-
番茄Lycopersicon esculentum青枯病是由病原菌青枯劳尔氏菌Ralstonia solanacearum (简称青枯菌)引起的病害,该菌被公认为最具侵染力和破坏力的土传病害之一[1−2]。侵染后引起植株维管束堵塞,严重时导致植物枯萎死亡。据估算,由青枯病造成的农业经济损失高达30万元·hm−2,山东、新疆、河北、河南、云南、江苏等地都是番茄青枯病的重灾区[3]。目前,对于番茄青枯病的防控措施主要有:抗病品种筛选,如BHN466和FL7514[4-5],但其果实普遍比正常果实小[6],且筛选抗病品种周期长;采用嫁接方式,虽能增强植物抗病能力,但经济成本较大,目前中国在栽培番茄上采用嫁接技术比例不足2%[7];化学农药喷施,易促进病源菌产生抗病性和造成土壤污染[8]。尚未有单一的物理化学防治方法能完全有效地抑制番茄青枯病[9]。
目前,关于利用生防菌进行生物防治研究集中在以根际促生菌作为拮抗微生物防治病源菌侵染[10]。其中芽孢杆菌Bacillus、青霉菌Penicillium、木霉菌Trichoderma等常用来作为生防菌应用于植物病害防治研究中。研究发现:芽孢杆菌中解淀粉芽孢杆菌B. amyloliquefaciens Am1和枯草芽孢杆菌B. subtilis PTS-394对番茄青枯病抑制率分别达88.98%[11]和80.00%[12]。木霉菌主要通过竞争作用、诱导宿主抗性、重寄生作用、抗生作用等机制抑制目标病原菌的生长繁殖[13],其中棘孢木霉T. asperellum是目前开发应用最多的菌种之一。平板对峙试验发现:棘孢木霉F2通过空间位置和营养竞争有效抑制三七Panax notoginseng灰霉病病菌Botrytis cinerea,其抑制率高达90%[14];棘孢木霉GDFS1009对秸秆腐烂病的抑制率达60%[15]。青霉菌对植物病原菌的抑制效果研究比木霉少,KOMAI等[16]从青霉菌P. simplicissimum IFM53375中分得的Penicillide(1-4)类化合物及其衍生物、嘌呤活性蛋白等具有抗真菌活性作用;夏汉祥等[17]发现淡紫拟青霉P. lilacinus产生的类植物生长素有防治病虫害、促进植物生长的作用;淡紫拟青霉能在香蕉Musa nana根际稳定定殖,对香蕉枯萎病有很强的防控效果。草酸青霉P. oxalicum通过营养竞争、重寄生等多种方式抑制尖孢镰刀菌Fusarium oxysporum生长发育,它对苹果Malus pumila连作土壤中常见的4种有害镰孢菌有抑制作用[18]。
木霉菌和青霉菌是有潜力的生防菌,但作为青枯菌的生防菌研究鲜见报道。本研究利用筛选获得的棘孢木霉QZ2和草酸青霉QZ8等2株生防菌探究对青枯菌的防治效果。
-
青枯劳尔氏菌 QL-RS 1115,GenBank: GU390462 (简称青枯菌)由南京农业大学资源与环境学院提供,从出现严重青枯病病症的番茄根际分离,经过科赫法则(Koch’ spostulates)[19]证实具有强致病性。棘孢木霉QZ2和草酸青霉QZ8由浙江农林大学浙江省森林生态系统碳循环与固碳减排重点实验室从衢州退化植被恢复试验区土壤中分离获得,经过ITS序列克隆测序、美国国家生物信息中心(NCBI)数据库比对鉴定,用甘油保存法−80 ℃保存[20]。
-
包括:①马铃薯葡萄糖培养基(PDA);②NA培养基:葡萄糖10 g,蛋白胨5 g,酵母粉0.5 g,牛肉膏3 g,1 L蒸馏水;③NB培养基:NB培养基基础上加20 g琼脂;④SMSA培养基:1 L NA培养基中加入质量浓度3%多黏菌素3 mL,质量浓度3%放线菌酮3 mL,质量浓度1%氯霉素450 μL,质量浓度为1%青霉素450 μL,质量浓度为1%结晶紫450 μL,质量浓度为1% TTC 4.5 mL,质量浓度为1%杆菌肽2.25 mL,于倒平板前加入到三角瓶中[17];⑤青霉选择培养基:孟加拉红(虎红)培养基;⑥木霉选择培养基:每升孟加拉红(虎红)培养基中加入氯霉素0.25 g,质量浓度为1%链霉素9 mL,于倒平板前加入到三角瓶中[21]。
-
参照严无瑕等[22]方法,用两点法进行平板对峙试验,以不接种生防菌为对照,每个处理设置3个重复。将青枯菌和棘孢木霉QZ2菌株分别接种于PDA固体培养基中,(28±1) ℃黑暗培养4 d后,用无菌枪头挑取棘孢木霉QZ2和青枯菌单菌落,接种于100 mL的PDA液体培养基中,28 ℃,170 r·min−1转速震荡培养14 h,得到棘孢木霉QZ2与青枯菌的种子液,取100 μL接种于PDA固体培养基,(28±1) ℃黑暗培养4 d,待平板长满后,用直径9 mm的打孔器获得青枯菌的菌饼,接种于平板中央,用同样方法得到棘孢木霉QZ2菌饼接种青枯菌左右间距40 mm位置。以只在中央接种青枯菌的平板作为对照,每个处理设置3个重复。接种后置于(28±1) ℃黑暗条件下培养,分别于培养4、6、8、10 d时测量青枯菌菌落直径。用同样方法进行草酸青霉QZ8两点法平板对峙试验。青枯菌生长抑制率=(对照菌落直径−处理的菌落直径)/对照菌落直径×100%。
-
生防菌发酵液的制备参照程敏等[23]的方法,生防菌种子液的配置参照1.2.1,将种子液按照体积分数为3%的接种量接种至500 mL的PDA液体培养基中,28 ℃、170 r·min−1震荡培养48 h,得到棘孢木霉QZ2和草酸青霉QZ8的发酵液。以6 000 r·min−1离心5 min发酵液后,收集上清液,分别与PDA液体培养基按照1∶1的体积比均匀混合,并加入质量浓度为20%的琼脂粉,115 ℃高压灭菌30 min,制成PDA与棘孢木霉QZ2发酵液的混合培养基。用直径9 mm的打孔器获取青枯菌菌饼,接种至棘孢木霉QZ2和草酸青霉QZ8的混合培养基的中央,用无菌水代替发酵液的PDA培养基平板作为对照,每个处理设置3个重复。分别于培养4、6、8、10 d时测量青枯菌菌落的直径,抑菌率计算公式同1.2.1。
-
生防菌发酵液灭菌后一些活性物质可能会失活,通过活性发酵液的液培试验进一步研究生防菌抑菌机制。液培试验参照谭军等[24]的方法,棘孢木霉QZ2和草酸青霉QZ8的初次发酵液制备参照1.2.1,然后通过无菌滤膜过滤,分别取100 mL棘孢木霉QZ2和青霉QZ8无菌发酵液,与NA液体培养基按照1∶1体积比混合均匀,制成含有棘孢木霉QZ2或草酸青霉QZ8发酵液的NA液体培养基。青枯菌种子液制备:用无菌枪头挑取青枯菌单菌落,接种至装有20 mL的NA液体培养基的三角瓶中,28 ℃、170 r·min−1摇床培养12 h,得到青枯菌种子液。取6 mL接种至生防菌发酵液NA混合培养基中,28 ℃、170 r·min−1摇床培养60 h。用灭菌的PDA代替发酵液与NA液体培养基混合均匀作为对照,每个处理3个重复。每隔6 h取样,测定各培养时间菌液的D(600),共计10次。通过D(600)比较不同环境中青枯菌的含量,并绘制青枯菌的生长曲线。
-
鉴于土壤中微生物丰富多样,本研究开展土培试验验证生防菌的生防效果。青枯菌菌液制备参照熊汉琴[25]的方法,青枯菌种子液的制备参照1.2.1,吸取2 mL青枯菌接种于200 mL NB液体培养基,28 ℃,170 r·min−1摇床培养36 h制作青枯菌的菌液,随后将青枯菌菌液分装于50 mL无菌离心管中,6 000 r·min−1离心5 min,去上清液,加无菌水,重复2次,最后加无菌水调整菌液含量,通过分光光度计测D(600),使其含量达1011 CFU·L−1。2株生防菌孢子液制备方法参考文献[26],首先分别将2种生防菌接种于PDA固体培养基中,在(28±1) ℃、黑暗条件下培养至孢子长满整个平板,将5 mL无菌水注射在平板上,用涂布棒刮下孢子溶解于无菌水中,用4层纱布过滤后得到孢子悬液,最后将孢子液稀释到1010 CFU·L−1。
采集无青枯菌污染的森林土,土壤pH 6.0~7.0,设置青枯菌+棘孢木霉(QZ2)、青枯菌+草酸青霉(QZ8)、青枯菌+无菌水(对照)3个处理,每处理9个重复,每个重复用100 g土。QZ2和QZ8处理分别将青枯菌菌液与棘孢木霉QZ2和草酸青霉QZ8孢子液按体积比1∶1混合后加入土中,每100 g土加13 mL混合液,置于(28±1) ℃的气候培养箱培养。分别在3、7、14、20、28 d,用SMSA培养基,采用稀释涂布平板法计数所有土壤中青枯菌菌落数量[25]。由于群体效应,孢子在土壤中数量达到峰值需要一定时间,因此从处理7 d开始用孟加拉红(虎红)培养基和木霉选择性培养基,采用稀释涂布平板法,获得棘孢木霉QZ2和草酸青霉QZ8菌落数量。
-
用Excel 2010统计数据,结果以平均值±标准差表示;用SPSS 22.0处理工作平台进行单因素方差分析,显著性水平取0.05;用Origin Pro 2019b绘图。
-
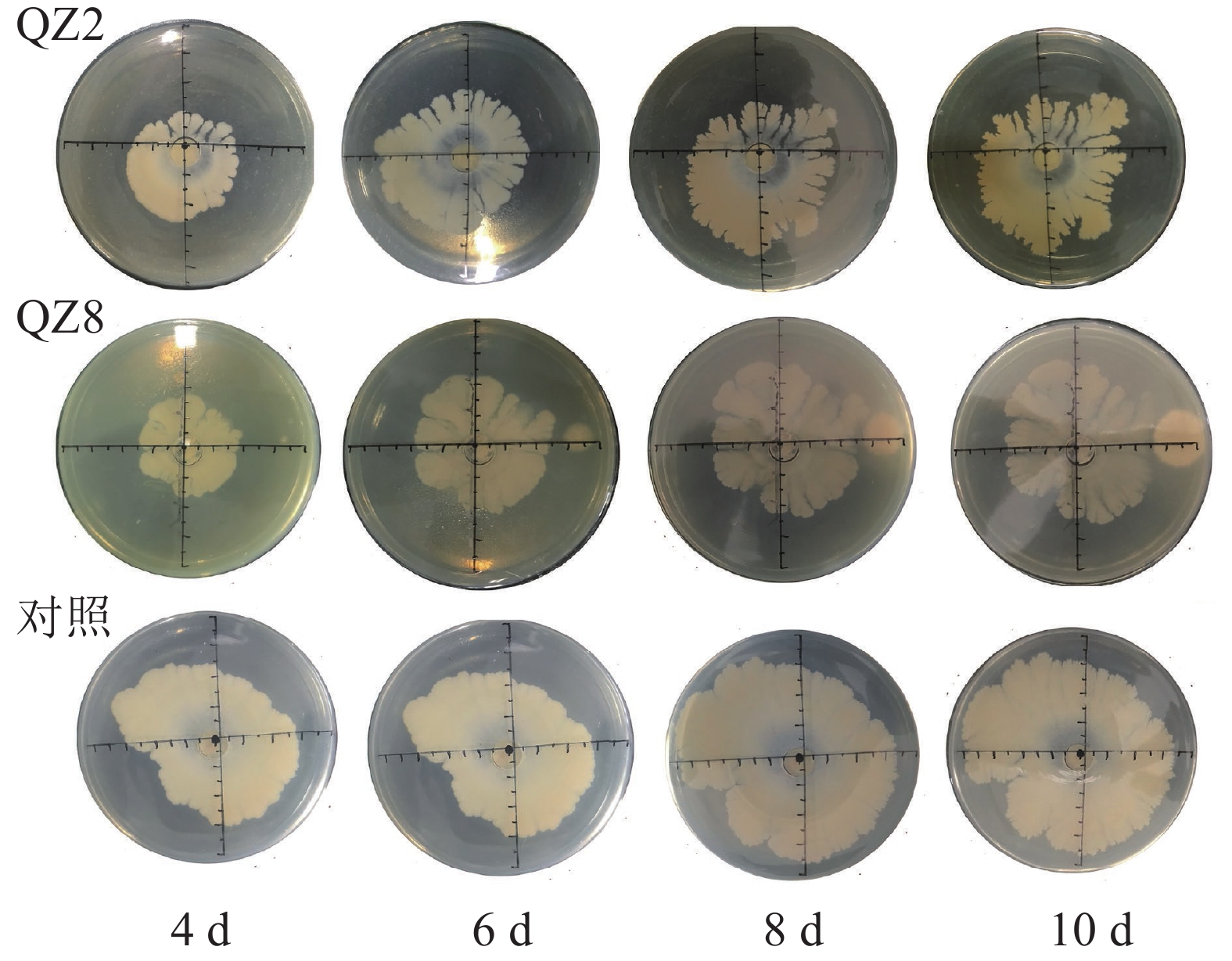
平板对峙结果(图1和表1)表明:棘孢木霉QZ2对青枯菌有显著的抑制效果(P<0.05),棘孢木霉QZ2的菌丝生长速度明显快于青枯菌生长速度,与对照相比,青枯菌的生长明显受到棘孢木霉QZ2抑制,对峙培养4 d时棘孢木霉QZ2菌丝布满了整个培养皿,包围了青枯菌,抑制率为45.5%,之后青枯菌停止扩大,对照青枯菌则持续生长,10 d对照青枯菌也停止扩大,此时棘孢木霉QZ2对青枯菌的抑制率达80.9%。草酸青霉QZ8对青枯菌也有显著抑制效果,对峙培养4 d时草酸青霉QZ8对青枯菌抑制率为30.5%,之后草酸青霉QZ8继续生长,10 d时草酸青霉QZ8与青枯菌菌落直径都不再扩大,此时草酸青霉QZ8对青枯菌生长抑制率达到45.9%。
培养时
间/d对照
菌落直
径/cmQZ2 QZ8 菌落直
径/cm抑制
率/%菌落直
径/cm抑制
率/%4 4.15±0.07 a 2.10±0.04 b 45.5 2.88±0.83 b 30.5 6 5.50±0.00 a 2.30±0.00 b 80.8 3.35±1.00 b 39.1 8 6.70±0.00 a 2.50±0.11 b 79.7 3.62±1.15 b 46.0 10 6.90±0.14 a 2.50±0.04 b 80.9 3.73±1.26 b 45.9 说明:数据代表平均值±标准差,同行数据后小写字母表示 不同处理间差异显著(P<0.05) Table 1. Inhibition rate of T. asperellum QZ2 and P. oxalicum QZ8 against R. solanacearum in plate confrontation
-
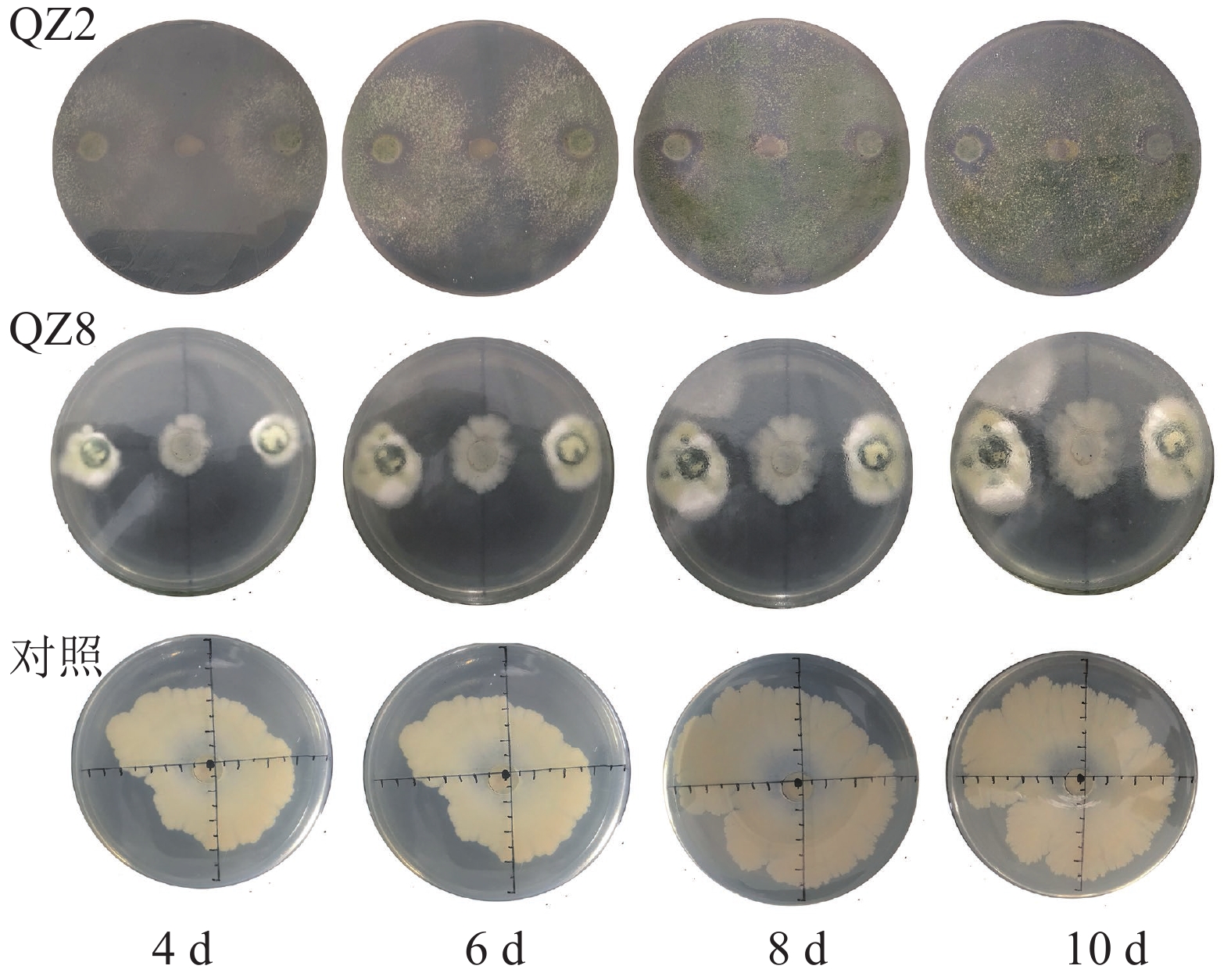
由图2和表2可见:2株生防菌制成的灭菌发酵液均对青枯菌产生显著的抑制效果(P<0.05)。棘孢木霉QZ2灭菌发酵液平板中的青枯菌菌落生长速度明显比对照慢,3个处理的青枯菌菌落均在培养10 d时停止扩大,故以10 d为试验结果的统计时间。2株生防菌发酵液的抑制率随着培养时间的增加而增加,4 d时棘孢木霉QZ2发酵液对青枯菌抑制率仅为9.6%,10 d时最大抑制率达33.3%。草酸青霉QZ8发酵液对青枯菌抑制作用与棘孢木霉QZ2相似,10 d时最大抑制率为34.8%,抑制率略高于棘孢木霉QZ2发酵液,差异不显著。
天数/d 对照菌落
直径/cmQZ2 QZ8 菌落直
径/cm抑制
率/%菌落直
径/cm抑制
率/%4 4.15±0.07 a 3.8±0.10 b 9.6 3.40±0.02 b 18.1 6 5.50±0.00 a 4.6±0.60 b 16.4 4.50±0.00 b 29.1 8 6.70±0.00 a 4.6±0.60 b 31.3 4.50±0.01 b 32.8 10 6.90±0.14 a 4.6±0.60 b 33.3 4.50±0.01 b 34.8 说明:数据代表平均值±标准差,同行数据后小写字母表示不 同处理间差异显著(P<0.05) Table 2. Inhibition effect of sterilized fermentation supernatant of biocontrol broth on the growth of R. solanacearum
-
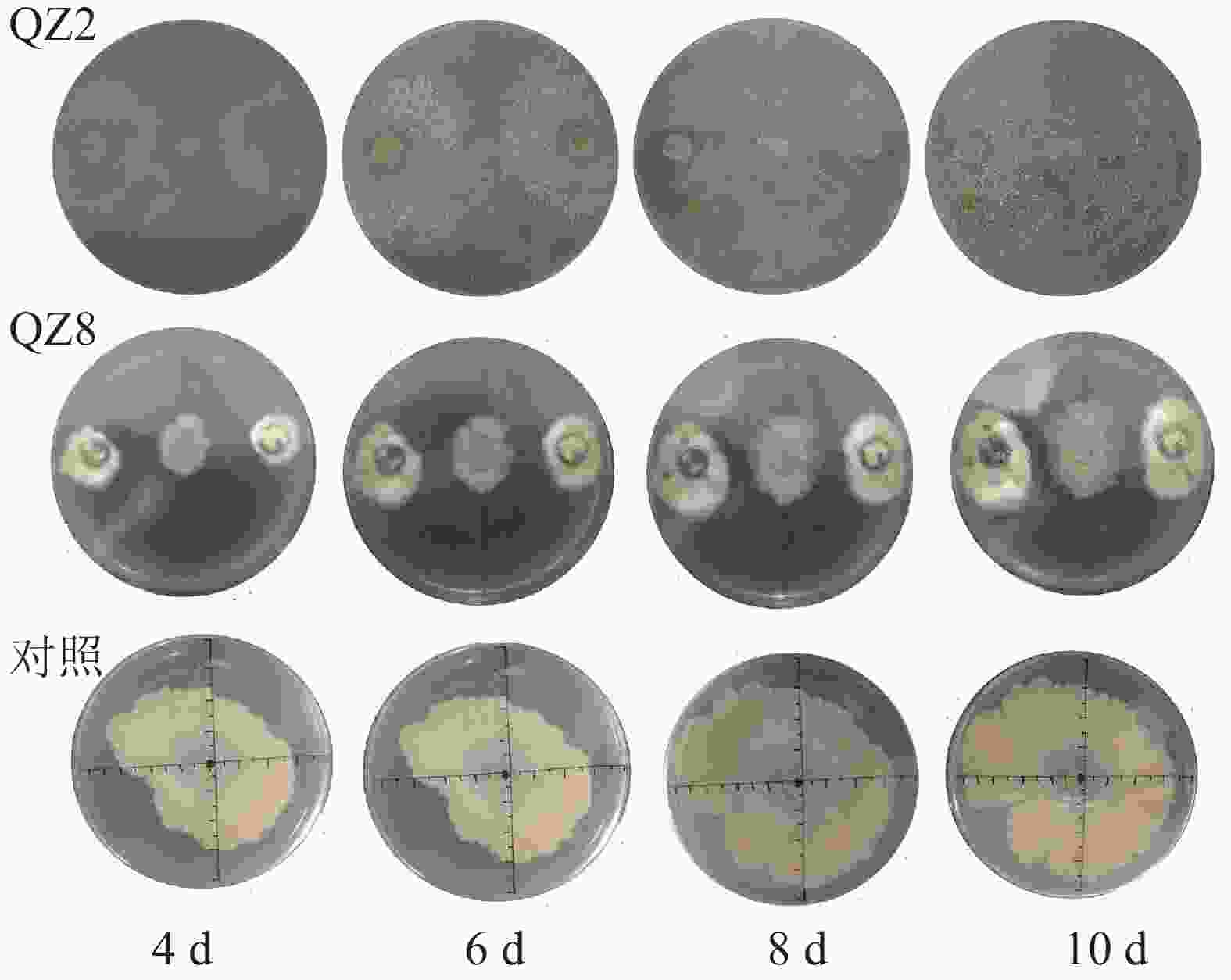
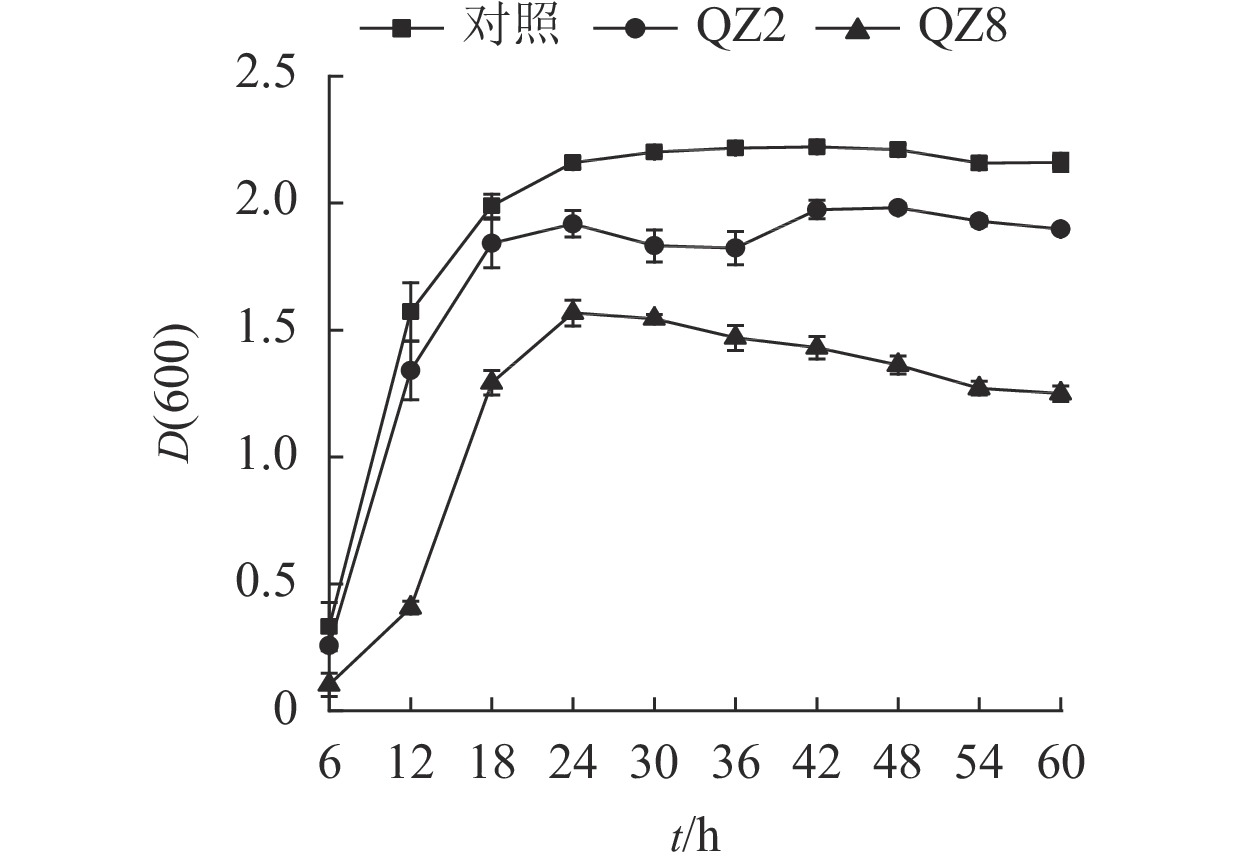
由图3可见:2株生防菌制成的活性发酵液均对青枯菌有显著的抑制效果(P<0.05)。对照培养6~18 h时青枯菌呈指数生长,18~24 h时逐渐趋向稳定,其生长曲线与大部分细菌相似。但棘孢木霉QZ2处理在30和36 h时青枯菌D(600)下降,而草酸青霉QZ8处理则在24 h青枯菌D(600)达到峰值后呈缓慢下降,青枯菌较早进入衰亡期。差异检验结果表明:24 h以后,2株生防菌活性发酵液处理的青枯菌D(600)均显著低于对照(P<0.05),其中草酸青霉QZ8处理的 D(600)低于棘孢木霉QZ2;60 h时,三者之间的D(600)差异均显著(P<0.05)。
-
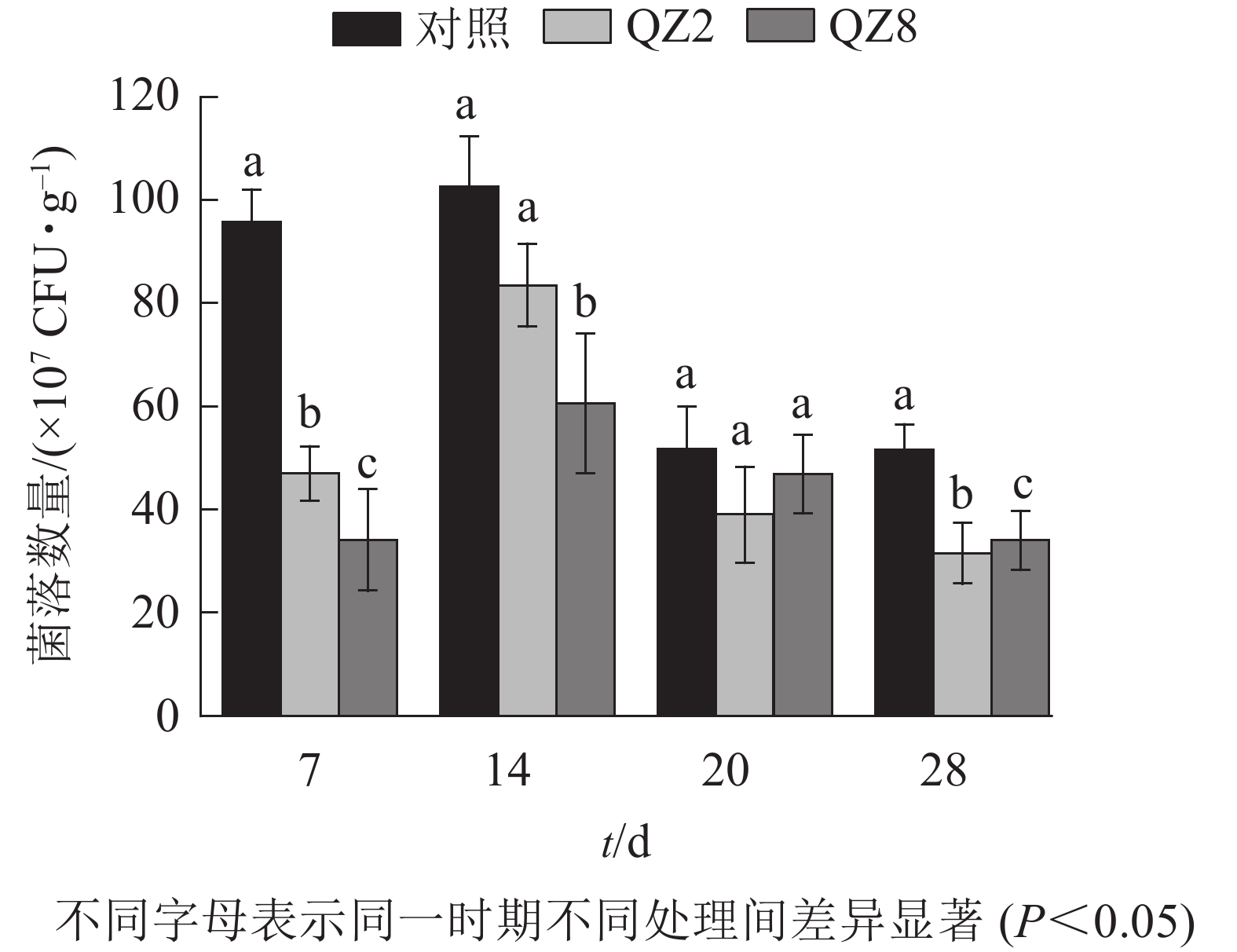
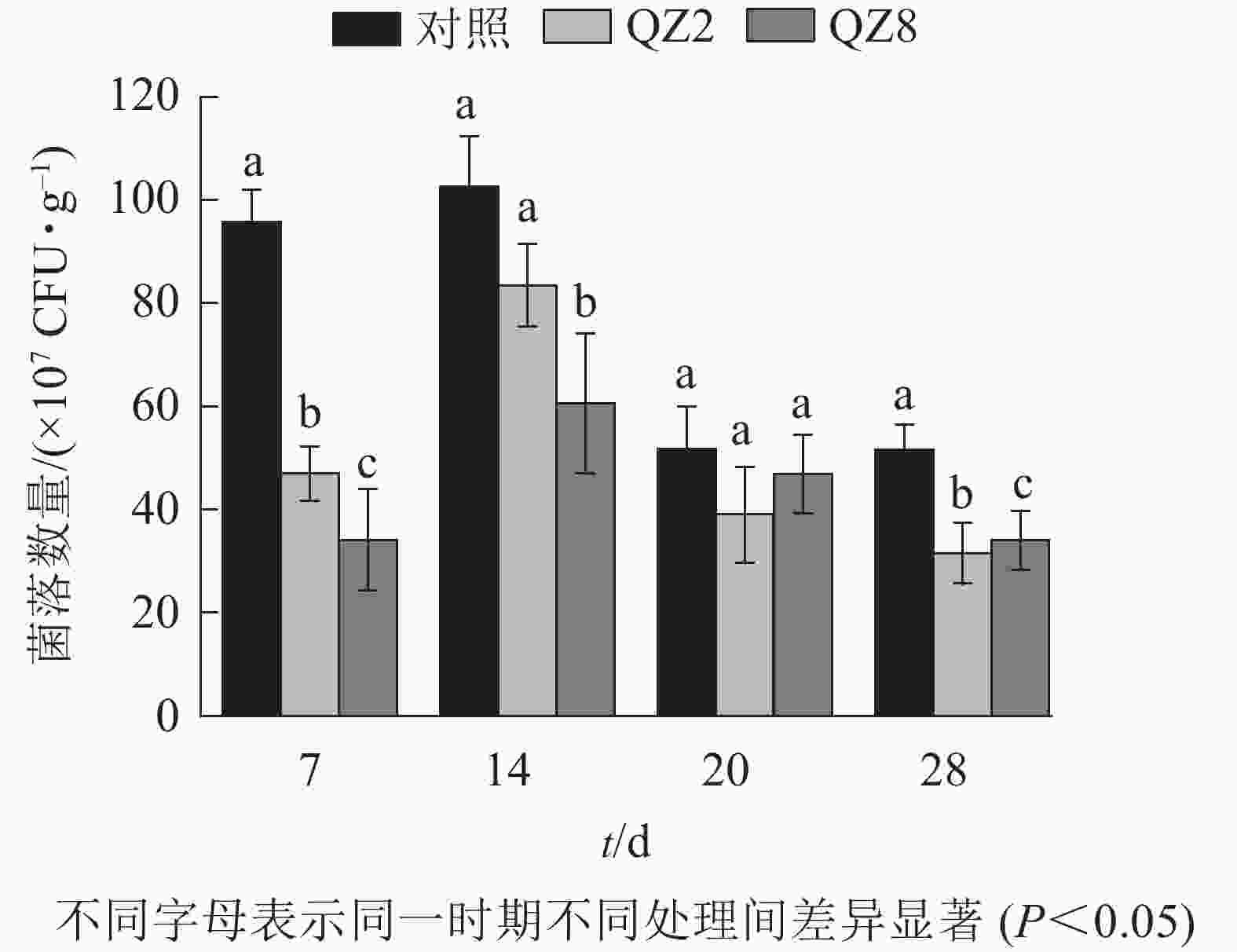
图4表明:棘孢木霉QZ2与草酸青霉QZ8在土壤中均能抑制番茄青枯菌的生长繁殖,其在4个测定时间的青枯菌数量均小于对照土壤;抑制效果最好的是7 d,QZ2与QZ8处理土壤中青枯菌数量均显著低于对照(P<0.05),分别下降了51.3%和64.7%;28 d,QZ2与QZ8处理青枯菌数量比对照显著下降了38.9%和34.1%(P<0.05);除20 d外,QZ8处理的青枯菌数量均显著低于对照(P<0.05);前期(7、14 d)的抑制效果是QZ8好于QZ2,而后期(20、28 d)则相反。
-
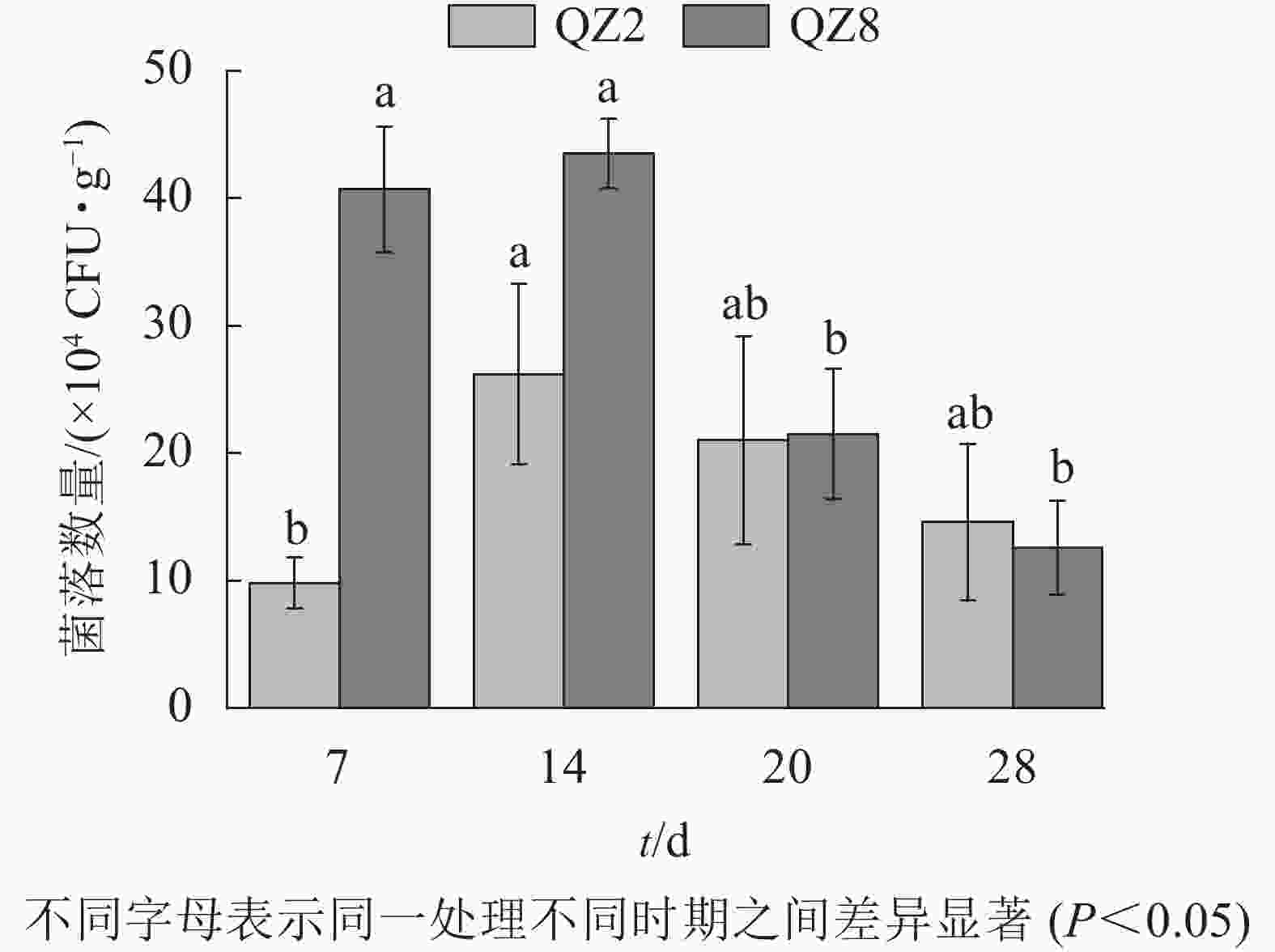
图5表明:棘孢木霉QZ2数量在处理7 d时处于低位,14 d达峰值,随后逐渐开始下降。草酸青霉QZ8数量在处理14 d时达到峰值,且明显多于棘孢木霉QZ2,但之后急剧下降,20 d时与棘孢木霉QZ2相近,28 d时反而少于棘孢木霉QZ2。
-
木霉菌的拮抗机制包括营养和空间竞争、重寄生、分泌有活性代谢产物、诱导宿主植物抗性等[27]。通过平板对峙试验发现:棘孢木霉QZ2生长速度明显快于青枯菌,8 d时已将青枯菌包围,10 d时占领整个生存空间和营养空间,对青枯菌抑制率达80.9%。黄远迪等[28]研究发现:棘孢木霉菌株GX004对尖孢镰刀菌在平板上的抑制率达85.2%;张晓梦等[29]发现棘孢木霉发酵11 d时的无菌滤液对枸杞炭疽病菌Collectotrichum gloeosporioides的抑制率达85.3%。可见棘孢木霉抑制效果良好。平板对峙培养过程中草酸青霉QZ8的生长速度与青枯菌基本一致,也没有发现明显的抑制带,可以推测草酸青霉QZ8能分泌某些代谢产物,从而抑制青枯菌生长。培养10 d时草酸青霉QZ8对青枯菌抑制率为45.9%,抑制效果较好。张先富[30]研究发现:草酸青霉A1对尖孢镰刀菌抑制率为47.1%,且草酸青霉A1与尖孢镰刀菌菌落交界处会产生抑菌带。说明草酸青霉具有较好的生防效果。
本研究结果表明:2株生防菌高温灭菌发酵液和无菌过滤发酵液均对青枯菌有显著的抑制作用。高温灭菌发酵液平板试验中,棘孢木霉QZ2和草酸青霉QZ8在混合培养基上培养10 d时最大抑制率分别为33.3%和34.8%。无菌过滤发酵液液培试验中,草酸青霉QZ8对青枯菌的抑制效果显著好于棘孢木霉QZ2。前人研究发现:木霉和青霉均能产生丰富的代谢产物,其中具有抑菌作用的有聚酮类、生物碱、萜类、大环内酯、纤维素酶和几丁质酶等物质[31-32]。棘孢木霉QZ2与草酸青霉QZ8灭菌发酵液对青枯菌的抑制效果差别不大,说明均存在耐高温的抑菌物质,而未灭菌发酵液青霉QZ8抑制作用明显强于木霉QZ2,说明草酸青霉QZ8发酵液中的某些不耐高温活性物质如生物碱、酶类化合物,对青枯菌的生长起到较强的抑制作用。
棘孢木霉QZ2和草酸青霉QZ8在土壤中的抑菌效果显著,在处理28 d时青枯菌数量显著下降,对照土壤中青枯菌数量始终高于2株生防菌处理的土壤中青枯菌数量。草酸青霉QZ8在处理7、14 d时的抑制效果好于棘孢木霉QZ2,后期则相反,这与土壤中草酸青霉QZ8和棘孢木霉QZ2数量的动态变化规律一致,说明草酸青霉QZ8比棘孢木霉QZ2能更快地在土壤中萌发、定殖,并发挥生防作用。
本研究的2株生防菌对青枯菌均有较好的抑制效果,抑制机制为营养和空间竞争、分泌有活性代谢产物等,其中棘孢木霉QZ2以营养和空间竞争作用为主,而草酸青霉QZ8则以代谢产物的抑制作用为主。两者均是防治番茄青枯病的潜在生防菌,它们在土壤以及番茄体内定殖情况需进一步研究。
Biocontrol effect of Penicillium oxalicum and Trichoderma asperellum on Ralstonia solanacearum
doi: 10.11833/j.issn.2095-0756.20210525
- Received Date: 2021-07-28
- Accepted Date: 2022-02-23
- Rev Recd Date: 2022-02-22
- Available Online: 2022-07-20
- Publish Date: 2022-08-20
-
Key words:
- Trichoderma asperellum /
- Penicillium oxalicum /
- Ralstonia solanacearum /
- fermentation broth /
- antibacterial effect
Abstract:
| Citation: | FANG Qihang, YAN Guzhe, FANG Wei, et al. Biocontrol effect of Penicillium oxalicum and Trichoderma asperellum on Ralstonia solanacearum[J]. Journal of Zhejiang A&F University, 2022, 39(4): 852-859. DOI: 10.11833/j.issn.2095-0756.20210525 |










 DownLoad:
DownLoad: