-
钙作为植物生长发育的重要调节因子及必需的矿质营养元素之一,不仅对维持细胞壁、细胞膜和膜结合蛋白具有一定的稳定性,而且还可作为细胞内生理生化反应的第二信使耦合外部信号[1]。钙能提高植物组织细胞的多种抗性,如植物抗寒[2]、抗旱[3]、抗病[4]、抗重金属毒害[5]及耐盐胁迫[6]等逆境。钙还具有改善植物光合作用,提高植物叶片中叶绿素含量的作用[7]。钙对增强植物叶片超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)及其他相关保护酶的活性起着重要作用[8]。植物种类不同,对钙的适宜浓度不同,钙浓度过高或过低都会影响植物的生长发育,当钙浓度适宜时才能促进植物生长发育。白枪杆Fraxinus malacophylla是木犀科Oleaceae梣属Fraxinus双子叶落叶乔木,树高约10 m,主要分布于云南和广西,是石漠化治理中优良的阔叶伴生树种[9]。其树皮灰白色,芽裸露,翅果匙形,根系发达,萌蘖力强。白枪杆不仅可以制作家具与农具,而且还具有极高的药用价值[10-12],有较好的发展前景[13]。白枪杆主要分布在中国石漠化治理重点区域,具有很强的适应性。该区域的生态治理也是国家高度重视的问题之一,土壤中钙含量是非岩溶地区的2~3倍。目前,有关白枪杆的相关研究集中在激素和肥料[13-14]、造林技术[15]和土壤水分[16-17]等方面,但关于白枪杆幼苗野外生长对环境中不同钙浓度的适应机制尚不清楚。本研究以1年生白枪杆幼苗为材料,研究了不同钙浓度对白枪杆幼苗叶片形态及生理特性的影响,以期为白枪杆培育提供科学依据。
-
研究材料为1年生白枪杆实生苗,2020年1月播种,2020年6月由云南建水移至西南林业大学树木园。炼苗15 d后植入营养钵中,营养钵上口径160 mm,下口径110 mm,高120 mm,基质配比为V(红壤)∶V(河沙)∶V(珍珠岩)=5∶3∶2,每盆种植1株幼苗。7月5日,选取长势均匀一致的苗木开始试验。于2020年7月5日至11月13日在西南林业大学格林温室(25°03′N,102°45′E)进行。温室海拔为1 904 m,温度为16~30 ℃,空气相对湿度为23%~67%,大气二氧化碳质量浓度为400~412 mg·L−1,光照充足。
-
采用单因素随机区组试验,4个施钙(氯化钙)处理分别为0 (对照)、25、50和75 mmol·L−1,每个处理10株苗,重复3次,共计120株苗。
在不同处理组每盆幼苗的根系周围浇灌50 mL不同钙浓度溶液,对照组则施加50 mL去离子水,每隔15 d施钙1次,连续施钙8次。试验期间,正常管理苗木。
-
处理120 d后,采用YMJ-C智能叶面积测量仪测定白枪杆幼苗的叶长、叶宽、叶长宽比、叶面积和叶周长。将白枪杆幼苗叶片剪碎混合进行相关生理指标测定,其中用乙醇浸提法测定叶绿素质量分数[18],用愈创木酚比色法测定POD活性[18],用氮蓝四唑光法测定SOD活性[19],用紫外吸收法测定CAT活性[19]。
-
利用Excel和SPSS进行数据整理与统计分析,利用单因素方差分析(one-way ANOVA)比较不同钙浓度处理下白枪杆幼苗叶片形态指标、叶片相关指标、叶绿素质量分数和酶活性的差异,运用LSD检验法进行多重比较;采用Graphpad Prism 8.0制图。
-
由表1可知:白枪杆幼苗的叶片形态指标随着钙浓度升高均呈先上升后下降的趋势。白枪杆幼苗的叶片数在不同钙浓度处理下与对照差异显著(P<0.05),在钙浓度为50 mmol·L−1时,叶片数达到最大值,且叶片数是对照的1.33倍。白枪杆幼苗的叶长、叶宽、叶长宽比、叶周长及叶面积在不同钙浓度处理下与对照均无显著差异(P>0.05),在钙浓度为50 mmol·L−1时,叶长、叶宽、叶长宽比、叶周长及叶面积均达到最大值,且叶长、叶宽、叶长宽比、叶周长和叶面积分别是对照的1.22、1.01、1.08、1.21和1.17倍。
钙浓度/(mmol·L−1) 叶片数/片 叶长/cm 叶宽/cm 叶长宽比 叶周长/cm 0 16.83±2.48 b 6.89±1.34 a 2.98±0.40 a 2.22±0.19 a 29.27±4.84 a 25 21.50±2.43 a 7.22±0.98 a 3.18±0.34 a 2.45±0.27 a 29.84±4.84 a 50 22.50±4.51 a 7.43±1.14 a 3.22±0.28 a 2.41±0.14 a 36.18±27.01 a 75 12.33±2.88 c 7.23±1.14 a 3.17±0.34 a 2.30±0.27 a 31.45±3.97 a 均值 18.29±5.10 7.19±1.10 3.13±0.33 2.34±0.23 31.69±5.98 钙浓度/(mmol·L−1) 叶面积/cm2 叶面积指数 叶生物量/g 比叶长/(cm·g−1) 比叶面积/(cm2·g−1) 0 184.36±68.61 a 0.92±0.34 a 1.09±0.34 a 4.90±1.14 b 123.07±47.71 b 25 192.52±66.84 a 0.96±0.33 a 1.17±0.50 a 7.41±4.31 b 216.69±162.82 b 50 216.71±99.31 a 1.08±0.49 a 1.53±0.33 a 7.49±3.71 b 235.38±203.47 b 75 164.78±35.31 a 0.82±0.18 a 0.82±0.26 b 23.82±15.39 a 554.86±361.36 a 均值 189.59±69.01 0.94±0.34 1.06±0.53 10.90±10.86 282.50±267.09 说明:数据为平均值±标准差,同列不同字母表示同一指标在不同钙浓度间差异显著(P<0.05) Table 1. Change of leaf shape determination result of F. malacophylla seedling under different calcium concentrations
白枪杆幼苗叶片生长发育过程中,不同钙浓度对白枪杆幼苗叶片的叶面积指数、叶生物量、比叶长、比叶面积有不同的影响。白枪杆幼苗在不同钙浓度处理间幼苗叶面积指数差异不显著(P>0.05),随着钙浓度的升高呈先上升后下降的趋势,当钙浓度为50 mmol·L−1时,叶面积指数达到最大值,且叶面积指数是对照的1.17倍;当钙浓度为75 mmol·L−1时,白枪杆幼苗叶生物量与其他3个处理有显著差异(P<0.05),随着钙浓度的升高呈先上升后下降的趋势。当钙浓度为50 mmol·L−1时,叶生物量达到最大值;当钙浓度为75 mmol·L−1时,白枪杆幼苗比叶长及比叶面积与其他3个处理差异显著(P<0.05),随着钙浓度的升高而升高,均在钙浓度为75 mmol·L−1时达到最大值。
-
由表2可知:白枪杆幼苗不同钙浓度处理间叶片叶绿素质量分数差异显著(P<0.05),随着钙浓度的增加,白枪杆幼苗叶片叶绿素质量分数均呈先上升后下降的趋势,均在钙浓度为50 mmol·L−1处理时达到最大值,其中,叶绿素a为(2.14±0.32) mg·g−1,叶绿素b为(0.62±0.10) mg·g−1,叶绿素(a+b)为(2.77±0.42) mg·g−1,叶绿素a/b为3.44±0.10,与对照相比,增幅分别为32.92%、21.57%、30.66%和8.52%。当钙浓度为50 mmol·L−1时,对白枪杆幼苗叶片的叶绿素质量分数有促进作用。
钙浓度/(mmol·L−1) 叶绿素a/(mg·g−1) 叶绿素b/(mg·g−1) 叶绿素(a+b)/(mg·g−1) 叶绿素a/b 0 1.61±0.11 c 0.51±0.05 b 2.12±0.12 c 3.17±0.35 b 25 1.91±0.34 b 0.59±0.09 a 2.53±0.43 ab 3.25±0.11 b 50 2.14±0.32 a 0.62±0.10 a 2.77±0.42 a 3.44±0.10 a 75 1.80±0.28 b 0.56±0.08 b 2.36±0.36 b 3.22±0.13 b 说明:数据为平均值±标准差,同列不同字母表示同一指标在不同钙浓度间差异显著(P<0.05) Table 2. Change of chlorophyll content determination of F. malacophylla seedling under different calcium concentrations
-
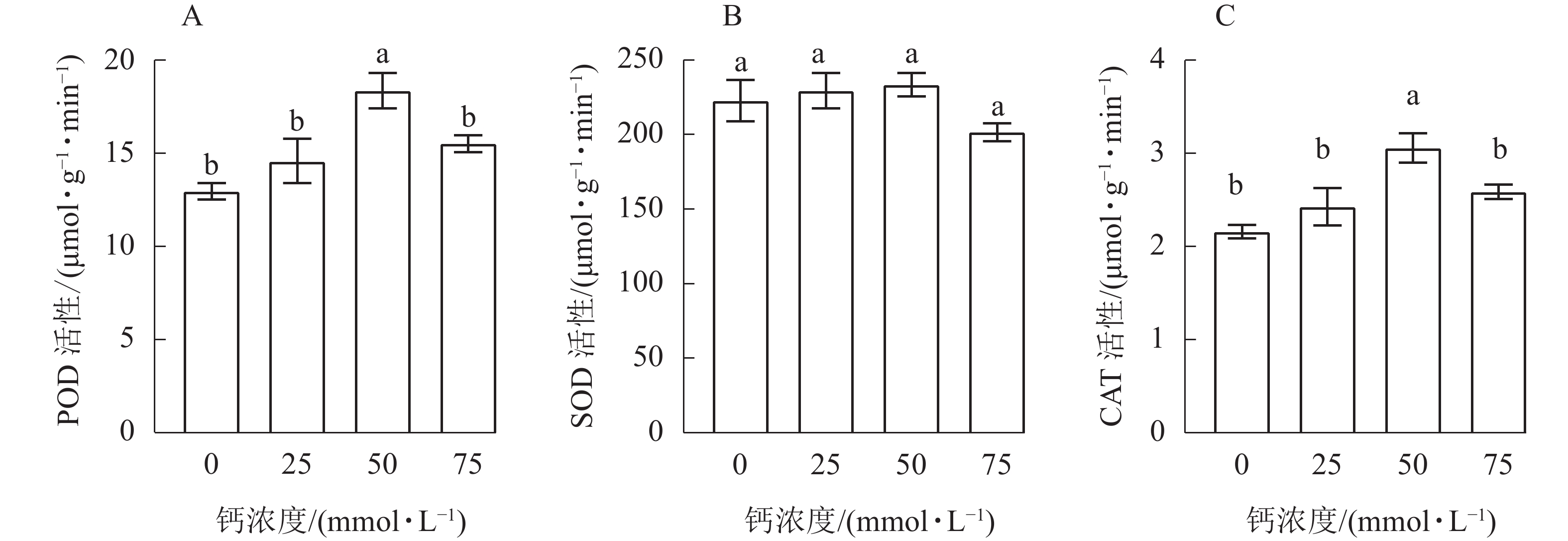
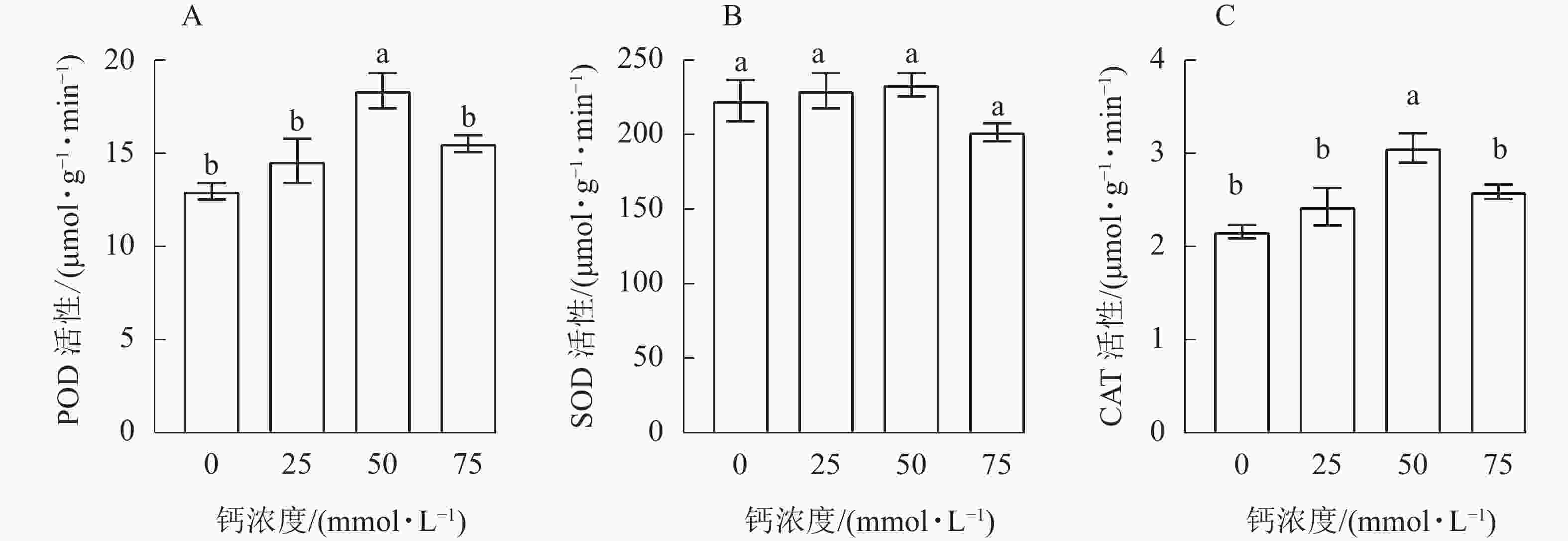
由图1A可知:当钙浓度为50 mmol·L−1时,白枪杆幼苗POD活性与其他处理差异显著(P<0.05),随着钙浓度的增加,白枪杆幼苗叶片中POD活性呈先增加后下降的趋势,施钙处理的POD活性均高于对照。与对照相比,20、50和75 mmol·L−1钙浓度处理的白枪杆幼苗叶片中的POD活性分别增加了12.42%、41.56%和19.63%。当钙浓度为50 mmol·L−1时,白枪杆幼苗叶片中POD活性最强。
-
由图1B可知:白枪幼苗不同钙浓度处理间SOD的活性差异不显著(P>0.05)。随着钙浓度的增加,白枪杆幼苗叶片中SOD活性呈先增加后下降的趋势。在钙浓度为75 mmol·L−1时,SOD活性最低。与50 mmol·L−1钙浓度处理相比,对照、25和75 mmol·L−1钙浓度处理下白枪杆幼苗叶片SOD活性分别降低了4.56%、1.69%和13.56%。当钙浓度为50 mmol·L−1时,白枪杆幼苗叶片中的SOD活性最强。
-
由图1C可知:随着钙浓度的增加,白枪杆幼苗CAT活性呈先增加后下降的趋势,50 mmol·L−1钙浓度处理与对照差异显著(P<0.05)。在4个处理中,50 mmol·L−1钙浓度处理的CAT活性最高,对照的CAT活性最低。钙浓度过高会抑制白枪杆幼苗CAT活性的增加,但施钙处理CAT活性均高于对照。因此,施钙处理能有效提高白枪杆幼苗叶片的CAT活性,在钙浓度为50 mmol·L−1时,白枪杆幼苗CAT活性最强。
-
在植物生长发育过程中需要相当数量的钙才能维持正常生长,土壤环境中钙较多的石漠化区域,植物亦经常受到钙胁迫抑制。部分植物通过减少叶片数、叶长、叶宽和叶生物量等来适应胁迫下的生长[20]。吴朝波等[8]研究表明:钙浓度过高会抑制槟榔Areca catechu幼苗生长。李香君等[21]研究发现:钙浓度过低或过高会抑制沙地樟子松Pinus sylvestris var. mongolica幼苗的生长,适宜的钙浓度可促进沙地樟子松幼苗生长发育。本研究表明:随着钙浓度的升高,白枪杆幼苗的叶片数、叶长、叶宽和叶面积等叶片形态指标均呈先增加后减少的趋势,当钙浓度为50 mmol·L−1时,白枪杆幼苗的叶片数、叶长、叶宽、叶面积、叶周长和叶生物量等达到最大值。当钙浓度为75 mmol·L−1时,对白枪杆幼苗的叶片形态及相关指标有抑制作用。综上所述,适宜的钙浓度可以促进白枪杆幼苗的生长,而钙浓度过高会抑制白枪杆幼苗的生长。
植物的叶面积直接影响着植物对光和碳的获取能力,比叶面积则反映不同植物获取资源的能力及对生存环境的适应性。江志标等[20]研究表明:比叶面积小对干旱贫瘠的地方适应能力较强,比叶面积大则获取资源和保持体内营养的能力越强。本研究表明:随着钙浓度的增加,叶面积呈先增加后减少的趋势。中、低钙浓度对白枪杆幼苗的生长有促进作用,当钙浓度过高时,白枪杆幼苗会通过减少叶片面积来适宜高钙环境。白枪杆幼苗叶片的比叶面积随着钙浓度的升高而增大,说明白枪杆幼苗在不同钙浓度处理下获取资源和保持体内营养的能力增强。
植物中的叶绿素则是光合作用的物质基础及重要色素[22],因此,叶绿素质量分数的高低在一定程度上反映了植物光合能力的强弱。孙悦[23]研究表明:钙浓度的增加会使油松Pinus tabuliformis幼苗叶片中叶绿素质量分数呈先增加后减少的趋势。李香君等[21]表明:钙浓度的增加会降低槟榔叶片中的叶绿素质量分数。本研究表明:随着钙浓度的增加,白枪杆幼苗叶片中叶绿素质量分数均呈先升高后降低的趋势。所以,适宜的钙浓度可以提高植物叶片中的叶绿素a、叶绿素b及叶绿素(a+b),过高的钙浓度可以抑制白枪杆幼苗中的叶绿素质量分数的增加。
钙在植物细胞中可以活化多种酶,还可以维持植物细胞生理平衡。SOD活性是鉴定植物抗逆性的重要生理生化指标,主要功能是将超氧阴离子自由基(O2 −)转化为双氧水(H2O2)和氧气(O2)[24]。POD和CAT是防止细胞内氧化的保护酶,主要功能是去除植物体内的过氧化物和H2O2,从而减少对细胞膜的损伤[25-27]。因此,SOD、POD和CAT的平衡有助于清除植物中的活性氧物种并维持细胞功能[28]。吴朝波等[8]、孙悦[23]研究发现:钙浓度过高会抑制植物体内酶活性。在本研究中,POD、SOD和CAT活性随钙浓度的增加先升高后降低;当钙浓度≤50 mmol·L−1时,白枪杆幼苗叶片的POD、SOD和CAT活性升高。当钙浓度>50 mmol·L−1时,白枪杆幼苗叶片的POD、SOD和CAT活性降低,说明钙浓度过高抑制了的POD、SOD和CAT活性的提高。白枪杆幼苗在25和50 mmol·L−1钙浓度处理下,酶活性均随钙浓度的增加而增加,表现出协同增效作用,可以维持细胞活性氧平衡。当钙浓度达75 mmol·L−1时,3种酶活性不同程度降低,表明超出了白枪杆的耐盐能力,植株体内活性氧过多对保护酶造成损害,导致酶活性降低,造成活性氧清除能力降低,抑制白枪杆幼苗生长。
-
钙浓度会影响白枪杆幼苗叶片的形态指标、叶绿素质量分数和酶活性,白枪杆的叶片形态指标除比叶长和比叶面积随钙浓度的增加而增加外,其余相关指标均呈先上升后下降的趋势,白枪杆幼苗的叶绿素质量分数、酶活性随着钙浓度的升高均呈先增加后降低的趋势。当钙浓度在50 mmol·L−1时,对白枪杆幼苗的叶片生长发育有一定促进作用,提高了白枪杆幼苗叶片中叶绿素质量分数,增加了白枪杆幼苗叶片中的POD、SOD、CAT活性。中、低钙浓度对白枪杆幼苗生长有不同程度的促进作用,说明白枪杆对中、低钙浓度处理具有一定的喜适性。
Response of leaf morphological and physiological traits of Fraxinus malacophylla seedlings to calcium
doi: 10.11833/j.issn.2095-0756.20210597
- Received Date: 2021-08-26
- Accepted Date: 2022-04-01
- Rev Recd Date: 2022-03-12
- Available Online: 2022-07-20
- Publish Date: 2022-08-20
-
Key words:
- Fraxinus malacophylla /
- calcium /
- leaf shape /
- physiological characteristics
Abstract:
| Citation: | ZHANG Mei, DONG Qiong, DUAN Huachao, et al. Response of leaf morphological and physiological traits of Fraxinus malacophylla seedlings to calcium[J]. Journal of Zhejiang A&F University, 2022, 39(4): 845-851. DOI: 10.11833/j.issn.2095-0756.20210597 |









 DownLoad:
DownLoad: