-
彩叶植物是指在整个生长季节或生长季节的某一阶段全部或部分叶片较稳定地呈现非绿色的植物[1],在园林中应用非常广泛。叶色作为观赏植物的重要性状之一,其主要的呈色物质是叶绿素、类胡萝卜素和花青素[2],它们的类型、比例和分布是叶片颜色发生变化的基础[3]。花青素是一类重要的类黄酮色素,通常积聚在叶片的表皮或表皮细胞、栅栏组织和海绵组织中[4],彩叶植物叶片颜色的呈现在很大程度上受花青素含量的影响。花青素的合成、降解以及稳定性均会影响叶片和花瓣等组织的色泽变化[5]。此外,可溶性蛋白是植物体内重要渗透调节物质,糖类是花青素合成的前体和信号物质,它们也间接影响花青素的形成[6];过氧化物酶(POD)是一种可能与花青素降解有关的酶,能够促进花青素的降解[7];液泡内pH值的变化可以影响液泡中花青素的稳定性进而改变植物颜色[8]。因此,植物的叶色变化涉及多种因素,阐明植物叶色形成及变化机制有助于人们更好地选择和培育具有理想叶色的植物,为园林绿化和观赏植物的应用提供更多可能性。
彩叶桂Osmanthus fragrans Colour Group主要特点在于枝条或叶片呈现鲜明的色彩变异,具有较高的观赏价值[9]。目前对于彩叶桂品种的分类主要是根据成型叶片的颜色[10],然而这种分类方式相对宽泛,对色系划分并不精确。对于彩叶桂叶片变色机制的研究集中在单个品种上[11],还未对不同品种之间的差异进行分析和比较。因此,本研究选取29份彩叶桂种质材料,运用量化标准并结合聚类分析方法对其进行分类[12]。同时,从中选择2个代表性品系(‘罗彩3号’‘Luocai 3’和‘罗彩28号’‘Luocai 28’)进行色素分布观察、组分定性、含量测定以及生理指标的测定,明确彩叶桂色素积累特征及变化规律,以期为彩叶桂的品种分类、筛选和改良提供理论依据。
-
于2023年3—6月随机选择29个生长健壮、长势一致的彩叶桂品系进行调查。根据叶片的生长状态和变色情况,划分为S1 (0%变色)、S2 (30%变色)、S3 (50%变色)、S4 (80%变色)和S5 (100%变色) 5个时期。从中选取2个代表性品系(‘罗彩3号’和‘罗彩28号’)进行色素分布观察、组分定性、含量测定以及生理指标的测定。取样时每个品系选取10株以上,每株植株取当年生枝条的第2至3轮叶片。
-
用便携式全自动色差仪(CR-10,日本)测定叶片上表皮的明度(L*)、红度(a*)、黄度(b*)、彩度(C*)和色相角(h)。采用英国皇家园艺学会标准比色卡(RHSCC)对29个不同品系的彩叶桂叶片的不同时期进行比对。每个品系5个生物学重复。
-
色素分布观察采用徒手切片法[13];色素组分定性参照白新祥等[14]的方法;叶绿素测定采用体积分数为80%的丙酮浸提法[15];类胡萝卜素测定参照陈建等[16]的方法;花青素的提取使用甲醇∶水∶甲酸∶三氟乙酸=70∶27∶2∶1(体积比)的提取液[17],采用UPLC-Triple-TOF/MS系统对叶片中花青素进行测定。色谱柱为ACQUITY UPLC BEH-C18柱(1.7 μm,2.1 mm×100.0 mm),以体积分数为1%的甲酸水溶液(A)和1%的甲酸乙腈(B)为流动相,进样量为3 μL,柱温为40 ℃,流速0.4 mL·min−1。洗脱程序为:0 min,95%A和5%B (体积分数);2 min,90%A和10%B (体积分数);12 min,5%A和95%B (体积分数);14 min,5%A和95%B (体积分数);15 min,95%A和5%B (体积分数);17 min,95%A和5%B (体积分数)。花青素的检测波长为520 nm,通过标准品半定量法计算叶片中花青素相对于标准品的含量。花青素标准品为矢车菊素-3-O-芸香糖苷。
-
可溶性蛋白测定采用考马斯亮蓝法[15];可溶性糖测定采用蒽酮比色法[18];POD活性测定采用愈创木酚法[19];pH值测定参照唐前瑞等[20]的方法。
-
叶片色素和生理指标测定均进行3个生物学重复。使用Excel 2020、Origin 2021以及SPSS 27.0软件进行数据统计分析和作图,采用邓肯新复极差法对不同时期各指标数据进行差异显著性分析,采用皮尔逊系数对叶色表型参数和影响因素进行相关性分析。
-
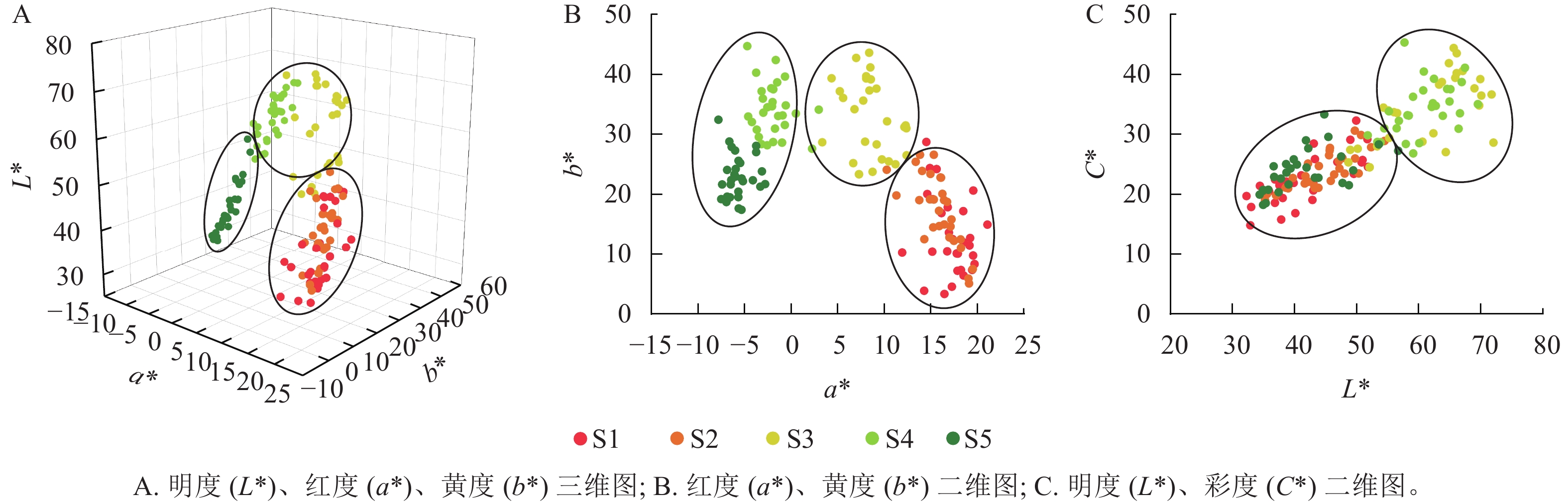
在变色前期(S1和S2时期),彩叶桂叶片颜色呈现多样性,包括紫粉色、红色、红棕色、红橙色、黄棕色和橄榄棕色等多种颜色;而在变色中后期(S3至S5时期),叶片颜色相对趋于一致,普遍呈现从黄色、黄绿色到橄榄绿色的变化(表1)。从图1A~B可见:明度、红度、黄度在S1和S2时期相对接近,S3和S4时期也相对接近。S1和S2时期具有较高的红度,而S4和S5时期则具有较高的黄度。此外,S1、S2和S5时期具有较高的重叠度,S3则和S4时期具有较高的重叠度,同时S3和S4时期的明度和彩度均高于S1、S2和S5时期(图1C)。
序号 品系 时期 S1 S2 S3 S4 S5 1 ‘罗彩2号’‘Luocai 2’ 紫粉色 红色 黄棕色 黄绿色 橄榄绿色 2 ‘罗彩3号’‘Luocai 3’ 红色 红棕色 黄棕色 黄绿色 橄榄绿色 3 ‘罗彩4号’‘Luocai 4’ 红棕色 红橙色 黄色 黄绿色 黄绿色 4 ‘罗彩6号’‘Luocai 6’ 红色 红色 黄绿色 黄绿色 橄榄绿色 5 ‘罗彩7号’‘Luocai 7’ 红橙色 红橙色 黄色 黄绿色 橄榄绿色 6 ‘罗彩22号’‘Luocai 22’ 红橙色 红橙色 黄色 黄绿色 黄绿色 7 ‘罗彩23号’‘Luocai 23’ 红棕色 红棕色 黄绿色 黄绿色 黄绿色 8 ‘罗彩26号’‘Luocai 26’ 红橙色 红色 橄榄棕色 黄绿色 橄榄绿色 9 ‘罗彩27号’‘Luocai 27’ 红橙色 黄棕色 橄榄棕色 黄绿色 橄榄绿色 10 ‘罗彩28号’‘Luocai 28’ 红色 红橙色 黄色 黄绿色 橄榄绿色 11 ‘罗彩29号’‘Luocai 29’ 红色 红棕色 橄榄棕色 黄绿色 橄榄绿色 12 ‘罗彩30号’‘Luocai 30’ 红色 红棕色 橄榄棕色 黄绿色 橄榄绿色 13 ‘罗彩31号’‘Luocai 31’ 红色 紫粉色 黄色 黄绿色 橄榄绿色 14 ‘罗彩33号’‘Luocai 33’ 红橙色 黄棕色 黄色 黄绿色 橄榄绿色 15 ‘罗彩34号’‘Luocai 34’ 红色 红色 黄色 黄绿色 橄榄绿色 16 ‘罗彩36号’‘Luocai 36’ 棕色 红橙色 橄榄棕色 黄绿色 橄榄绿色 17 ‘罗彩37号’‘Luocai 37’ 红色 红橙色 黄色 黄绿色 橄榄绿色 18 ‘罗彩46号’‘Luocai 46’ 红色 紫粉色 黄色 黄绿色 橄榄绿色 19 ‘罗彩47号’‘Luocai 47’ 红橙色 橄榄棕色 黄色 黄绿色 橄榄绿色 20 ‘罗彩55号’‘Luocai 55’ 红棕色 红棕色 黄色 黄绿色 橄榄绿色 21 ‘罗彩59号’‘Luocai 59’ 棕色 红棕色 黄棕色 黄绿色 橄榄绿色 22 ‘罗彩62号’‘Luocai 62’ 红色 红色 黄色 黄绿色 橄榄绿色 23 ‘罗彩65号’‘Luocai 65’ 红棕色 红棕色 黄色 黄绿色 黄绿色 24 ‘罗彩66号’‘Luocai 66’ 红橙色 红橙色 黄色 黄绿色 橄榄绿色 25 ‘罗彩77号’‘Luocai 77’ 红橙色 红橙色 黄色 黄绿色 黄绿色 26 ‘罗彩82号’‘Luocai 82’ 红棕色 红色 黄色 黄绿色 橄榄绿色 27 ‘罗彩88号’‘Luocai 88’ 红色 红棕色 黄色 黄绿色 黄绿色 28 ‘罗彩151号’‘Luocai 151’ 红棕色 橄榄棕色 黄色 黄绿色 黄绿色 29 ‘罗彩153号’‘Luocai 153’ 红色 红棕色 黄色 黄绿色 黄绿色 说明:不同时期的叶色均是通过英国皇家园艺学会标准比色卡(RHSCC)测定得来。 Table 1. Leaf color changes of 29 cultivars of O. fragrans Colour Group
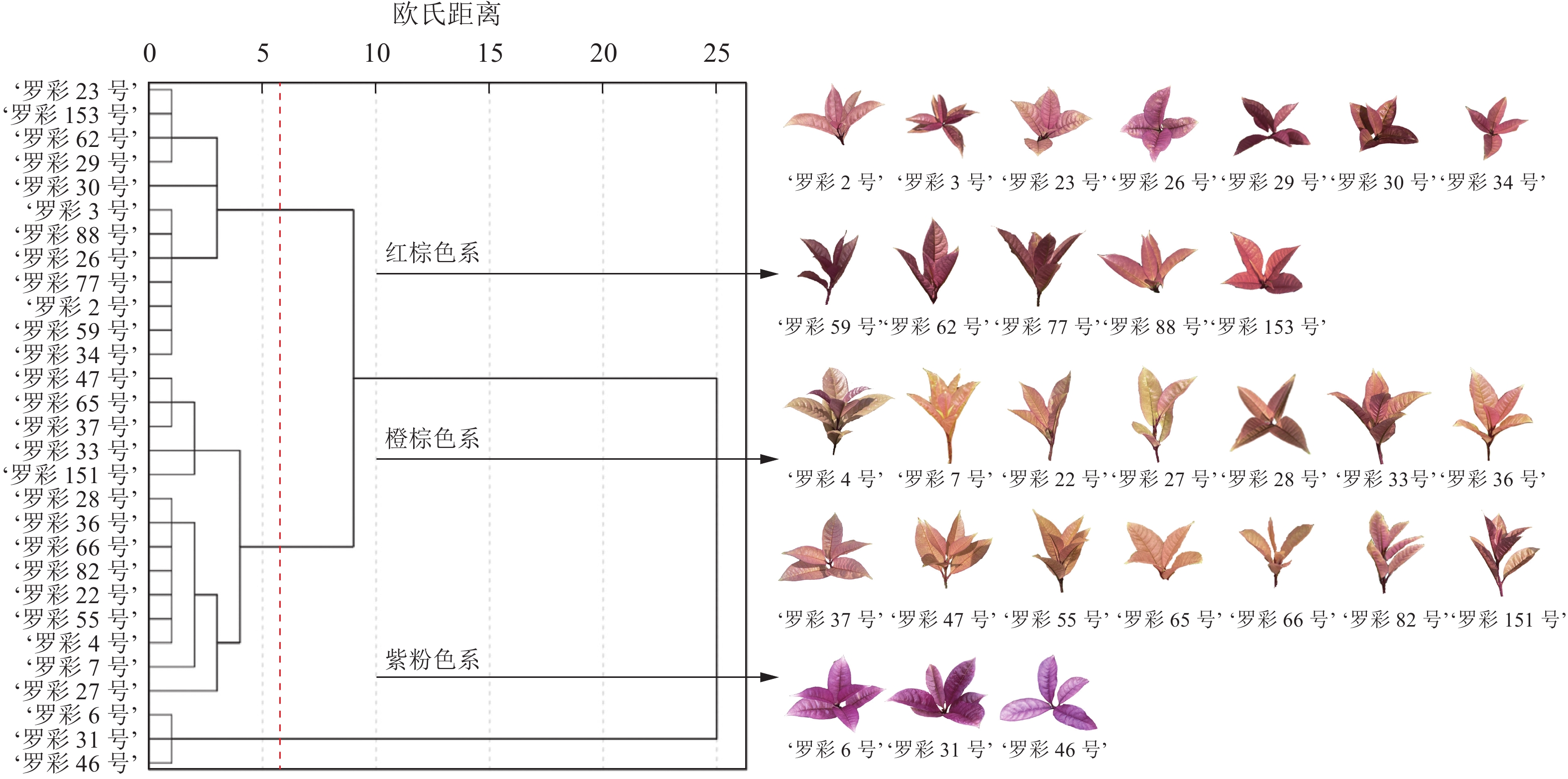
在S2时期,彩叶桂的叶色呈现出丰富的色彩,而且叶片发育相对成熟。因此,基于S2时期的叶色参数,对29个彩叶桂品系进行聚类分析,将其分为两大类共3个色系(图2):即红棕色系(第Ⅰ类第1亚类,包括‘罗彩23号’‘Luocai 23’至‘罗彩34号’‘Luocai 34’等12个品系)、橙棕色系(第Ⅰ类第2亚类,包括‘罗彩47号’‘Luocai 47’至‘罗彩27号’‘Luocai 27’等14个品系)和紫粉色系(第Ⅱ类,包括‘罗彩6号’‘Luocai 6’至‘罗彩46号’‘Luocai 46’等3个品系)。彩叶桂叶色参数在红度、黄度坐标上均分布在正数范围内,且各色系之间可明显区分(图3A)。在以明度、红度和黄度构建的三维象限图中,各色系集中分布在1条主线附近,呈带状分布(图3B)。通过对3个色系的明度、红度和黄度分析发现:橙棕色系的明度和黄度最高,红棕色系次之,而紫粉色系最低。尽管橙棕色系与红棕色系部分样本的明度存在重叠,但是可以通过红度和黄度进行区分。
-
对红棕色系和橙棕色系这2个颜色相近的色系进行研究发现:在叶片生长过程中,红棕色系的‘罗彩3号’叶片呈红色—红棕色—黄棕色—黄绿色—橄榄绿色的变化(图4A),橙棕色系的‘罗彩28号’‘Luocai 28’叶片呈红色—红橙色—黄色—黄绿色—橄榄绿色的变化(图4B)。这2个色系在叶片生长过程中的颜色变化略有不同,其中‘罗彩3号’在颜色过渡中呈现出较多的红色和红棕色调,而‘罗彩28号’则有更多的橙色和橙棕色调。解剖结构发现:在S1和S2时期,2个品系叶片的叶肉和叶脉横截面细胞中以红色色素为主,且主要分布在表皮细胞中。其中‘罗彩3号’细胞颜色逐渐加深,‘罗彩28号’的红色色素由多变少。随着叶片生长到S3至S5时期,叶肉细胞中的绿色色素逐渐增加,表皮细胞中红色色素逐渐减少。
此外,显色反应表明:‘罗彩3号’和‘罗彩28号’在S1至S3时期石油醚的提取液呈接近透明的状态,推测叶片中可能不含或仅含少量类胡萝卜素;然而,在S4至S5时期,提取液逐渐呈现黄色,表明类胡萝卜素质量分数逐渐增加。在体积分数为10%的盐酸反应中,‘罗彩3号’和‘罗彩28号’在S1和S2时期的提取液呈现不同程度的红色,说明叶片中含有较多的花青素;然而,在S3至S5时期,提取液的颜色逐渐减淡,说明花青素质量分数逐渐减少。在体积分数为30%的氨水反应中,所有样品的提取液呈现浅棕色,表明叶片中均含有黄酮类或黄酮醇类化合物(图4C和图4D)。
-
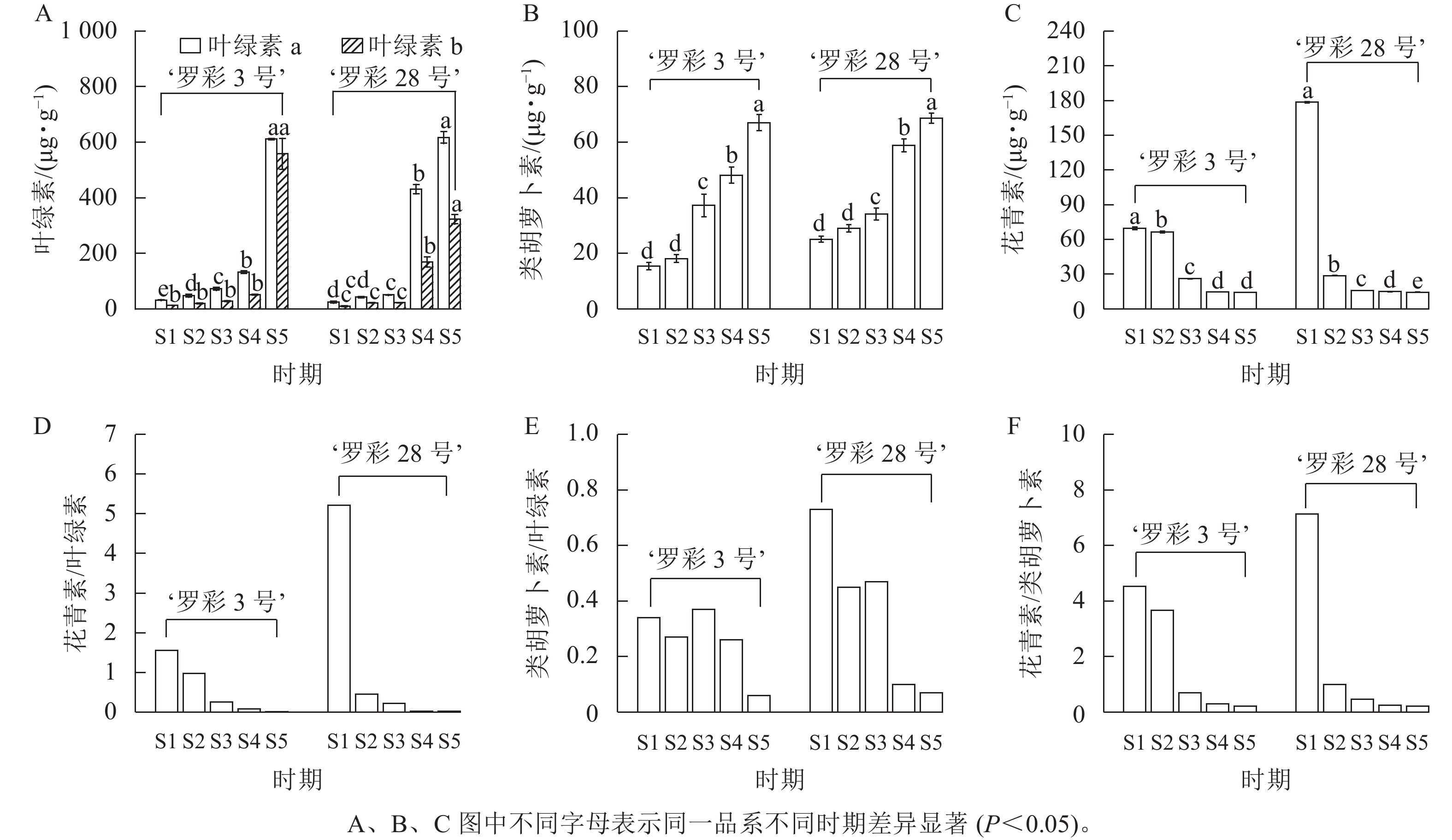
色素测定(图5)发现:在叶片生长过程中,‘罗彩3号’和‘罗彩28号’的叶绿素a、叶绿素b呈明显的增加趋势,‘罗彩3号’在S5时期显著增加(P<0.05),而‘罗彩28号’在S4时期显著增加(P<0.05),这与叶片变绿的过程一致;同样,类胡萝卜素质量分数也随着叶片生长逐渐增加。相反的,花青素质量分数在叶片生长过程中显著减少(P<0.05)。2个品系的花青素/叶绿素以及花青素/类胡萝卜素均呈下降趋势。‘罗彩3号’在S1和S3时期类胡萝卜素/叶绿素相对较高,而‘罗彩28号’则在S1时期相对较高。
-
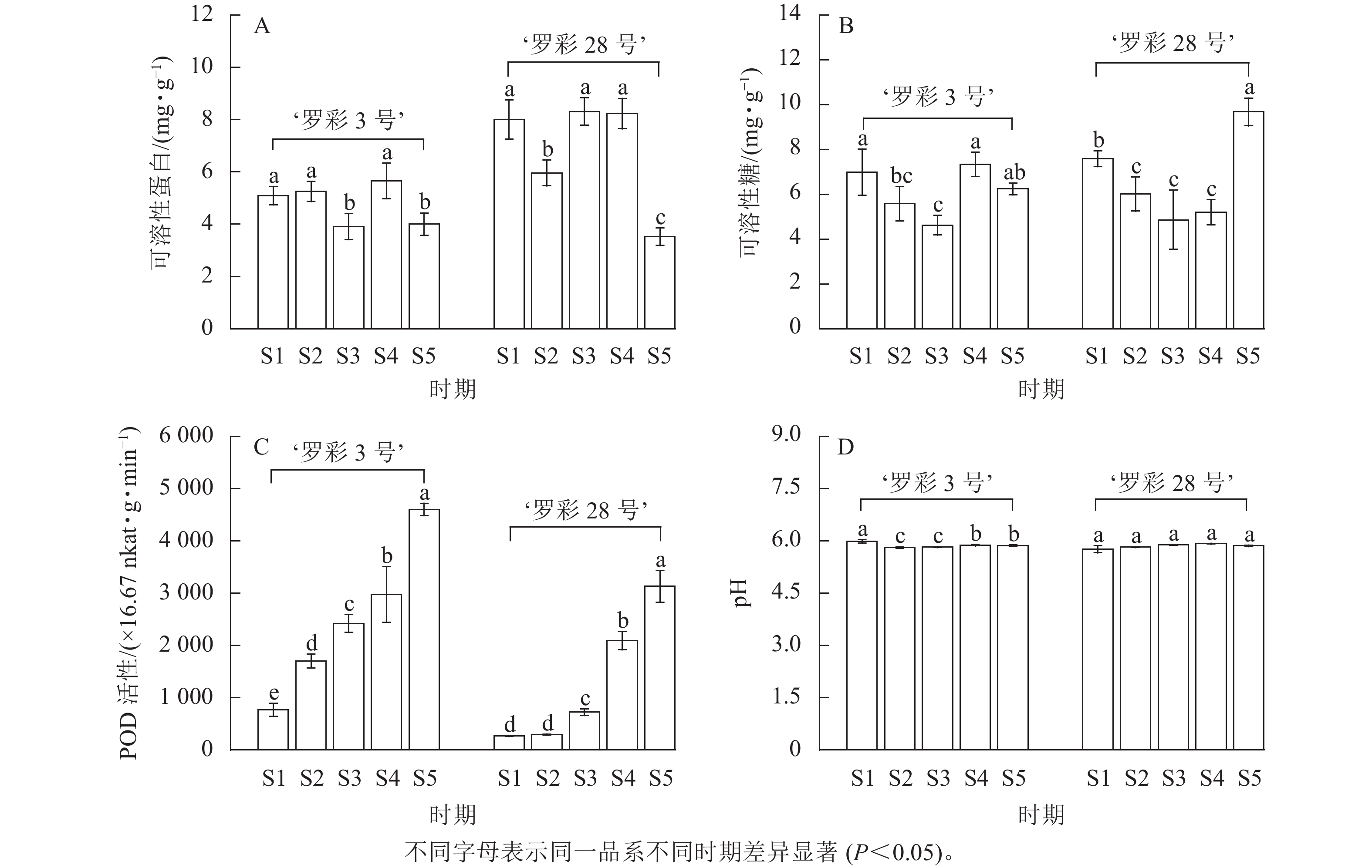
生理指标测定发现:在叶片生长过程中,可溶性蛋白质量分数没有明显的变化规律(图6A),但可溶性糖质量分数整体上呈先下降后上升的趋势。从S3时期开始,2个品系叶片中的可溶性糖质量分数开始增加,但‘罗彩3号’在S5时期出现轻微下降趋势(图6B)。此外,POD活性在叶片生长过程中呈上升趋势,‘罗彩3号’的POD活性持续增加,而‘罗彩28号’的POD活性前期上升较小,中后期上升幅度较大(图6C)。2个品系叶片细胞液的pH在整个生长过程中保持弱酸性水平,整体上无明显变化(图6D)。
-
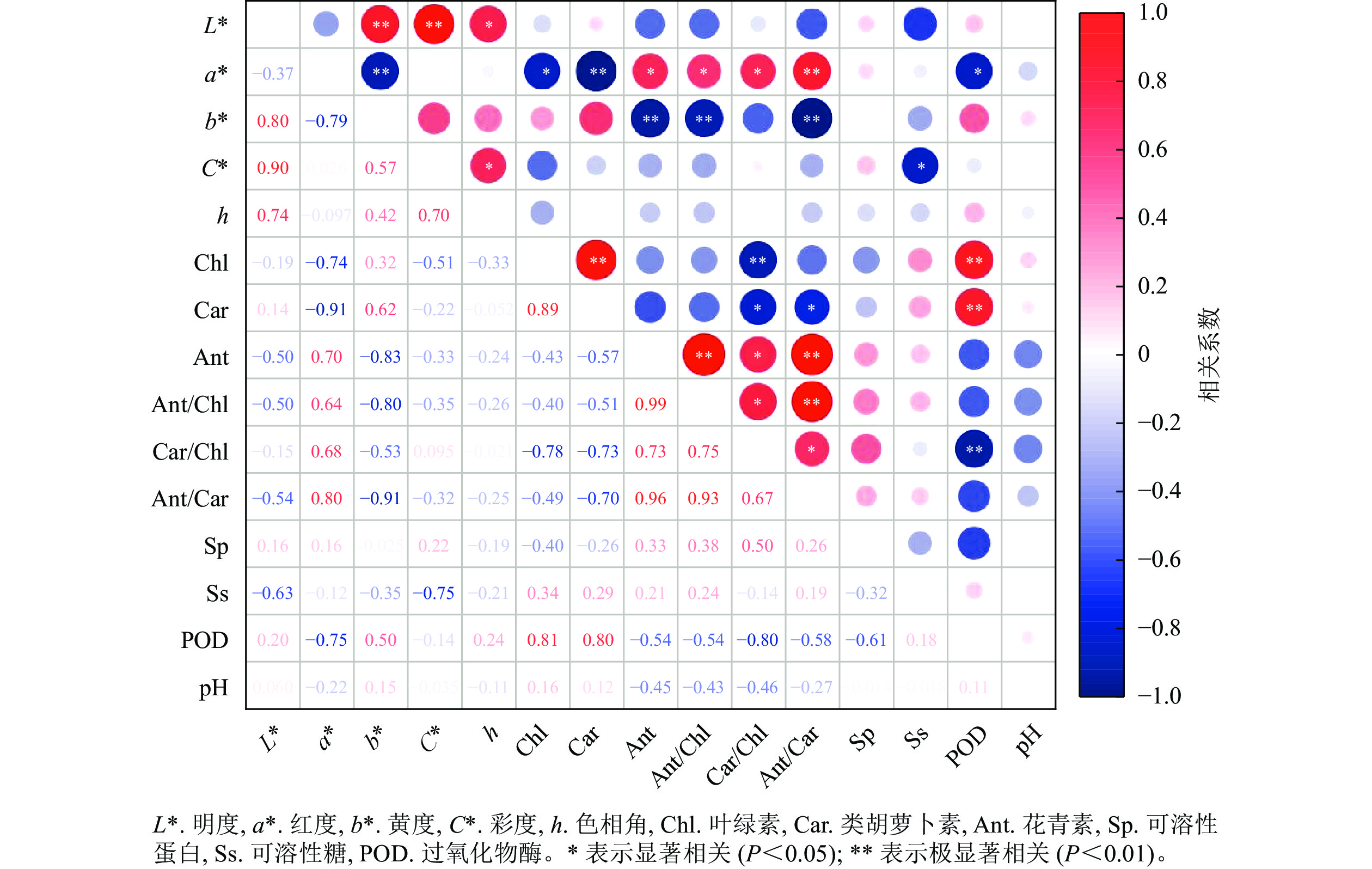
相关性分析(图7)显示:彩叶桂叶片的明度、色相角与各色素及生理指标间均无显著相关;红度与花青素、花青素/叶绿素、类胡萝卜素/叶绿素间呈显著正相关(P<0.05),与花青素/类胡萝卜素间呈极显著正相关(P<0.01),与叶绿素和POD活性呈显著负相关(P<0.05),与类胡萝卜素呈极显著负相关(P<0.01);黄度与花青素、花青素/叶绿素、花青素/类胡萝卜素间呈极显著负相关(P<0.01);彩度与可溶性糖呈显著负相关(P<0.05)。此外,叶绿素与类胡萝卜、POD活性呈极显著正相关(P<0.01),与类胡萝卜素/叶绿素呈极显著负相关(P<0.01);类胡萝卜素与POD活性呈极显著正相关(P<0.01),与类胡萝卜素/叶绿素、花青素/类胡萝卜素间呈显著负相关(P<0.05);花青素与类胡萝卜素/叶绿素呈显著正相关(P<0.05),与花青素/叶绿素、花青素/类胡萝卜素间呈极显著正相关(P<0.01)。
-
彩叶桂以其丰富的叶色变化而备受青睐,具有较高的观赏价值。冯园园[10]根据成型叶叶片颜色将彩叶桂划分为红色系和黄色系,其中红色系在特定时期转为黄色。然而,这种分类方式相对宽泛,对色系划分不够精细。本研究通过对S2时期叶片颜色变化分析发现:叶片不仅呈色丰富,而且发育相对成熟。通过聚类分析将彩叶桂划分为两大类共3个色系,即红棕色系(第Ⅰ类第1亚类)、橙棕色系(第Ⅰ类第2亚类)和紫粉色系(第Ⅱ类)。其中,橙棕色系具有较高的明度和黄度,红棕色系次之,而紫粉色系则具有较高的红度。以上结果表明:彩叶桂整体上可分为2个明显的颜色类别,但第Ⅰ类可以进一步区分,这表明即使在同一主色系下,彩叶桂叶色仍存在差异。
叶片中的色素种类、质量分数、比例及分布位置等的差异都是影响叶色变化的关键因素[1]。叶绿素、类胡萝卜素和花青素变化时将导致叶片颜色发生改变[21]。在本研究中,2个品系的花青素主要分布在彩叶桂幼嫩叶片的上、下表皮细胞中。随着叶片的生长,红色色素逐渐减少,而绿色色素不断增加,最终导致成熟叶片呈现出稳定的绿色形态。通过对不同变色时期叶片的色素质量分数比较发现:花青素的减少伴随着叶绿素的持续上升,这可能是叶片颜色变化的重要因素之一;与此同时,虽然类胡萝卜素质量分数逐渐上升,但其变化并不显著,可能在叶片颜色形成过程中的作用相对较小。类似的变化也在其他植物如芍药Paeonia lactifora[22]、岭南槭Acer tutcheri[23]和红枫Acer palmatum‘Atropurpureum’ [24]中观察到。在S2时期,‘罗彩3号’和‘罗彩28号’叶片的叶绿素质量分数分别为67.42、64.04 μg·g−1;类胡萝卜素质量分数分别为18.14、29.01 μg·g−1;花青素质量分数分别为66.40、28.81 μg·g−1。2个品系的叶绿素质量分数相对接近,但花青素和类胡萝卜素的质量分数和比值存在差异。‘罗彩3号’的花青素/类胡萝卜素大于‘罗彩28号’,表明红棕色系和橙棕色系的区别在于花青素和类胡萝卜素的质量分数和比值。
糖类不仅是花青素合成的前体,还扮演着信号物质的角色;可溶性蛋白质在植物体内被认为是重要的渗透调节物质,通过一定生理代谢来调节花青素的形成[6]。聂庆娟等[25]对美国红栌Cotinus coggygria ‘Royal Purple’研究发现:变色期的红色叶片中具有较高的可溶性蛋白和可溶性糖。类似地,本研究2个品系在S1时期的红色叶片中也显示出相对较高的可溶性蛋白和可溶性糖。然而,两者均与叶片内花青素的相关性不显著,这与唐生森等[26]在枫香Liquidambar formosana中的研究结果相似。POD是以花青素为底物的酶,其作用是催化花青素的氧化过程,导致花青素失去颜色[7]。在本研究中,2个品系的POD活性均呈上升趋势。与此同时,POD活性与叶绿素和类胡萝卜素呈显著正相关,但与花青素的相关性则不显著,这与枫香[27]的研究结果相似。这暗示着POD可能不是彩叶桂叶片中影响花青素降解的主要酶,但对叶绿素和类胡萝卜素质量分数的积累具有正向促进作用,作用强弱因品系不同而略有差异。pH是液泡环境的重要特征,对水溶性色素的稳定性和活性产生影响;随着pH的升高,花色苷稳定性降低[28]。以红罗宾石楠Photinia × fraseri ‘Red Robin’[29]为例,其叶色变绿主要与叶片细胞液中的pH上升引起的花青素质量分数下降有关。然而,在整个变色期内,2个品系彩叶桂叶片的pH基本保持稳定,且始终保持在弱酸性水平,与叶色参数及色素间均不存在显著相关,这说明彩叶桂的叶色变化与pH无关。
-
综上所述,基于S2时期的叶色参数可对彩叶桂进行详细的色系划分。彩叶桂叶色变化主要受花青素质量分数减少和叶绿素质量分数增加的影响,而POD在其中发挥了重要作用。花青素和类胡萝卜素的质量分数及其比值可区分红棕色系和橙棕色系,不同品种的彩叶桂叶色变化受其自身特性的影响。
Color change and physiological characteristics in Osmanthus fragrans Colour Group
doi: 10.11833/j.issn.2095-0756.20240160
- Received Date: 2024-02-01
- Accepted Date: 2024-05-23
- Rev Recd Date: 2024-05-16
- Available Online: 2024-07-03
- Publish Date: 2024-09-25
-
Key words:
- Osmanthus fragrans Colour Group /
- cluster analysis /
- pigment /
- anthocyanin /
- physiological characteristics
Abstract:
| Citation: | JIN Xiaoyu, WANG Yiguang, ZHAO Hongbo, et al. Color change and physiological characteristics in Osmanthus fragrans Colour Group[J]. Journal of Zhejiang A&F University, 2024, 41(5): 1056-1065. DOI: 10.11833/j.issn.2095-0756.20240160 |















 DownLoad:
DownLoad: