-
四照花Cornus spp.为山茱萸科Cornaceae四照花属Cornus常绿或落叶小乔木或灌木,花序外有2对黄白色花瓣状大型苞片[1]。四照花主要包括东亚和北美2个大类群[2]。由于北美类群具有极高的观赏价值,已被大量引种栽培和推广应用,但成活率不高、抗病性弱[3]。因此,对东亚类群的资源开发和引种栽培显得尤为重要。其中,作为东亚类群的东京四照花Cornus hongkongensis subsp. tonkinensis因分布广、适应性强和独特观赏价值而备受关注[4−6];其种内不仅变异丰富,而且具有耐盐、耐高温等抗性[7−8]。东京四照花也称西南四照花,为常绿小乔木或灌木,主要分布于中国、日本和越南等东亚地区,在中国主要分布于贵州、四川、广西及云南等地,常发现于海拔900~2300 m的森林中。目前,东京四照花种群数量有限,扩大繁殖加快其推广和应用已显得尤为重要。然而东京四照花扦插育苗中存在扦插生根困难、成活率低等较多问题。
树木插穗生根不仅受内在因素影响,如树种种类、插穗年龄和位置效应、插穗的养分状况[9−12]等,还受到扦插基质、环境因素和生长阶段[13−15]等外在因素的影响。插穗的酶活性、营养物质与生根密切相关,如过氧化物酶(POD)和超氧化物歧化酶(SOD)具有清除超氧自由基的作用,从而增强插穗对逆境的抵抗能力[16],且POD与不定根的诱导和生长密切相关[17];多酚氧化酶(PPO)通过催化酚类物质与吲哚乙酸(IAA)形成IAA-酚酸复合物,从而促进根原基的诱导和不定根的形成[18]。鉴于此,本研究以4年生东京四照花母树的半木质化嫩枝为插穗材料,测定不同处理插穗的生根率、生根数量和根长等变化,观察不同时期插穗茎段营养物质质量分数和酶活性变化,旨在建立东京四照花繁殖体系,并探究生根的生理机制。
-
扦插样地设在江苏农林职业技术学院内(31°14′~33°38′N,118°23′~119°24′E),该区属北亚热带中部气候区,扦插池室温控制在35~38 ℃,空气相对湿度保持在80%~85%。
-
插穗采自于江苏农林职业技术学院试验地的4年生东京四照花实生苗。采集健康饱满的枝条,选择带有2对芽的插穗,剪制为直径0.35~0.55 cm、长度8.00~12.00 cm的枝条;插穗上切口平剪,下切口斜剪成45°~50°的斜面,上下切口离芽距离均2.00~3.00 cm;保留插穗上部1~2片叶,叶片保留1/3~1/2大小。
-
包括调节剂种类(A)、调节剂质量浓度(B)及浸泡时间(C) 3个因素,每个因素设3个水平,采用正交L9(34)试验设计;以清水处理为对照(ck),共10个处理(表1)。2021年7月12日进行扦插;扦插基质配比为V(泥炭土)∶V(蛭石)∶V(珍珠岩)=2∶2∶1。
处理 因素水平 处理 因素水平 A B C A B C ck 0 0 0 A2B2C3 2 2 3 A1B1C1 1 1 1 A2B3C1 2 3 1 A1B2C2 1 2 2 A3B1C3 3 1 3 A1B3C3 1 3 3 A3B2C1 3 2 1 A2B1C2 2 1 2 A3B3C2 3 3 2 说明:A1~3分别是生根粉1号(ABT-1)、萘乙酸 (NAA) 和吲哚乙酸 (IAA);B1~3分别是100、300 和 500 mg·L−1;C1~3分别是浸泡30、60和180 min。 Table 1. Orthogonal test design for hormone treatment L9 (34)
-
共设置4种扦插基质,分别为:蛭石(S1)、河沙(S2)、V(泥炭土)∶V(蛭石):V(珍珠岩)=2∶2∶1(S3)和V(泥炭土)∶V(黄土)=1∶1(S4)。于2021年8月15日进行试验,插穗用A3B2C1处理。
-
于2021年选取生长初期(7月12日)、生长中期(8月15日)、生长末期(9月1日) 3个生长阶段,以S3为扦插基质,插穗用A3B2C1处理。
上述3个试验均以当年半木质化枝条为扦插材料,每处理3次重复,每重复30根插穗。
-
以当年生半木质化枝条为扦插材料,于2021年7月12日选用S3为扦插基质,插穗用A3B2C1处理,以清水处理为对照(ck)。每重复150根插穗,重复3次。根据预试验观察到的插穗的动态变化,将生根过程分为5个时期:扦插前(0 d)、芽萌动阶段(10 d)、插穗基部膨大阶段(20 d)、不定根发生阶段(30 d)、不定根大量发生阶段(40 d)。每个阶段从各重复中取15根插穗,剥取插穗基部1~3 cm处韧皮部材料,保存在−80 ℃冰箱中用于生理指标测定。
-
扦插株行距为10 cm×15 cm,深度为插穗长度的1/3~1/2。扦插后浇透水,并覆盖透光率为50%的遮光网,每2周用600倍液多菌灵喷洒消毒,及时清除杂草。采用自动喷雾系统保持适宜的基质含水量和空气湿度。
-
扦插60 d后统计生根率(%)、不定根数(条);测量不定根长(cm),计算平均根长(cm)和生根指数,生根指数=生根率×平均根数×平均根长。
-
采用蒽酮-硫酸比色法测定可溶性糖和淀粉,采用G-250考马斯亮蓝法测定可溶性蛋白;采用愈创木酚法测定POD活性,采用邻苯二酚法测定PPO活性,采用氮蓝四唑(NBT)法测定SOD活性[19]。
-
利用Excel 2017和SPSS 19.0统计分析数据,并进行差异显著性分析和Duncan多重比较。
-
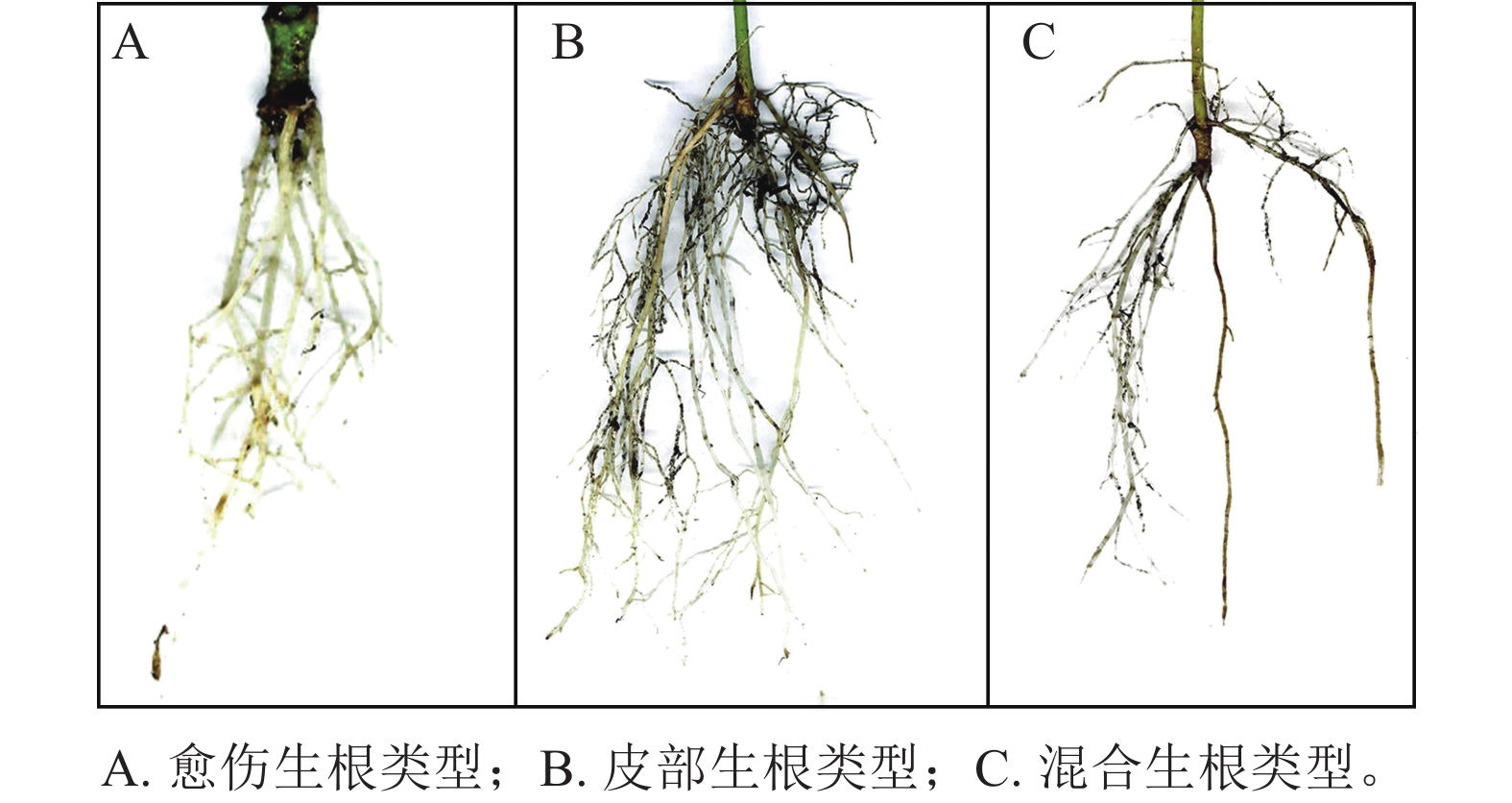
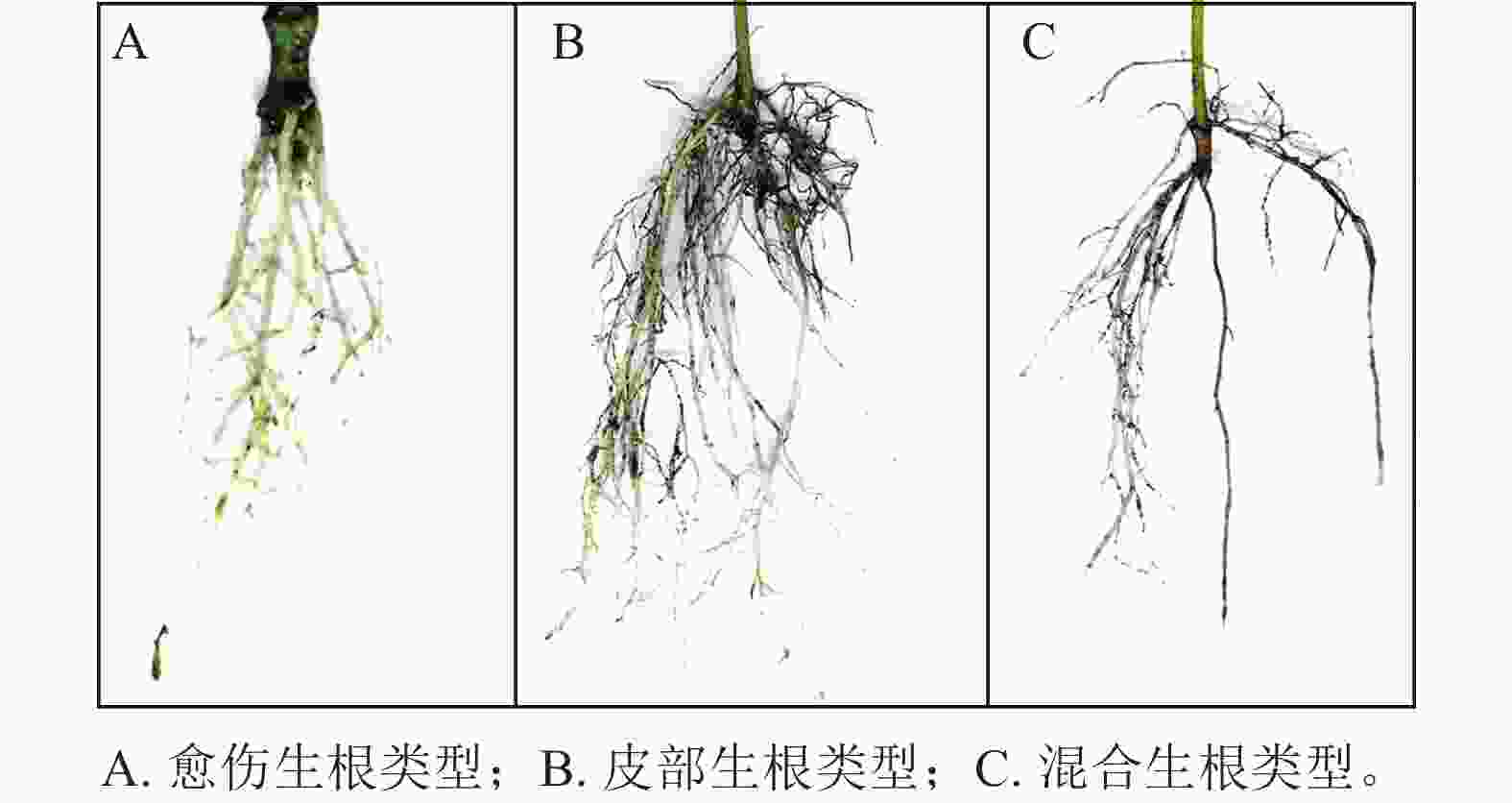
根据插穗的外部形态观察(图1),东京四照花嫩枝插穗的愈伤组织部位和皮部均可以产生不定根,其中以皮部生根类型为主,占生根数的58.6%;混合生根类型次之,占生根数的30.1%;愈伤生根比例最少,仅为11.3%。
-
方差分析(表2)表明:植物生长调节剂种类和浸泡时间对生根率的影响都达到了显著水平(P<0.05);相比较而言,植物生长调节剂种类对生根率的影响大于浸泡时间,而植物生长调节剂质量浓度对生根率无显著影响。浸泡时间对不定根数的影响差异极显著(P<0.01),而植物生长调节剂种类和质量浓度对不定根数的影响差异不显著。浸泡时间对生根指数的影响差异极显著(P<0.01),而植物生长调节剂种类和质量浓度对生根指数的影响差异不显著。尽管植物生长调节剂种类、质量浓度和浸泡时间对最长根长和平均根长有一定影响,但差异不显著。
指标 方差来源 平方和 均方 F P 指标 方差来源 平方和 均方 F P 生根率 A 1 513.702 756.851 4.735 0.021 C 3.384 1.692 0.808 0.458 B 1 097.004 548.502 3.432 0.052 C 1 207.954 603.977 3.779 0.041 平均根长 A 0.736 0.368 0.275 0.762 B 0.148 0.074 0.054 0.947 不定根数 A 0.178 0.089 0.032 0.968 C 1.844 0.922 0.713 0.500 B 4.038 2.019 0.776 0.472 C 28.877 14.438 9.209 0.001 生根指数 A 39.233 19.616 0.823 0.453 B 26.276 13.138 0.551 0.585 最长根长 A 0.114 0.072 0.032 0.968 C 400.006 200.003 8.391 0.002 B 0.473 0.236 0.107 0.899 说明:表中各项指标自由度均为2。A. 调节剂种类;B. 调节剂质量浓度;C. 浸泡时间。 Table 2. Variance analysis of different factors on rooting indexes of softwood cutting on C. hongdongensis supsp. tonkinensis
从表3可以看出:A3B2C1处理的生根率最高,达77.78%,而A2B2C3处理的生根率最低,仅为41.11%;从不定根数来看,A1B2C2处理最高,达6.89条,而A1B3C3处理最低,仅有3.61条;生根指数方面,A3B3C2处理最高,达21.32,而A1B3C3处理最低,仅有8.76。植物生长调节剂对最长根长和平均根长影响差异不显著;最长根长的变异范围为7.49~8.80 cm,平均根长的变异范围为5.00~6.25 cm。
处理 生根率/% 不定根数/条 最长根长/cm 平均根长/cm 生根指数 ck 45.56±8.32 b 4.28±0.96 bc 8.40±1.31 a 5.49±0.17 a 10.46±1.95 c A1B1C1 75.56±11.33 a 3.76±0.61 c 8.02±2.09 a 5.71±1.41 a 15.75±3.71 abc A1B2C2 52.00±13.49 b 6.89±2.01 a 8.06±1.16 a 5.99±0.53 a 20.03±3.49 ab A1B3C3 46.56±16.64 b 3.61±0.80 c 7.50±1.63 a 5.68±2.07 a 8.76±3.02 c A2B1C2 55.56±8.32 ab 5.21±0.72 abc 8.80±0.44 a 6.25±0.62 a 17.79±2.32 abc A2B2C3 41.11±11.33 b 4.98±0.18 abc 7.49±0.46 a 5.54±0.54 a 11.46±3.83 bc A2B3C1 43.33±8.16 b 3.84±0.37 bc 7.64±0.47 a 5.92±0.64 a 10.00±2.98 c A3B1C3 61.11±3.14 ab 3.78±1.66 c 7.60±2.30 a 5.00±1.31 a 10.94±4.74 c A3B2C1 77.78±4.16 a 3.67±0.85 c 8.16±0.85 a 5.64±0.48 a 15.78±2.68 abc A3B3C2 55.56±9.56 ab 6.22±0.87 ab 8.34±1.14 a 5.90±0.62 a 21.32±7.37 a 说明:同列不同小写字母表示差异显著(P<0.05)。各处理所表示具体含义见表1。 Table 3. Effects of hormone treatment on rooting traits of softwood cutting on C. hongdongensis subsp. tonkinensis
-
由表4可知:各因素对生根率的影响从大到小依次为植物生长调节剂种类、浸泡时间、植物生长调节剂质量浓度;对于不定根数和最长根长的影响从大到小依次为浸泡时间、植物生长调节剂质量浓度、植物生长调节剂种类;对于平均根长和生根指数的影响从大到小依次为浸泡时间、植物生长调节剂种类、植物生长调节剂质量浓度。由此可见,植物生长调节剂种类对生根率的影响最大,对于不定根数、最长根长、平均根长、生根指数来说,浸泡时间的影响则更为突出。
因素 生根率/% 不定根数/条 最长根长/cm k1 k2 k3 R k1 k2 k3 R k1 k2 k3 R 调节剂种类(A) 58.04 46.67 64.81 18.14 4.75 4.68 4.56 0.19 7.86 7.98 8.03 0.17 调节剂质量浓度(B) 64.07 56.96 48.48 15.59 4.25 5.18 4.56 0.93 8.33 7.90 7.82 0.51 浸泡时间(C) 65.55 54.37 49.59 15.96 3.73 6.11 4.12 2.38 7.94 8.47 7.53 0.94 Table 4. Range analysis of various factors in orthogonal test on rooting traits of softwood cutting on C. hongdongensis subsp. tonkinensis
因素水平 平均根长/cm 生根指数 k1 k2 k3 R k1 k2 k3 R 调节剂种类(A) 5.79 5.90 5.51 0.39 14.85 13.08 16.01 2.93 调节剂质量浓度(B) 5.65 5.72 5.83 0.18 14.83 15.76 13.16 2.60 浸泡时间(C) 5.76 6.05 5.41 0.64 13.84 19.71 10.39 9.32 说明:k1、k2、k3为各因素3水平对应各试验结果之和的平均值,R表示极差。 影响生根率的最优组合为A3B1C1 (100 mg·L−1IAA浸泡30 min);影响不定根数的最优组合为A1B2C2 (300 mg·L−1ABT-1浸泡60 min);影响最长根长的最优组合为A3B1C2 (100 mg·L−1IAA浸泡60 min);影响平均根长的最优组合为A2B3C2 (500 mg·L−1NAA浸泡60 min);影响生根指数的最优组合为A3B2C2 (300 mg·L−1IAA浸泡60 min)。
-
如表5所示:扦插基质对嫩枝扦插生根率影响差异显著(P<0.05)。其中,混合基质S3的生根率最高,达82.22%:其次为单一基质S1,生根率为74.41%;最低的是混合基质S4,生根率仅为44.44%。基质对不定根数的影响差异显著(P<0.05),单一基质S1的不定根数最多,达6.43条;其次是混合基质S3,不定根数为4.87条;混合基质S4的不定根数最少,仅有3.33条。相应的,扦插基质对生根指数的影响差异显著(P<0.05),混合基质S3的生根指数最高,达19.34;其次是单一基质S1,生根指数为16.01;最低的是混合基质S4,仅为5.56。扦插基质对生根插穗的最长根长和平均根长影响差异不显著。
基质种类 生根率/% 不定根数/条 最长根长/cm 平均根长/cm 生根指数 S1 74.41±9.52 a 6.43±0.42 a 5.29±0.36 a 3.36±0.18 a 16.01±1.81 ab S2 51.12±3.10 ab 4.38±1.04 b 6.14±1.18 a 4.46±0.82 a 10.86±5.27 bc S3 82.22±4.16 a 4.87±0.31 ab 7.18±1.34 a 4.89±0.88 a 19.34±2.55 a S4 44.44±6.85 b 3.33±0.97 b 6.18±1.53 a 3.89±0.93 a 5.56±2.09 c 说明:同列不同小写字母表示差异显著(P<0.05)。4种基质分别为蛭石(S1)、河沙(S2)、V(泥炭土)∶V(蛭石)∶V(珍珠岩)=2∶2∶1(S3)和V(泥炭土)∶V(黄土)=1∶1 (S4)。 Table 5. Effect of substrates on rooting traits of softwood cutting on C. hongdongensis subsp. tonkinensis
-
如表6所示:生长阶段对嫩枝扦插生根率影响差异显著(P<0.05)。其中,生长中期扦插的生根率最高,为82.22%;其次是生长初期扦插的生根率(77.78%);生长末期的生根率最低,仅为55.56%。生长阶段对不定根数影响差异显著(P<0.05), 其中生长中期生根数最多(4.87条),其次是生长初期(3.67条),而生长末期不定根数最少,为3.30条。生长阶段对根长的影响显著(P<0.05),生长初期扦插生根的最长根长(8.16 cm)和平均根长(5.64 cm)最大;其次是生长中期,分别为7.18和4.89 cm;而生长末期的最长根长(4.53 cm)和平均根长(3.09 cm)都最低。生长阶段对生根指数的影响差异显著(P<0.05),其中生根指数最高的是生长中期(19.34);其次是生长初期(15.78);而生长末期的生根指数最低,仅有5.63。
生长阶段 生根率/% 不定根数/条 最长根长/cm 平均根长/cm 生根指数 初期 77.78±4.16 a 3.67±0.85 ab 8.16±0.85 a 5.64±0.48 a 15.78±2.68 a 中期 82.22±4.16 a 4.87±0.31 a 7.18±1.34 a 4.89±0.88 ab 19.34±2.55 a 末期 55.56±8.32 b 3.30±0.36 b 4.53±0.70 b 3.09±0.86 b 5.63±1.81 b 说明:同列不同小写字母表示差异显著(P<0.05)。 Table 6. Effects of growth phase on rooting traits of softwood cutting on C. hongdongensis subsp. tonkinensis
-
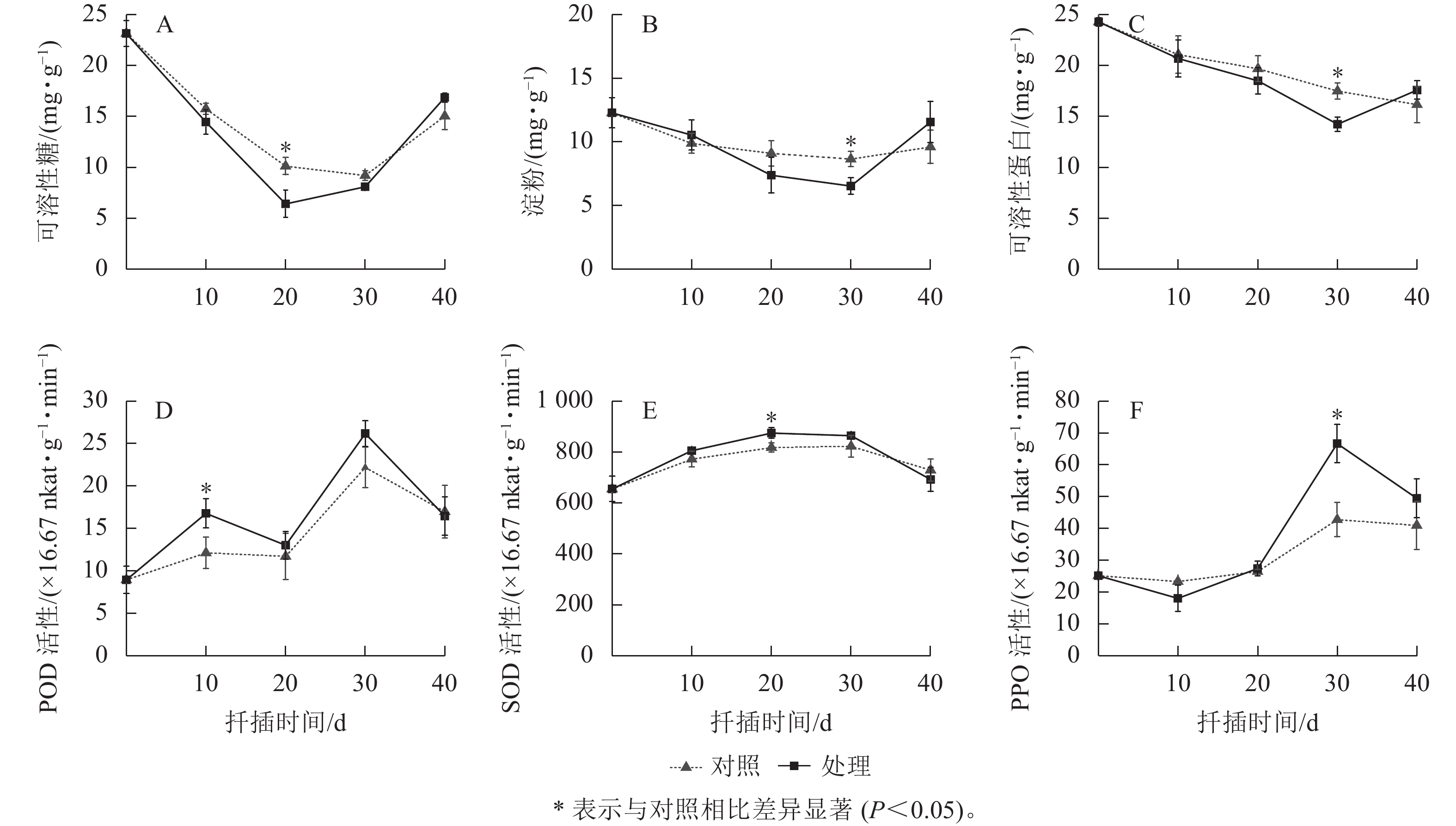
如2A所示:对照组和处理组插穗的可溶性糖均呈“V”字型的变化趋势。0~20 d时可溶性糖急剧下降,此期间插穗经过萌芽、愈伤组织形成,插穗基部代谢活跃,消耗了大量的营养物质;在20~40 d时,淀粉水解补充可溶性糖,叶片光合产物运输养分,可溶性糖缓慢上升。处理组可溶性糖的变化幅度大于对照组,特别在20 d时,处理组可溶性糖显著低于对照组(P<0.05),说明IAA处理加快了愈伤组织和不定根的形成,从而消耗了更多的养分;后期则由于根系较早形成,从而促进了光合产物的积累。
-
从图2B所示:淀粉的变化趋势与可溶性糖一致,均表现出先降低后上升的趋势,但时间稍有滞后。0~30 d,插穗淀粉均在逐渐下降,且在30 d时达到谷值。前20 d已有大量的可溶性糖及部分淀粉降解产生的可溶性糖用于根原基的形成与分化,但无功能性根系,需继续降解淀粉;30~40 d,具备根系插穗的光合产物能提供生长所需并合成淀粉,导致淀粉上升。经过IAA处理的插穗淀粉变化趋势大于对照组,特别在30 d时,对照组对淀粉的消耗小于植物生长调节剂,说明对照组生根量少且所需养分少。
-
如图2C所示:0~30 d,对照和处理组的可溶性蛋白逐步下降,为根系形成过程中的细胞增殖、DNA复制提供物质条件;30~40 d,处理组已形成的根系开始合成可溶性蛋白并加以积累,而对照组可溶性蛋白持续下降,说明对照组生根较慢,不定根的形成仍然需要消耗可溶性蛋白。经过IAA处理,插穗的可溶性蛋白在生根前始终低于对照组,说明植物生长调节剂通过加快利用可溶性蛋白促进插穗生根。
-
对照组和处理组插穗的POD活性均呈现“M”型变化趋势,均在10和30 d出现高峰(图2D)。0~10 d,插穗离体处于逆境胁迫,POD活性大幅上升以消除产生的自由基,避免插穗受害;10~20 d,POD活性下降,使体内IAA质量浓度升高,有利于皮部膨大处开裂,从而形成愈伤组织及根原基诱导;20~30 d时根原基诱导完成,此时POD活性上升,进而氧化过多的IAA,促进了根系的伸长与生长;30~40 d,由于插穗形成完整根系成为独立植株,致使植株抵抗能力增强,POD活性又开始降低。相比较而言,处理组在愈伤膨大期(10~20 d)的POD活性显著大于对照组(P<0.05),说明IAA处理提高了插穗的抗逆能力。
-
对照组和处理组插穗的SOD活性均呈先升后降的变化趋势(图2E)。0~20 d,SOD活性逐渐上升,消除了插穗因离体而产生的大量自由基,降低自由基对植物细胞的伤害;20~40 d,处理组SOD活性开始下降,说明处理组插穗逐渐恢复完整植株的功能,对不利环境的抵抗能力逐渐增强,体内自由基降低,SOD活性逐渐降低。而对照组SOD活性的峰值比处理组晚了约10 d,说明对照组插穗根系形成时间晚于处理组。处理组SOD活性在生根前都高于对照组,表明IAA处理能提高SOD活性,清除插穗体内的自由基来提高抗逆性,从而有利于生根。
-
对照组和处理组插穗的PPO活性均呈先降低后升高再降低的变化趋势,但处理组插穗的PPO活性变化趋势更加明显(图2F)。0~10 d,通过使插穗发生褐变,PPO活性降低,避免插穗感染;10~30 d,PPO活性显著上升(P<0.05),此时PPO通过催化苯酚氧化生成高活性的邻醌类化合物,促进生根;30~40 d,PPO活性呈现下降趋势,此时根系进入伸长和生长。由此可见,PPO不仅反映了插穗自身的防御能力和褐化过程,还可能与愈伤组织的形成及不定根的形成密切相关。
-
从表7可见:生根率与生根指数呈显著正相关(P<0.05);生根率与淀粉和可溶性蛋白呈显著负相关(P<0.05),与可溶性糖呈极显著负相关(P<0.01);生根率与POD和SOD活性呈极显著正相关(P<0.01)。此外,各生理指标之间也表现出一定的相关性,淀粉与可溶性糖呈显著正相关(P<0.01),两者与POD和SOD活性呈显著负相关(P<0.05);可溶性蛋白与POD和SOD活性呈极显著负相关(P<0.01);POD与SOD活性呈极显著正相关(P<0.01)。结合前期分析,本研究认为插穗在20和30 d内养分含量越低(即消耗越大),更多的养分则用于根原基诱导和愈伤组织形成,生根率越高;在测定的3种酶活性中,POD和SOD活性与生根率呈极显著正相关(P<0.01),说明东京四照花扦插生根过程中消除自由基是影响生根的重要因子。
指标 生根率 生根指数 可溶性糖 淀粉 可溶性蛋白 PPO活性 POD活性 生根指数 0.830* 可溶性糖 −0.918** −0.823* 淀粉 −0.861* −0.820* 0.911* 可溶性蛋白 −0.896* −0.850* 0.909* 0.844* PPO活性 0.619 0.384 −0.389 −0.359 −0.214 POD活性 0.928** 0.889* −0.953** −0.903* −0.988** 0.298 SOD活性 0.947** 0.729 −0.932** −0.879* −0.940** 0.390 0.948** 说明:*表示显著相关(P<0.05);**表示极显著相关(P<0.01)。 Table 7. Correlation analysis between rooting index and physiological index
-
植物生长调节剂通过调节插穗生根过程中内源激素水平和氧化酶的活性,刺激根原基的启动,有助于提高插穗的生根率,但不同的植物生长调节剂对树种生根的影响不同[19]。胡涛等[20]研究发现:吲哚丁酸(IBA)处理的美国流苏Chionanthus virginicus,其插穗生根率要显著高于IAA和NAA处理。此外,植物生长调节剂的质量浓度和浸泡时间也显著影响插穗生根。SABATINO等[21]研究发现:银香科科Teucrium frutican插穗在IBA中浸泡5 min的生根率最高,而浸泡7 min的不定根数最多。本研究结果证明了这一点,合理的选择植物生长调节剂可以有效提高东京四照花插穗的生根率。
扦插基质由于理化性质的差异,导致其在保温性、保湿性、透气性、透水性等方面有所不同[22]。研究表明:混合基质的生根效果优于单一基质的生根效果[23]。本研究也得到了相似的观点,插穗在S3混合基质中的生根率和根长显著高于其他处理,说明泥炭土、蛭石和珍珠岩的混合基质土壤结构疏松、透气状态和营养状况良好,有利于插穗的生根与生长,也与东京四照花不耐水湿的特性有关。
处于不同生长发育阶段的插穗由于内源激素水平、生根相关酶的活性和养分储备存在差异,从而影响插穗的不定根形成[24]。KIM等[25]发现:在5月采集的草莓Fragaia×ananassa插穗生根成活率最高,并随着插穗的成熟,生根率逐渐降低。本研究发现:东京四照花在生长中期扦插生根率最高,为82.22%;此前的穗条木质化程度不够且养分储备不足,此后的穗条内可能产生了过多的抑制类物质,以上情况均影响了根系的产生。
-
本研究发现:东京四照花插穗在生根前,可溶性糖和淀粉均呈现“先下降、后上升”的趋势,这是由于刚扦插时会消耗插穗体内部分可溶性糖和淀粉以提供生根所需养分;随着根系形成及光合能力的提升,促进了可溶性糖和淀粉的合成和转化[26]。在生根过程中,可溶性蛋白呈持续下降趋势,一方面提供插穗生根所需的能量,另一方面以蛋白酶类的形式参与调控生根生理生化。
POD与SOD不仅参与植物的氧化胁迫反应,而且在植物体内的多种生理生化反应中发挥重要的作用[27]。本研究中,POD与SOD在插穗扦插后上升,有助于清除插穗体内的自由基,提高插穗的抗逆性。此外,POD还通过调节IAA水平来影响插穗愈伤组织的形成和不定根的诱导与生长,相似的结果在楸树Catalpa bungei[28]扦插生根研究中也有报道。PPO通过褐变插穗,避免其切口感染,同时催化IAA形成的IAA-酚酸复合物有助于插穗生根[29]。本研究发现:在插穗生根过程中,PPO活性在前期升高,在不定根发育时期出现峰值,说明PPO有效促进了插穗根系的生长,这与YANG等[30]的研究结果一致。
-
采用质量浓度为300 mg·L−1的IAA溶液浸泡东京四照花生长中期(8月中旬)的半木质化插穗30 min,扦插在V(泥炭土)∶V(蛭石)∶V(珍珠岩)=2∶2∶1的基质中,生根率和生根指数分别高达82.22%和19.34;生根类型主要以皮部生根为主。IAA处理可以加快插穗可溶性糖、淀粉和可溶性蛋白的代谢速度,并提高POD、SOD和PPO的活性以促进生根。
Cutting propagation of softwood and dynamic changes in physiological indicators during the rooting process of Cornus hongdongensis subsp. tonkinensis
doi: 10.11833/j.issn.2095-0756.20230457
- Received Date: 2023-09-07
- Accepted Date: 2024-01-19
- Rev Recd Date: 2023-12-22
- Available Online: 2024-05-22
- Publish Date: 2024-05-22
-
Key words:
- softwood cuttings /
- IAA /
- cutting substrate /
- enzyme activity /
- Cornus hongkongensis subsp. tonkinensis
Abstract:
| Citation: | YUAN Zhen’an, DU Wenting, LIU Guohua, et al. Cutting propagation of softwood and dynamic changes in physiological indicators during the rooting process of Cornus hongdongensis subsp. tonkinensis[J]. Journal of Zhejiang A&F University, 2024, 41(3): 624-633. DOI: 10.11833/j.issn.2095-0756.20230457 |










 DownLoad:
DownLoad: