-
菌根是植物根系与菌根真菌形成的共生体,广泛分布于陆地生态系统中,90%以上的维管植物根系能够形成菌根[1]。菌根真菌能帮助植物吸收水分及氮、磷等营养物质,促进植物生长,同时从宿主植物获取碳水化合物以满足自身的生长需求[2]。由于菌根真菌和植物之间存在碳源和养分的交换[2],能显著影响土壤有机碳的积累与转化[3],因此,了解菌根在土壤碳循环中的作用具有重要意义。根据宿主植物以及菌根真菌种类不同,菌根具有7种不同的类型,其中丛枝菌根(arbuscular mycorrhiza,AM)和外生菌根(ectomycorrhiza,ECM)是分布最为广泛、物种最为多样的菌根类型[1, 4]。据估算,AM和ECM植被在地上生物量中的碳储量分别为(241.0±15.0)和(100.0±17.0) Gt,而非菌根植被的碳储量仅有(29.0±5.5) Gt[5]。因此,菌根真菌在陆地生态系统碳氮循环过程中发挥着重要作用[6],其中对土壤碳循环的影响主要有3种方式:①将植物光合作用固定的产物运输到土壤,从而增加碳输入[7-8];②菌根共生体本身的碳汇功能及对土壤中有机碳的固定作用[9-10];③通过激发效应或控制土壤养分有效性调控有机碳矿化过程[11-13]。由于AM和ECM在形态和生理功能(如营养获取方式、菌丝周转速率等)上有很大的不同[2],所以菌根介导的碳循环过程和方向在很大程度上取决于菌根类型和群落组成[14-15],以及与菌根存在相互作用的微生物群落[16-17]。为了深入理解不同类型菌根对土壤碳循环的影响,本研究综述了外生菌根和丛枝菌根对宿主光合产物分配、有机碳固定、有机碳矿化等土壤碳循环过程的差异并分析其作用机制,最后总结该方向今后研究的重点。
-
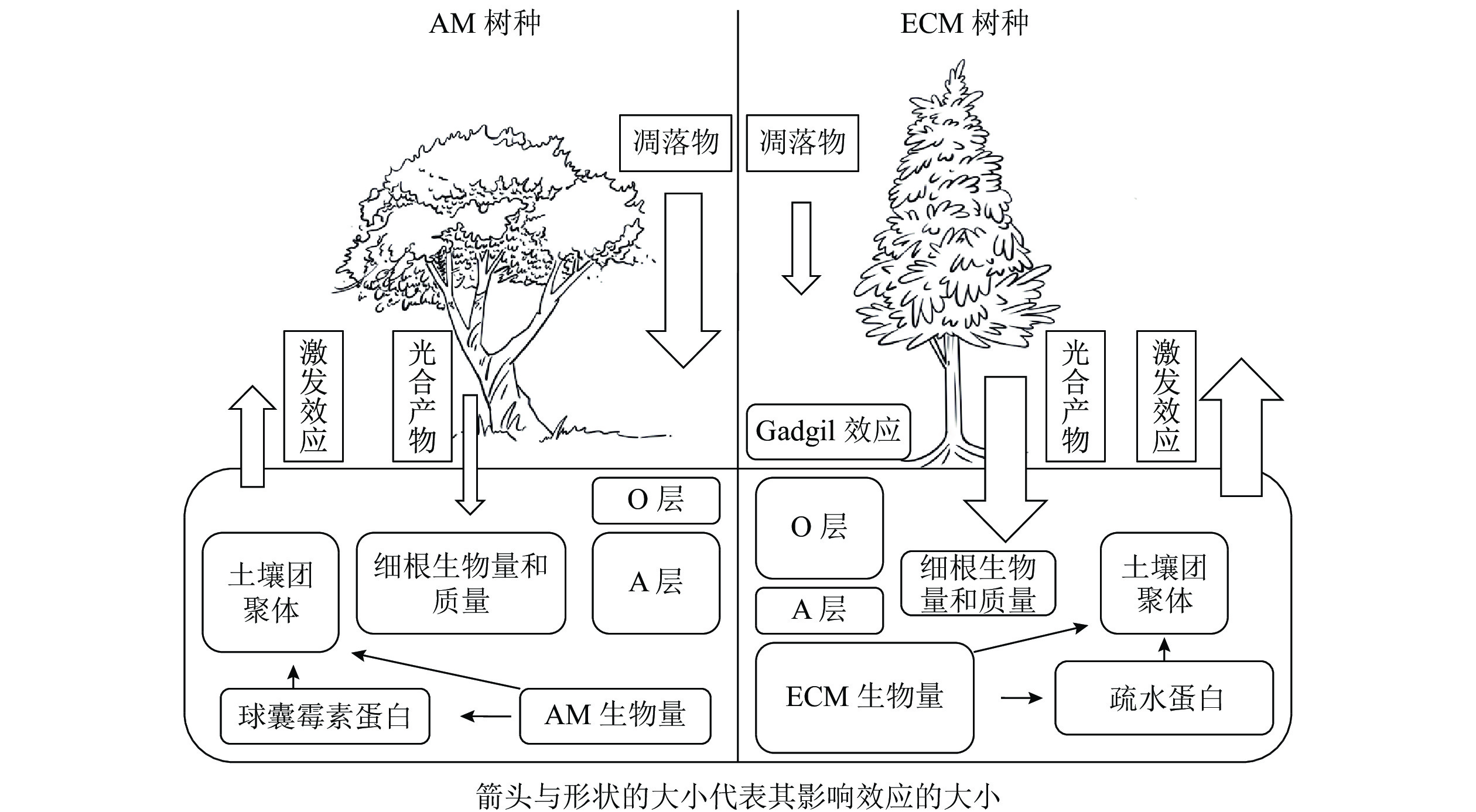
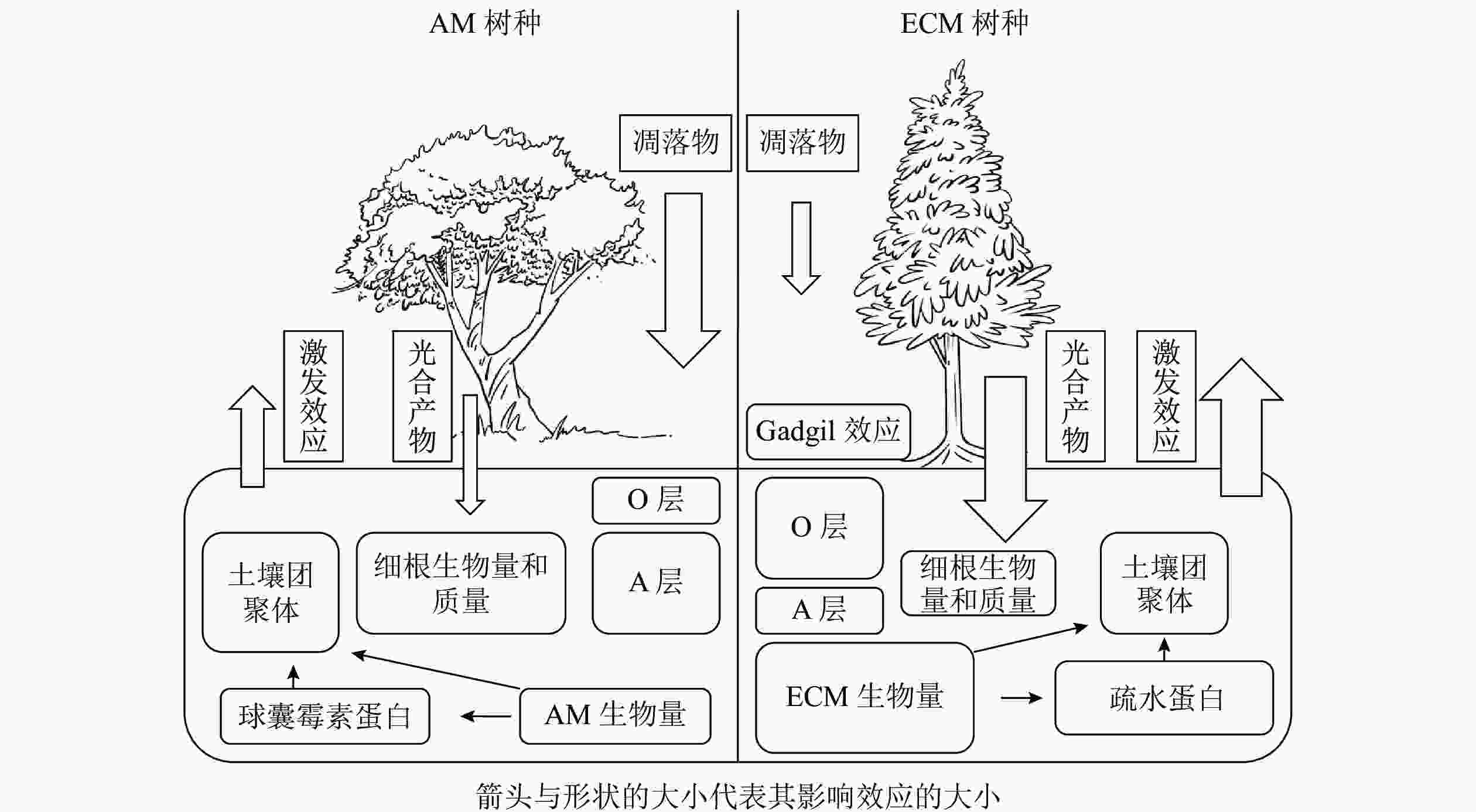
植物主要通过光合产物的分配以及凋落物的输入来影响土壤碳循环过程。一般来说,菌根真菌主要由宿主提供碳水化合物以完成其整个生命周期[2],并作为一种重要载体将碳从植物运输到土壤中。不同菌根类型宿主给菌根提供碳水化合物的数量存在一定差异,ECM宿主通常可分配更多的碳水化合物[4],因此ECM真菌通常比AM真菌具有更高的生物量,最终ECM宿主比AM宿主通过菌根释放到土壤中的碳更多[18-19]。对于AM真菌而言,宿主主要通过脂肪酸的形式提供碳源[20-21]。由于AM真菌无法分泌脂肪酸合成酶,因此不能在缺乏宿主的情况下合成脂肪酸,这也是目前其作为专性营养生物的一种解释[22]。脂质主要以三酰甘油、棕榈酸等脂肪酸的形式存储碳,广泛参与AM共生体的生命过程[23],是孢子(脂质占总干质量的95%)、根外菌丝(47%)、囊泡(58%)等结构的重要成分[24],但是目前碳以脂质形式从宿主转移到菌根真菌对土壤碳库的贡献尚未定量计算。因此,不同菌根类型宿主对于光合产物分配的差异会对土壤碳循环过程造成影响,其中菌根真菌起着重要的调控作用。不同菌根类型宿主除向菌根分配光合产物存在差异外,在凋落物输入量上也有显著差异,进而对土壤碳循环过程产生不同的影响。以AM为主导的生态系统一般具有更高的初级生产力,比以ECM占优势的生态系统产生更多的凋落物[14, 25-28]。但也有研究表明:两者的凋落物输入量差异并不显著[29-30]。ECM和AM宿主的根系特性差异也会显著影响地下凋落物输入量。由于ECM真菌的菌丝与幼根形成共生体,在植物根外形成菌丝套,在细胞间形成哈氏网结构[31],一定程度上代替了细根的作用,导致细根生物量降低。WITHINGTON等[32]发现:AM宿主细根生物量显著高于ECM宿主,且细根周转迅速。有研究也同样发现:AM宿主的细根质量显著高于ECM宿主[33-34]。但也有研究发现:AM和ECM宿主细根的生物量和质量差异并不显著[35-36]。因此,不同菌根类型地上和地下凋落物输入量差异尚待进一步研究。
-
菌根真菌的根外菌丝本身就是土壤中重要的碳源,在根外菌丝生物量上通常认为ECM真菌比AM真菌高出1个数量级[4, 15]。据估算,ECM真菌根外菌丝占地下碳库总输入量的50%~60%,可能超过树叶凋落物及细根周转的量[4, 25, 37-38]。相对于AM真菌,ECM真菌的菌丝死亡后产生更多次生代谢物,如几丁质、黑色素等,更难以被分解[7]。因此,ECM真菌根外菌丝对于土壤碳汇的贡献大于AM真菌。尽管AM真菌的生物量较低,但它对土壤有机碳输入的贡献可能是巨大的[39]。①AM真菌的宿主植物超过20万种[40],相较于ECM宿主而言更加丰富。②AM真菌生物量最高可占微生物生物量的20%以上[6, 20, 41-42],并且周转迅速(平均5~6 d),这会导致大量的菌丝残体(细胞壁等)产生并积累,并且远离微生物活动强烈的根际区域[20],形成的有机碳不易分解。③AM真菌衰亡和降解后释放到土壤中的球囊霉素相关土壤蛋白(glomalin-related soil protein,GRSP)在不同的生态系统中可占土壤总有机碳的3%~12%[6, 10, 43-44],也是一类重要的土壤有机碳来源。由此可见,ECM和AM真菌生物量是土壤有机碳输入的重要驱动因素之一。
-
土壤有机碳重要稳定机制之一是团聚体的物理保护作用所导致的生物与有机碳的空间隔离[44-45]。菌根真菌对于土壤团聚体的促进作用可以用“黏袋”(sticky-string-bag)机制来解释,即菌丝通过缠绕和胶结土壤颗粒形成土壤团聚体以减少有机碳分解[20, 46]。在根系对团聚体形成的贡献方面,由于AM宿主细根生物量显著高于ECM宿主,所以AM宿主的细根在促进土壤团聚体形成过程中具有更大的贡献。根外菌丝也可以起到类似于根系缠绕的作用[47],ECM根外菌丝生物量显著高于AM,因此ECM菌丝对土壤团聚体形成的贡献可能更大。相较于AM优势系统,ECM优势系统土壤中有机氮(特别是可溶性有机氮)含量较高,导致其土壤微生物群落中真菌占主导地位,而真菌对于土壤中大团聚体(粒径>250 μm)形成的作用可能更大[48],因此,ECM优势系统中微生物对大团聚体的贡献可能更高。除菌丝缠绕外,胶黏剂在土壤团聚体形成过程中的作用也不可忽视。AM真菌产生的球囊霉素(GRSP)可在土壤中维持6~42 a,能够作为胶结剂直接影响土壤团聚体的形成和稳定[43, 47-48]。研究表明:土壤团聚体稳定性与GRSP含量呈显著正相关[43, 49],所以AM真菌对土壤团聚体的形成可能在时间尺度上具有更深远的影响,但是目前具体有多少GRSP来自AM真菌尚不清楚[50]。除此之外,ECM真菌也可以通过产生疏水蛋白影响土壤团聚过程[47]。
-
AM和ECM宿主凋落物在化学性质上的差异导致其分解速率不同[51],从而对土壤碳循环产生影响。有研究表明:ECM凋落物碳氮比较高,其质量相对较低[52-53],ECM宿主凋落物比AM宿主凋落物的分解速率慢1倍以上[29-30],ECM优势系统土壤表层会有更多凋落物碳积累[54]。目前一般认为AM宿主的凋落物分解过程中微生物受到氮限制的影响较小,因此AM占优势的生态系统中通常含有较多的腐殖质,而ECM占优势的生态系统中有较多半分解的凋落物积累[4, 14, 55]。与AM优势生态系统相比,ECM优势生态系统土壤有机氮占比较大,宿主需要ECM真菌分泌胞外酶来矿化有机氮。菌根真菌与腐生微生物相互作用可以抑制凋落物的分解,这种抑制效应被称为“切换(gadgil)效应”[12]。目前被广泛接受的机制是养分竞争假说,即认为ECM真菌可以分泌大量的氧化酶降解有机物,使ECM真菌可以直接利用土壤中的有机氮(如甲壳素、蛋白质等),进而降低残留凋落物的碳氮比;这使得土壤中菌根真菌和其他微生物分解者直接竞争有机氮,从而加剧了微生物分解者的氮限制,抑制了凋落物的降解[14, 55]。但是在ECM森林中,“切换效应”的大小也不是固定的,还受土壤肥力、气候等因素影响[56]。对于土壤肥力相对高的区域,由于氮相对丰富,缓解了微生物氮限制,减轻了“切换效应”[57]。气候的影响体现在温度和水分的变化,氧化酶(主要由ECM真菌分泌)比水解酶(主要由自由分解者分泌)需要相对更多的能量,因此氧化酶对温度的变化可能更加敏感,从而影响“切换效应”的大小[58]。
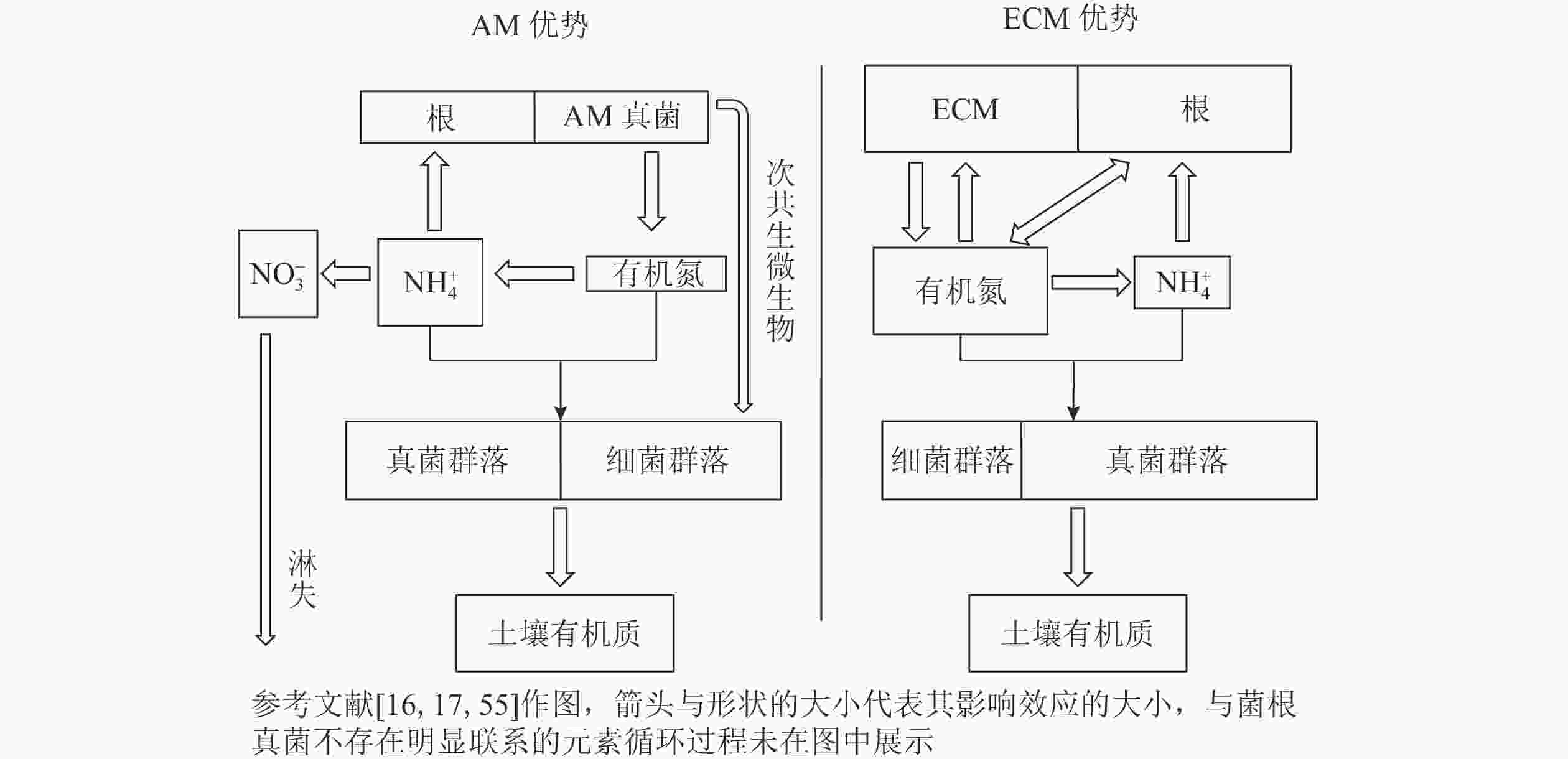
不同菌根真菌类型及其与土壤微生物之间的相互作用对土壤养分循环的影响具有差异,从而影响土壤有机碳的矿化[15, 59]。相比于ECM真菌,AM真菌优势系统凋落物中含有相对丰富的氮源,受到氮限制的影响较小。因而AM优势系统具有更快的碳循环速率,从而具有相对较薄的枯枝落叶层(O层)和较厚的富含腐殖质的腐殖质层(A层)。另外,目前大部分研究都认为,AM真菌会促进凋落物分解[15]。虽然有研究证明:AM真菌无法产生降解大分子所需要的酶[60],但是AM真菌可能会将不稳定碳释放到它们的菌丝际土壤中[61],从而刺激土壤微生物分解者的活性[62-63],间接促进有机质的分解[64]。因此学者对于菌根真菌和微生物的相互关系,提出了“次共生微生物假说”。AM真菌刺激微生物活性,可能针对的是一类具有特异性的微生物类群,两者之间形成一种次生共生关系[16]。有研究表明:特异性微生物联合AM真菌,负责分解菌丝际有机质,以获取能量和养分,与此同时AM真菌的菌丝可以吸收释放到土壤溶液中的矿质养分[61],两者形成一种类似互惠共生的关系。如DRIGO等[17]利用稳定同位素的方法,发现AM从植物获得碳源后,优先分配给放线菌和芽孢杆菌,因此AM在植物向微生物群落释放碳的过程中处于关键地位。
在某些特定的环境中,AM真菌也可能存在抑制凋落物分解的情况。一般来说,AM优势系统土壤中,无机氮的占比较高[55],无机氮虽然是植物和AM真菌获得氮源的主要形式[65-66],但是同时也是氮淋失的主要途径。如BRZOSTEK等[15]发现环割后AM优势系统凋落物分解加速,认为尽管AM优势系统土壤氮丰富,但并不是所有的氮都可以被菌根或土壤微生物所利用,也可能通过淋溶损失,而AM真菌菌丝对土壤速效养分的“搜刮”造成了AM真菌和土壤微生物的养分竞争,从而抑制了分解过程。此外,AM真菌与土壤微生物也会在凋落物降解过程中竞争磷。有研究表明:AM真菌侵染率与磷尤其是速效磷含量呈负相关[67],并且在养分充足时会转向寄生生长[68],因此在贫瘠的土壤中可能会存在AM真菌与其他微生物竞争磷。综上所述,土壤中氮、磷等养分成分的比例对AM真菌介导的有机质分解过程有显著影响。
-
植物根系以及共生真菌向周围土壤中分泌活性有机碳会引起根际激发效应,这是植物适应土壤养分状况的重要机制[11, 69-70]。SULMAN等[71]发现:ECM真菌能提升根系分泌物的分泌速率,使得其对有机质分解的激发效应大于AM真菌。研究发现:ECM真菌可以通过给菌根际微生物供应有机碳从而激发有机质的微生物分解[15, 70, 72]。ECM宿主凋落物分解速率较低[14, 55],导致土壤有效氮含量降低,为了适应这种低有效氮环境,ECM可能通过加大其根系分泌物量以激发有机质的矿化过程。因此,相比于AM,ECM宿主在微生物生物量、无机氮含量和氮循环速率上具有更强烈的根际效应[73]。ECM真菌死亡后的残体可能也是激发效应的一个重要来源,它与菌丝分泌的活性有机质有类似的效果,可以刺激土壤中的微生物分解者[74]。因为生物量的差异,ECM菌丝周转对激发效应的贡献可能也大于AM菌丝。目前多数研究采用挖壕、环割等方法研究ECM真菌对土壤微生物及有机质降解的影响,切断了根系对水分的吸收,这可能会增加土壤水分并加速土壤有机质分解,并且新产生的根系和ECM菌丝凋落物可能会促进有机碳的分解。尽管这些研究结果还存在一定争议,但是目前大部分的研究都认为ECM真菌对激发效应的贡献大于AM真菌。关于AM真菌对土壤有机质分解是否具有激发效应目前仍有争议。有研究表明:AM真菌能促进土壤自持有机质的矿化[11, 62, 75],但SHAHZAD等[76]发现:根系分泌物、地下凋落物是植物诱导的激发效应的主要贡献者,而AM真菌没有任何直接影响。有研究发现:AM优势系统土壤中通常含有更多与矿物结合态有机质[54, 77],这对微生物主导的激发效应有缓冲作用[29, 55, 78-79]。今后,关于AM真菌是否存在激发效应及其正负方向还需进一步研究。
-
除光合作用外,土壤呼吸是生态系统中第二大碳通量[80]。早先的研究将菌根呼吸和宿主根系呼吸作为同一个呼吸来源,在很大程度上忽略了菌根呼吸对土壤呼吸的贡献[81]。有研究表明:有4%~6%的光合产物在21 h内通过菌根呼吸的形式释放[82]。AM和ECM在同一条件下对土壤呼吸贡献的研究并不多。在AM优势生态系统中,利用根际微宇宙同位素标记等方法观察到AM真菌呼吸占土壤呼吸的8%~14%[83-85]。在ECM优势系统中,ECM呼吸为1.3 mg·hm−2·a−1,此时的根系呼吸为2.8 mg·hm−2·a−1[86],这可能超过了宿主对于构建ECM生物量的碳分配[87]。也有研究表明:ECM对于土壤呼吸的贡献可能较小[86, 88]。但是目前菌根呼吸的研究对象多是单一类型的菌根,关于不同菌根对于土壤呼吸的影响的比较研究还较少。
-
一半以上土壤有机碳来自土壤微生物,主要以代谢物和残留物的形式存在[3]。AM真菌无法直接利用土壤中有机养分,而是偏向于“无机”营养模式以及具有快速的有机质周转速率[55],有学者提出了关于菌根真菌对土壤碳积累贡献的假说,即“微生物周转-底物稳定(microbial efficiency-matrix stabilization)”假说[54]。与ECM优势系统相比,AM优势系统通常具有高养分和高质量的凋落物,土壤微生物利用效率高,有利于微生物残体碳的积累,并且微生物残体碳容易受到黏土矿物的物理保护而稳定存在[89]。根据这个假说预测,AM优势系统土壤的微生物源碳储量可能高于ECM优势系统土壤。与AM宿主相比,ECM宿主可能保持相对封闭的“有机”营养循环模式[55],以促进具有特定氮降解酶功能的微生物[90]。在ECM宿主凋落物累积情况下,大多数土壤氮库中有机氮占比较大,与之相对应可能是一个由真菌(包括ECM真菌)主导的土壤微生物群落[55],这将限制微生物对氮的利用效率(图1)。因此,有学者提出了“缓慢分解(slow decay)”假说,即ECM优势系统中的营养循环相对封闭以及具有较慢的凋落物分解速率,植物源有机碳在土壤中的积累相对容易[77]。基于这个假说以及模型估算,ECM优势系统土壤每单位土壤氮储存的碳是AM优势系统土壤的1.7倍[14]。因此,这种假说预测ECM占优势的生态系统储存在土壤中的有机碳更多。事实上,以上2种假说的机制可能都存在于森林生态系统中。“缓慢分解”假说认为:有机碳在土壤表面缓慢分解,因此可能在土壤表层中储存更多的有机碳;而“微生物周转-底物稳定”假说认为:有机碳在表层土壤中有较快的降解速率,同时也会更有利于有机碳在土壤深层中的积累[54]。
-
陆地生态系统中菌根类型及其群落组成是碳循环过程和方向的重要驱动因素[14, 15, 91](图2),阐明菌根类型演替对土壤碳循环的影响及其调控机制已成为亟待解决的重要科学问题,对于丰富生态系统碳循环过程理论、预测生态系统演替对全球气候变化的影响等具有重要意义。虽然菌根真菌对营养获取至关重要,并且可以获得很大一部分的净初级生产量,但大多数研究没有明确地将菌根真菌的碳通量包括在生态系统碳估算中[92]。不同类型菌根碳通量的差异如何,其关键控制因素是什么,至今仍不清楚。因此,今后的研究也不应仅限于菌根真菌类型的差异,由于菌根真菌群落间本身存在差异,因此单一的从菌根真菌类型来解释其在生态系统中的功能具有局限性,应该与其菌根真菌群落与丰度联系起来[93]。对于估计菌根有关碳储量,应当把菌根真菌群落组成也纳入驱动因素之中,利用高通量测序和同位素等技术,跟踪真菌群落的组成变化,有助于识别参与这个过程的特定真菌,并逐步理解到土壤碳周转中的资源分配。迄今为止,大多数关于真菌-真菌相互作用对生态系统过程的影响的研究都没有认真考虑如何将结果在空间和时间上扩大到生态系统水平。土壤中碳的周转是一个发生在广泛的时间尺度上的过程,目前绝大多数的试验都只进行了几个月或者1 a,这并不能准确反映整个过程。
当前和今后的研究工作应着眼于6个方面:①不同类型菌根真菌对土壤碳输入贡献的直接比较,以及对矿质土层碳输入的贡献的研究;②不同类型菌根对土壤有机碳稳定性的影响机制存在差异,其关键控制因素还有待于进一步研究;③不同菌根类型其激发效应的大小及方向如何调控土壤有机质的矿化过程;④不同菌根优势系统中,有机质积累的能力及其机制具体如何,是否符合“微生物周转-底物稳定”和“缓慢分解”假说;⑤增加养分有效性通常会减少植物对菌根真菌的光合产物投入[94],那么养分化学计量学的变化如何影响菌根真菌功能,继而如何影响凋落物分解仍将是今后研究的重点;⑥不同类型菌根对生态系统碳循环产生长远影响如何,菌根真菌群落是否是其关键影响因子仍有待于进一步阐明。
Research progress in the impact of different mycorrhizal types on soil carbon cycling
doi: 10.11833/j.issn.2095-0756.20210531
- Received Date: 2021-07-29
- Rev Recd Date: 2021-08-18
- Available Online: 2021-10-12
- Publish Date: 2021-10-20
-
Key words:
- ectomycorrhiza /
- arbuscular mycorrhiza /
- soil carbon cycling /
- soil aggregation /
- litter decomposition /
- priming effect
Abstract: Mycorrhiza is a bridge between plants and soil in terrestrial ecosystems, and acts on soil carbon cycling by affecting litter decomposition, soil aggregation, and root exudates. Different types of mycorrhiza have different physiological functions, among which ectomycorrhiza (ECM) and arbuscular mycorrhiza (AM) are the most widely distributed mycorrhizal types. Previous studies showed that different mycorrhizal types affected soil organic carbon input through the distribution of host photosynthetic products. The stability of soil organic carbon was affected by the differences of metabolites and winding action, and the soil organic carbon mineralization was affected by regulating the litter decomposition characteristics and interrelationship between mycorrhizae and microbe. In order to understand how ECM and AM affect soil carbon cycling and its key regulatory factors, this study reviewed the effects of different types of mycorrhiza on soil carbon cycling from four aspects and discussed the influence mechanisms: differences in the distribution process of photosynthetic products such as providing carbon and litter quantity to mycorrhizae, carbon sink functions of different mycorrhizal types and the impacts on soil aggregation, differences in soil organic carbon mineralization such as litter decomposition, priming effect and soil respiration in different dominant mycorrhizal ecosystems, and different accumulation capacity for soil carbon and corresponding microbial communities in different dominant mycorrhizal ecosystems. Finally, the future research direction is proposed, aiming to provide theoretical basis for how to enhance the carbon sink functions of ecosystems by relying on mycorrhiza in the context of “carbon neutrality”. [Ch, 2 fig. 94 ref.]
| Citation: | JIN Wenhao, SHAO Shuai, CHEN Junhui, et al. Research progress in the impact of different mycorrhizal types on soil carbon cycling[J]. Journal of Zhejiang A&F University, 2021, 38(5): 953-962. DOI: 10.11833/j.issn.2095-0756.20210531 |










 DownLoad:
DownLoad: