样性指数为0.856,表明不同观赏石榴品种群之间,表型性状的多样性指数均有不同程度的差异。其中,重瓣品种群表型多样性指数最高,除枝型外,其他12个性状均高于所有品种相应性状的平均值,特别是花瓣数、瓣化雄蕊数、花萼宽、花宽和1年生小枝颜色。单瓣品种群的株型、枝型、1年生小枝颜色、花萼色、花长和花萼宽的多样性指数均高于所有品种相应性状的平均值。由此可见:3个品种群中,重瓣品种群的表型性状遗传多样性最丰富,其次是单瓣品种群,复瓣品种群的表型遗传多样性最低。
性状 单瓣品种群 复瓣品种群 重瓣品种群 平均值 性状 单瓣品种群 复瓣品种群 重瓣品种群 平均值 株型 0.562 0.000 0.586 0.383 枝型 0.974 0.562 0.689 0.742 花型 0.000 0.000 0.000 0.000 1年生小枝颜色 0.900 0.693 1.030 0.874 花瓣色 1.082 1.040 1.720 1.281 花萼色 1.321 1.040 1.290 1.217 花长 1.560 1.040 1.666 1.422 花宽 0.900 0.562 1.295 0.919 花萼长 1.321 1.040 1.673 1.344 花萼宽 0.974 0.562 1.067 0.868 花萼瓣数 0.974 0.693 1.594 1.087 花瓣数 0.000 0.000 1.264 0.421 瓣化雄蕊数 0.000 0.562 1.162 0.575 平均值 0.813 0.600 1.157 0.856 Table 5. Morphological diversity index of different ornamental pomegranate cultivars
-
供试观赏石榴表型性状的KMO检验值为0.622(高于0.600),Bartlett球形度检验显著性为0.000(低于0.050),符合主成分分析条件。以特征值大于1为标准提取主成分,前4个主成分的累计贡献率为80.10%,认为这4个主成分能充分反映13个表型性状(表6)。第1主成分的特征值为4.52,贡献率为34.74%,特征向量绝对值较大的为枝型、1年生小枝颜色、花长、花萼宽,说明第1主成分是枝的形态颜色与花大小的综合反映。第2主成分的特征值为2.62,贡献率为20.17%,特征向量绝对值较大的为花型、株型、瓣化雄蕊数,说明第2主成分是花瓣数及花瓣形成、株型的综合反映。第3主成分的特征值为2.06,贡献率为15.82%,特征向量绝对值较大的为花瓣数、花萼瓣数、花瓣色,说明第3主成分是花瓣数、花大小、花色的综合反映。第4主成分的特征值为1.22,贡献率为9.39%,特征向量绝对值较大的为花萼长,说明第3主成分是花大小的综合反映。由此得出:枝的形态与颜色、花大小、花瓣数及花瓣形成、株型、花色是造成观赏石榴表型差异的主要影响因子。
性状 主成分 性状 主成分 PC1 PC2 PC3 PC4 PC1 PC2 PC3 PC4 株型 0.16 −0.48 0.21 −0.24 枝型 0.43 0.07 −0.14 −0.09 花型 0.10 −0.53 0.20 −0.13 1年生小枝颜色 0.43 0.00 −0.22 −0.02 花瓣色 0.25 0.17 0.42 0.02 花萼色 0.39 0.14 −0.23 0.12 花长 0.41 −0.11 0.13 0.11 花宽 −0.11 0.29 −0.32 −0.36 花萼长 0.01 0.00 0.09 0.81 花萼宽 0.42 0.08 −0.25 −0.04 花萼瓣数 −0.10 −0.24 −0.42 0.33 花瓣数 0.14 0.18 0.43 0.03 瓣化雄蕊数 0.01 0.48 0.27 −0.03 特征值 4.52 2.62 2.06 1.22 累积贡献率/% 34.74 20.17 15.82 9.39 累积贡献率/% 34.74 54.91 70.73 80.12 Table 6. Principle component analysis of morphological diversity of ornamental pomegranate cultivars
-
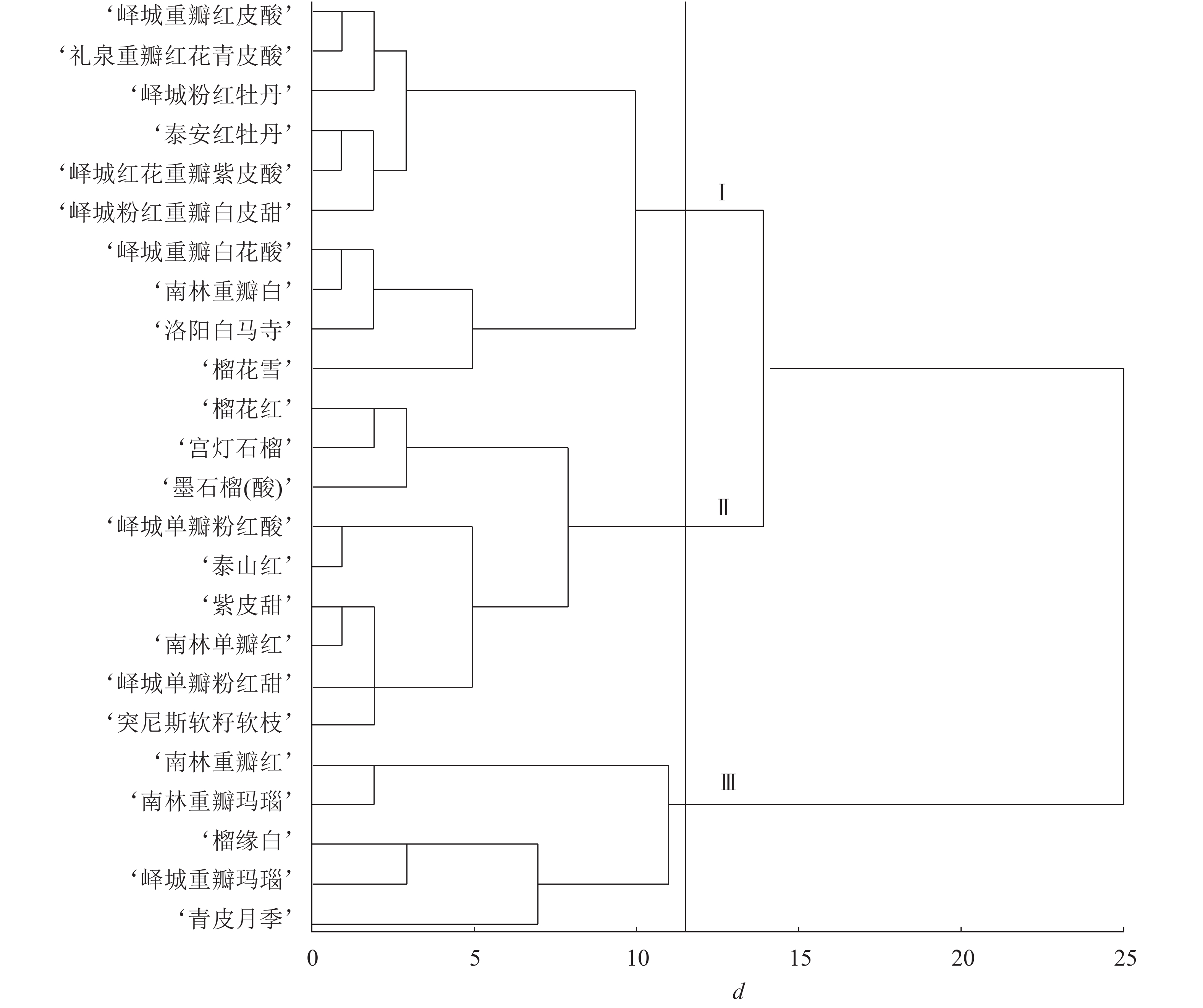
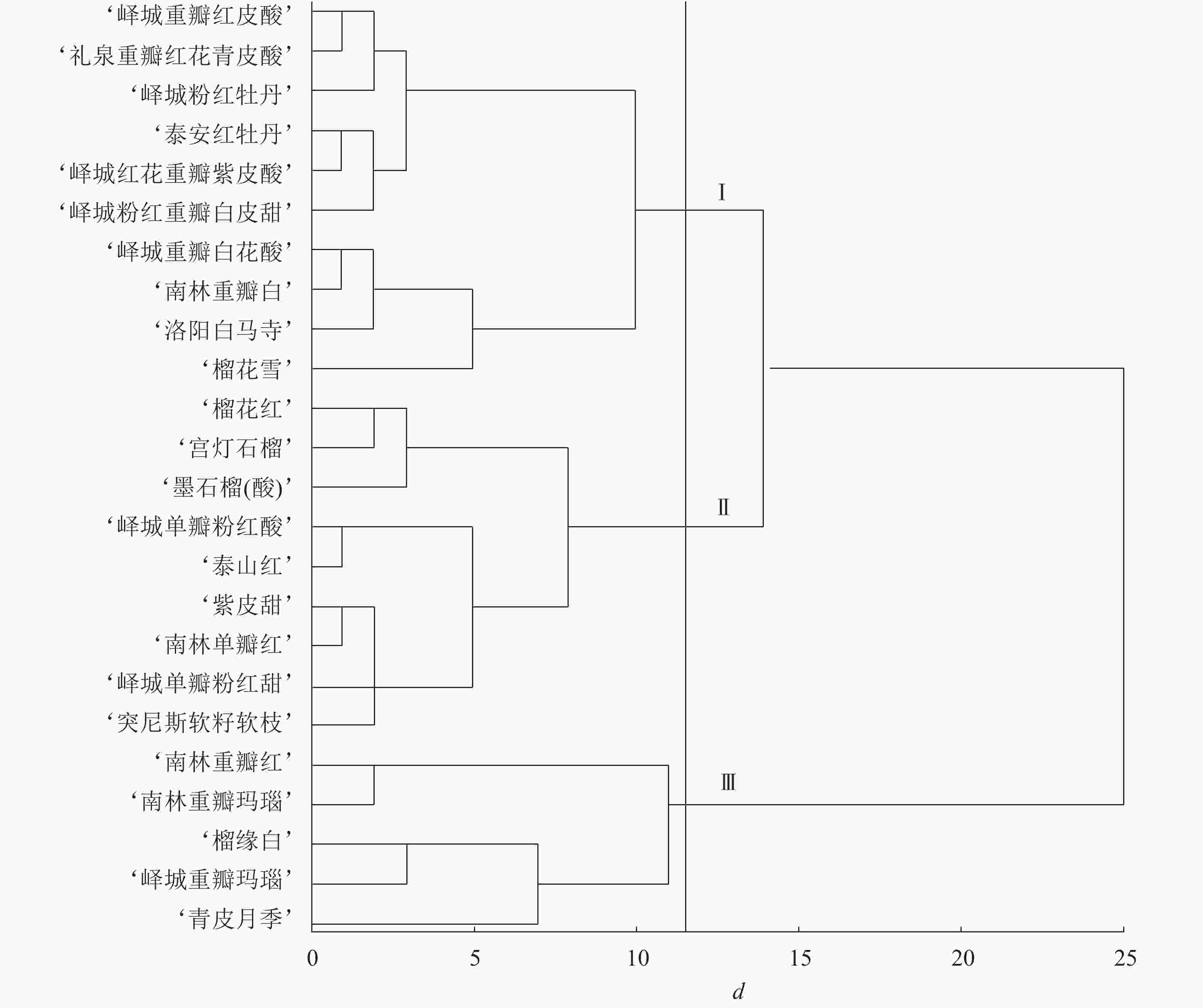
根据表型性状和主成分分析结果进行变量筛选,单因素方差分析发现:对聚类贡献较大的变量为花萼长、花萼瓣数、花瓣数、瓣化雄蕊数、株型、枝型、花型、1年生小枝颜色和花瓣色9个指标,构建聚类图(图1)。在遗传距离为11~12时,观赏石榴品种分为3个组群:组群Ⅰ、组群Ⅱ、组群Ⅲ。其中,组群Ⅰ包括6个重瓣品种群和4个复瓣品种群;组群Ⅱ包括8个单瓣品种群和1个重瓣品种群;组群Ⅲ包括4个重瓣品种群和1个台阁品种群。在遗传距离14~15时,组群Ⅰ和组群Ⅱ聚在一起,说明组群Ⅰ和组群Ⅱ遗传关系较近,两者与组群Ⅲ的遗传关系均较远。进一步分析,4个白花类品种(‘峄城重瓣白花酸’‘南林重瓣白’‘洛阳白马寺’‘榴花雪’)聚在一起,1年生小枝颜色为浅绿色的品种也都是白花品种,全部聚在一起,说明白花品种的遗传关系较近。3个复色品种(‘榴缘白’‘峄城重瓣玛瑙’‘南林重瓣玛瑙’)也聚在一起。除了‘青皮月季’以外,4个矮生品种(‘榴花雪’‘榴花红’‘墨石榴’‘宫灯石榴’)聚在一起,表明矮生品种间遗传关系较近。
对各类组群个体的数量性状取平均值,质量性状计算个数,得到各组群的特征(表7)。组群Ⅰ的特征为:花长最大、花萼长最大、花萼瓣数最少,绝大多数为乔木型,主要为开张型,重瓣略多于复瓣,1年生小枝主要为粉红色和浅绿色,花瓣色主要为白色、粉色、橙红,花萼色主要为橙黄、黄色。组群Ⅱ的特征为:花长最小、花宽最小、花萼长最小、花萼宽最小、花瓣数最小、瓣化雄蕊数最小,主要为乔木型,直立型和开张型各占一半,绝大多数为单瓣,1年生小枝颜色主要为粉红色,花瓣色主要为橙黄和橙红,花萼色主要为橙黄。组群Ⅲ的特征为:花宽最大、花萼宽最大、花萼瓣数最大、花瓣数最大、瓣化雄蕊数最大,主要为乔木型、开张型、重瓣,1年生小枝颜色主要为粉红色,花瓣色主要为复色,花萼色主要为橙黄和红色。
性状 花长/cm 花宽/cm 花萼长/cm 花萼宽/cm 花萼瓣数/片 花瓣数/片 瓣化雄蕊数/片 组群Ⅰ 4.56 5.15 3.21 4.00 6.04 53.62 63.66 组群Ⅱ 4.29 3.50 2.97 2.64 6.26 9.70 3.15 组群Ⅲ 4.41 5.95 3.06 4.53 7.73 76.60 141.13 Table 7. Morphological characteristics of different ornamental pomegranate cultivars groups after cluster
性状 株型 枝型 花型 1年生小枝颜色 花瓣色 花萼色 组群Ⅰ 1(9),2(1) 1(4),2(6) 2(4),3(6) 1(5),4(1),5(4) 1(4),2(1),3(3),5(2) 1(2),2(4),4(4) 组群Ⅱ 1(6),2(3) 1(4),2(4),3(1) 1(8),3(1) 1(6),3(2),4(1) 2(4),3(3),5(2) 3(2),4(4),5(1),6(2) 组群Ⅲ 1(4),2(1) 1(1),2(4) 3(4),4(1) 1(4),2(1) 2(1),3(1),6(3) 4(2),5(1),6(2) 说明:括号中数值表示品种数,括号外数值表示类型 -
流式细胞仪测定结果表明(表8):24个观赏石榴品种均为二倍体,C值范围为0.28~0.39 pg,品种间C值差异较大。其中,单瓣品种群、复瓣品种群、重瓣品种群及台阁品种群的平均C值分别为0.34、0.32、0.34和0.33 pg,可见由花型划分的各品种群之间C值相差不大。白花品种群、粉花品种群、红花品种群及复色花品种群的平均C值分别为0.34、0.32、0.34和0.32 pg,可见由花色划分的各品种群之间C值相差不大。中国品种、美国品种、突尼斯品种的平均C值分别为0.33、0.35和0.34 pg,各地品种C值相差不大。
序号 品种名 荧光强度 变异系数/% C值/pg 倍性 序号 品种名 荧光强度 变异系数/% C值/pg 倍性 1 ‘泰安红牡丹’ 9 560 6.66 0.32 二倍体 13 ‘峄城单瓣粉红酸’ 9 645 4.54 0.32 二倍体 2 ‘榴缘白’ 9 951 4.61 0.33 二倍体 14 ‘榴花红’ 11 142 6.66 0.37 二倍体 3 ‘青皮月季’ 11 614 6.84 0.39 二倍体 15 ‘紫皮甜’ 10 368 6.47 0.34 二倍体 4 ‘峄城重瓣红皮酸’ 8 435 5.04 0.28 二倍体 16 ‘峄城单瓣粉红甜’ 9 370 7.42 0.31 二倍体 5 ‘峄城粉红牡丹’ 10 024 6.17 0.33 二倍体 17 ‘突尼斯软籽软枝’ 10 151 5.82 0.34 二倍体 6 ‘峄城红花重瓣紫皮酸’ 9 437 4.58 0.31 二倍体 18 ‘墨石榴’ 9 257 7.10 0.31 二倍体 7 ‘榴花雪’ 10 622 5.67 0.35 二倍体 19 ‘宫灯石榴’ 11 138 5.51 0.37 二倍体 8 ‘峄城粉红重瓣白皮甜’ 9 801 6.56 0.33 二倍体 20 ‘泰山红’ 10 331 7.28 0.34 二倍体 9 ‘峄城重瓣玛瑙’ 9 967 5.99 0.33 二倍体 21 ‘南林重瓣红’ 9 511 6.57 0.32 二倍体 10 ‘洛阳白马寺’ 11 078 5.94 0.37 二倍体 22 ‘南林单瓣红’ 10 580 7.34 0.35 二倍体 11 ‘礼泉重瓣红花青皮酸’ 10 168 5.71 0.34 二倍体 23 ‘南林重瓣白’ 10 329 4.26 0.34 二倍体 12 ‘峄城重瓣白花酸’ 9 160 4.88 0.30 二倍体 24 ‘南林重瓣玛瑙’ 9 100 6.80 0.30 二倍体 Table 8. Ploidy level and C-value of 24 ornamental pomegranate cultivars
-
种质资源的调查和评价是研究品种起源、演化、驯化的基础,对优异资源筛选、品种选育具有重要意义[32]。利用表型性状分析种质资源的遗传多样性直观易行,能快速了解植物的遗传变异水平[33]。本研究发现:24个观赏石榴品种具有丰富的表型多样性,平均形态多样性指数为1.453,数量性状多样性指数(1.715)大于质量性状(1.148),与前人对紫薇Lagerstroemia indica[28]、香瓜Cucumis melo[34-35]等的表型多样性研究观点基本相符。其中,花瓣色、花萼色2个质量性状和花长、花宽、花萼长、花萼宽、花萼瓣数、花瓣色、瓣化雄蕊数7个数量性状变异明显,多样性指数高于1.400。
值得注意的是,各数量性状的多样性指数与变异系数变化趋势不一致。与其他数量性状相比,瓣化雄蕊数和花瓣数的多样性指数较小,分别为1.456和1.630,小于平均值(1.715);但两者的变异系数最大,分别为117.59%和78.86%,大于平均值41.82%。多样性指数代表变异分布的均匀度,变异系数代表变异的离散程度。不一致的变化趋势说明变异的范围很大,且变异的分布不均匀,与张海平等[36]对睡莲Nymphaea tetragona的表型研究类似,同时符合TILMAN[37]关于多样性指数和变异系数之间关系的观点。另外,变异系数在一定程度上反映了进化的快慢,瓣化雄蕊数和花瓣数的变异系数大说明重瓣和雄蕊瓣化程度高的品种,自然品种形成较晚;人工育种加速了雄蕊瓣化和花瓣数量的增多,这与杜鹃花Rhododendron simsii[38]、矮牵牛Petunia hybrid等[39]、日本龙胆花Gentiana scabra等[40]等观赏植物品种选育方向一致,说明观赏石榴的选育也是朝着花瓣数增多、雄蕊瓣化的方向进行。
胡标林等[41]认为:利用株高、芒等表型性状能够明确水稻Oryza sativa核心种质的亲缘关系。本研究聚类结果表明:24个观赏石榴品种可划分为3个组群,其遗传聚类与花型、颜色、株型关系密切。其中,单瓣品种与复瓣品种、部分重瓣品种的亲缘关系较近,另一部分重瓣品种与台阁品种的亲缘关系较近,这与陈俊愉[42]的花卉品种分类体系提出的花型演化观点相同。而依据花瓣色划分,橙色花品种与粉色花品种、白色花品种的亲缘关系较近,与复色花品种的亲缘关系较远,与张启翔[43]对紫薇品种群的划分方法相同。
MOSLEMI等[44]、JBIR等[45]在对突尼斯和伊朗石榴的研究中认为:石榴品种间亲缘关系与地理环境的相关性不强。本研究聚类结果表明:来源南京的品种并没有聚在一起,分散在3个组群中,该结果同样支持上述观点。王庆军等[21]通过内部简单序列重复(ISSR)将峄城35个观赏石榴分为6个组群,发现依据分子标记划分的大类中有不同的花色和花型,认为基于分子性状的亲缘关系与基于花色、花型等表型性状的亲缘关系不一致。本研究中,基于多个表型性状的聚类结果只有小部分与王庆军等[21]的分子标记划分结果一致;赵丽华等[46]在利用ISSR对47个石榴栽培品种的聚类研究中也发现了同样的矛盾。观赏石榴品种在繁殖栽培过程中大量的地区间引种交流以及长期的自然选择和人工选择下积累了丰富的遗传变异,使得某些品种在遗传信息上可聚为一类,但形态特征和地理来源却有较大差异,因此,对观赏石榴品种的遗传关系的研究时,需进一步选择合适的分子标记开展分子水平的亲缘关系分析,结合本研究的形态聚类结果共同评判与讨论。
千屈菜科倍性复杂,大多为多倍体物种[47]。该科的紫薇属Lagerstroemia中,桂林紫薇L. guilinensis是四倍体,云南紫薇L.intermedia、大花紫薇L.speciosa和西双紫薇L.venusta是六倍体,毛萼紫薇L. balansae为非整倍体[48]。本研究发现:引种自各地的24个观赏石榴品种均为二倍体,说明从倍性来看,石榴属品种遗传相对稳定,可进一步探究石榴多倍体育种的可能性,比较二倍体石榴与多倍体石榴的表型差异与遗传差异。
本研究中观赏石榴品种主要是单瓣类、复瓣类、重瓣类,而台阁类品种的数量较少。故还需广泛收集不同类别的品种资源,并结合分子生物学证据,开展更为广泛的观赏石榴遗传多样性、品种分类研究。
-
观赏石榴种质资源具有丰富的表型遗传多样性,以花的数量性状最为突出,其中瓣化雄蕊数和花瓣数的变异系数分别为117.59%和78.86%。聚类分析可分为3大组群,组群Ⅰ和组群Ⅱ聚在一起,两者与组群Ⅲ的亲缘关系均较远,其遗传聚类与花型、颜色、株型关系密切,暗示着花色、花型在栽培观赏石榴的驯化育种过程中的重要指示性作用。24个观赏石榴品种均为二倍体,显示出石榴观赏品种较强的遗传稳定性。
Phenotypic genetic diversity of ornamental pomegranate cultivars
doi: 10.11833/j.issn.2095-0756.20190619
- Received Date: 2019-10-21
- Rev Recd Date: 2020-05-13
- Available Online: 2020-10-10
- Publish Date: 2020-08-20
-
Key words:
- horticulture /
- pomegranate /
- ornamental cultivars /
- phenotypic diversity /
- principle component analysis /
- clustering analysis
Abstract:
| Citation: | HUO Yan, ZHAO Xueqing, HUANG Houyi, et al. Phenotypic genetic diversity of ornamental pomegranate cultivars[J]. Journal of Zhejiang A&F University, 2020, 37(5): 939-949. DOI: 10.11833/j.issn.2095-0756.20190619 |









 DownLoad:
DownLoad: