-
在植物细胞中,内膜系统(endomembrane system)是一个由多种细胞器组成的精密网络,负责囊泡运输和细胞内物质的精确分配。这一系统在植物的生长发育以及应对各种环境胁迫(如高温、干旱等)起着不可或缺的作用[1]。这些细胞器包括内质网(endoplasmic reticulum, ER),高尔基体(golgi),反式高尔基体网络/早期内吞体(trans-golgi network/early endosome, TGN/EE),液泡前体/多囊泡体/晚期内吞体(prevacuolar compartment/multivesicular body/late endosome, PVC/MVB/LE),自噬体(autophagosome)和液泡(vacuole)等[2]。在液泡转运途径(vacuolar transport pathway)中,蛋白质在内质网中合成,然后被包装进COPⅡ (coat proten Ⅱ)囊泡中,运送到高尔基体进行进一步的修饰。经过TGN/EE的转运,这些蛋白质被分选到MVB/PVC/LE中,最终运输至液泡行使功能或降解。内吞体分选转运复合物(endosomal sorting complex required for transport, ESCRT)是一类在真核细胞内质膜系统中高度保守的蛋白质复合物[3]。ESCRT特异性调控PVC/MVB/LE的生成以及从PVC/MVB到液泡/溶酶体的蛋白质分选[4−5]。它在细胞质或质膜中的蛋白质修饰和运输中发挥关键作用,对蛋白质分泌途径、内吞作用以及各种重要的信号通路都有重要贡献[3, 6−7]。
作为固着生物,植物需要不断监测环境变化,这些变化的环境往往不利于植物的生长发育,包括非生物胁迫如盐胁迫、干旱胁迫,生物胁迫如病原体感染。植物为了应对这些不利条件,已经进化出有效而复杂的响应系统[8−9]。在干旱条件下,植物细胞中的脱落酸(abscisic acid, ABA)开始积累,形成ABA-PYRABACTIN RESISTANCE (PYR)/PYR-LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR)-2C型蛋白磷酸酯酶(2C-type protein phosphatases, PP2C)三元复合物和SnRK2 (SNF1-related protein kinase 2) 结合。这种结合导致SnRK2被磷酸化,激活下游基因,积极调控ABA信号响应基因表达[10]。在盐胁迫条件下,植物感知到过量的Na+ 并激活盐过度敏感(salt over-sensitivity, SOS)途径,随后SOS2激酶和质膜定位的Na+ 抗转运蛋白SOS1发挥作用,将多余的Na+ 排出细胞,促进植物的生存和生长[8−9, 11]。植物在与病原体长期的“博弈”中演化出2套免疫系统,即模式诱导免疫(pattern-triggered immunity, PTI)和效应诱导免疫(effector-triggered immunity, ETI)。植物通过细胞膜上的受体蛋白识别病原体携带的一些分子,激活PTI以抵抗病原体入侵。另一类受体,核苷酸结合亮氨酸重复蛋白(nucleotide-binding leucine-rich repeat protein, NLR),感知毒性蛋白,触发ETI,随后激活更强烈的免疫反应[12−13]。
植物细胞中,内膜运输和泛素介导的蛋白质降解途径在胁迫相关货物分子的正确递送中起着重要作用。ESCRT复合体是调节货物蛋白运输和降解的蛋白复合物之一。本研究总结了近年来ESCRT介导的内膜运输系统在植物胁迫响应中的研究进展,以期为构建精准的ESCRT介导逆境响应分子调控网络提供参考。
-
在后生动物和真菌中,ESCRT复合体主要由5个亚基组成:ESCRT-0、ESCRT-Ⅰ、ESCRT-Ⅱ、ESCRT-Ⅲ和VPS4 (vacuolar protein sorting 4)/SKD1 (suppressor of K+ transport growth defect 1)[7, 14]。然而,在植物中没有发现ESCRT-0亚基的同源蛋白,但是拟南芥Arabidopsis thaliana基因组编码9种TOLs蛋白 (TOM1-like proteins)[15−16]。TOLs蛋白具有保守的VHS[VPS27, HRS (hepatocyte growth factor-regulated tyrosine kinase substrate)]和STAM (signal transducing adaptor molecule)结构域,能有效地与泛素结合并行使与ESCRT-0类似的功能[16−18]。此外,在植物中鉴定了其他ESCRT相关蛋白,包括FREE1 (FYVE domain protein required for endosomal sorting 1)[2, 4]、ALIX (apoptosis-linked gene-2 interacting protein X)[19]、PROS (SKD1 positive regulatory factor)[20−21] 和BRAF (Bro1-domain protein as FREE1 suppressor)[22]。
-
ESCRT-0复合物是由2个异聚亚基VPS27/HRS和Hse1 (Hbp STAM, EAST1)/STAM按照数量1∶1构成的多价泛素结合蛋白复合体[5, 23]。VPS27含有1个PSAP基序,可与ESCRT-Ⅰ亚基Vps23结合,从而募集异四聚体ESCRT-Ⅰ [24]。ESCRT-0含有GAT (GGA and Tom1)结构域[25]和多个泛素结合结构域,包括UIM (ubiquitin-interacting motif)结构域和位于氨基末端的VHS结构域[23]。它的主要功能是识别泛素化蛋白并富集底物[2],除此以外,还具有招募网格蛋白(clathrin)、泛素化连接酶及去泛素化酶的作用[26]。
-
ESCRT-Ⅰ复合物由亚基VPS23/TSG101、VPS28、VPS37和MVB12 (MVB sorting factor of 12 kDa)按照1∶1∶1∶1 的数量比组装而成[14, 27]。ESCRT-Ⅰ与位于复合物两端的ESCRT-0和ESCRT-Ⅱ相互作用[28]。酵母细胞中,在VPS23/TSG101的N末端的UEV (ubiquitin E2 variant)结构域与ESCRT-0的P[S/T]-AP基序相互作用[2]。VPS28通过C末端四螺旋束与ESCRT-Ⅱ组分VSP36中的GLUE (GRAM-like ubiquitin binding in EAP45)结构域相互作用[29]。VPS37含有位于N端的碱性α螺旋(N-terminal basic helix, NTBH),能结合酸性膜脂,通过高度柔性区域连接到核心结构[25]。ESCRT-Ⅰ负责将泛素化的膜货物分子分拣到MVB[14, 27]。
-
ESCRT-Ⅱ复合物是一种Y型异源四聚体,由亚基VPS22/EAP30、VPS36/EAP45和VPS25/EAP20以1∶1∶2的数量比例组成的。VPS22通过CC (coiled-coil)结构域与VPS36的C末端结合形成主干[30]。VPS36通过其N端GLUE结构域与ESCRT-Ⅰ组分蛋白VPS28相互作用,从而识别泛素化蛋白。VPS25通过与VPS20相互作用,促进ESCRT-Ⅲ复合物组分的募集和组装[30]。ESCRT-Ⅱ主要通过与ESCRT-Ⅰ协同,有选择地招募泛素化货物分子。
-
ESCRT-Ⅲ复合物由4个核心亚基组成,即VPS2/CHMP2 (charged multivesicular body protein 2)、VPS20/CHMP6、VPS24/CHMP3和SNF7/CHMP4。此外还有3种辅助蛋白,即Did2 (Doa4-independent degradation2)/CHMP1、VPS60/CHMP5和IST1 (increased sodium tolerance 1)。ESCRT-Ⅲ的MIM (MIT interacting motif )可以与VPS4结合,中间柔性区域包括至少1个CC结构域[31−32]。ESCRT通路激活后,VPS20首先被VPS25招募并激活,然后SNF7被多聚化[28]。随后,VPS24桥接SNF7与VPS2[33],进入内吞体后VPS24与VPS2结合。最后,VPS2招募ATP酶VPS4,而Did2招募Vta1或IST1完成组装[26]。ESCRT-Ⅲ主要驱动PVC/MVB腔内囊泡 (intraluminal vesicle, ILV)的形成[31−32]。
-
VPS4/SKD1复合物属于AAA-ATP酶蛋白家族。该复合物也被称为VPS4/SKD1-LIP5 (lyst-interacting protein 5),通过水解ATP促进ESCRT-Ⅲ复合物从PVC/MVB上脱落,分解进入下一个循环[33]。SKD1通过C端的富含α螺旋结构域与LIP5相互作用[34]。LIP5丰度的增加可能会增强SKD1的活性,有助于植物在逆境条件下的耐受性[34]。VPS4/SKD1在维持细胞内ESCRT复合物的稳定性和参与细胞内物质的降解中起着至关重要的作用。
-
FREE1是双子叶植物中特有的ESCRT蛋白,在酵母和动物中均未发现其同源蛋白。FREE1的N端区域具有富含脯氨酸和谷氨酰胺的内在无序区(intrinsically disordered region, IDR)、1个保守的FYVE结构域和C端的CC结构域[4, 35]。FREE1通过N端富含脯氨酸的区域(proline-rich region)与ALIX相互作用[21],通过FYVE结构域,FREE1选择性地识别并结合磷脂酰肌醇-3-磷酸(phosphatidyl inositol-3-phosphate, PI3P),最终定位于MVB [36]。FREE1在植物的生长发育过程、泛素化蛋白的内吞体分选等细胞活动中都是必不可少的[36]。此外,FREE1能与植物自噬调节蛋白SH3P2 (SH3 domain-containing protein 2)发生相互作用,并与磷脂酰肌醇-3-激酶(phosphatidyl inositol-3-kinase, PI3K)复合物结合,调节植物自噬降解[37−38]。
-
ALIX代表了一种进化上保守的ESCRT-Ⅲ相关蛋白,可介导G蛋白偶联受体PAR1和 P2Y1的泛素非依赖性分选到ILV进行降解[39]。在结构上,ALIX具有N端Bro1结构域、中央V结构域和柔性的C端富含脯氨酸的区域[40]。Bro1结构域负责将SNF7募集到囊泡膜上[33],V结构域充当泛素结合结构域,促进ALIX与泛素化蛋白的相互作用[41]。在货物蛋白运输过程中,ALIX严格调节了ESCRT-Ⅰ和ESCRT-Ⅲ复合物的活性,这在控制ABA受体蛋白的丰度和促进其降解等方面起着重要作用[41]。最近的研究阐明了ALIX的非常规ESCRT功能,ALIX通过与逆转运复合体(retromer complex)的核心亚基相互作用来调节可溶性蛋白质的液泡分选[2, 42]。这项研究进一步揭示了植物中液泡运输和内吞体循环途径之间存在交互关系。
-
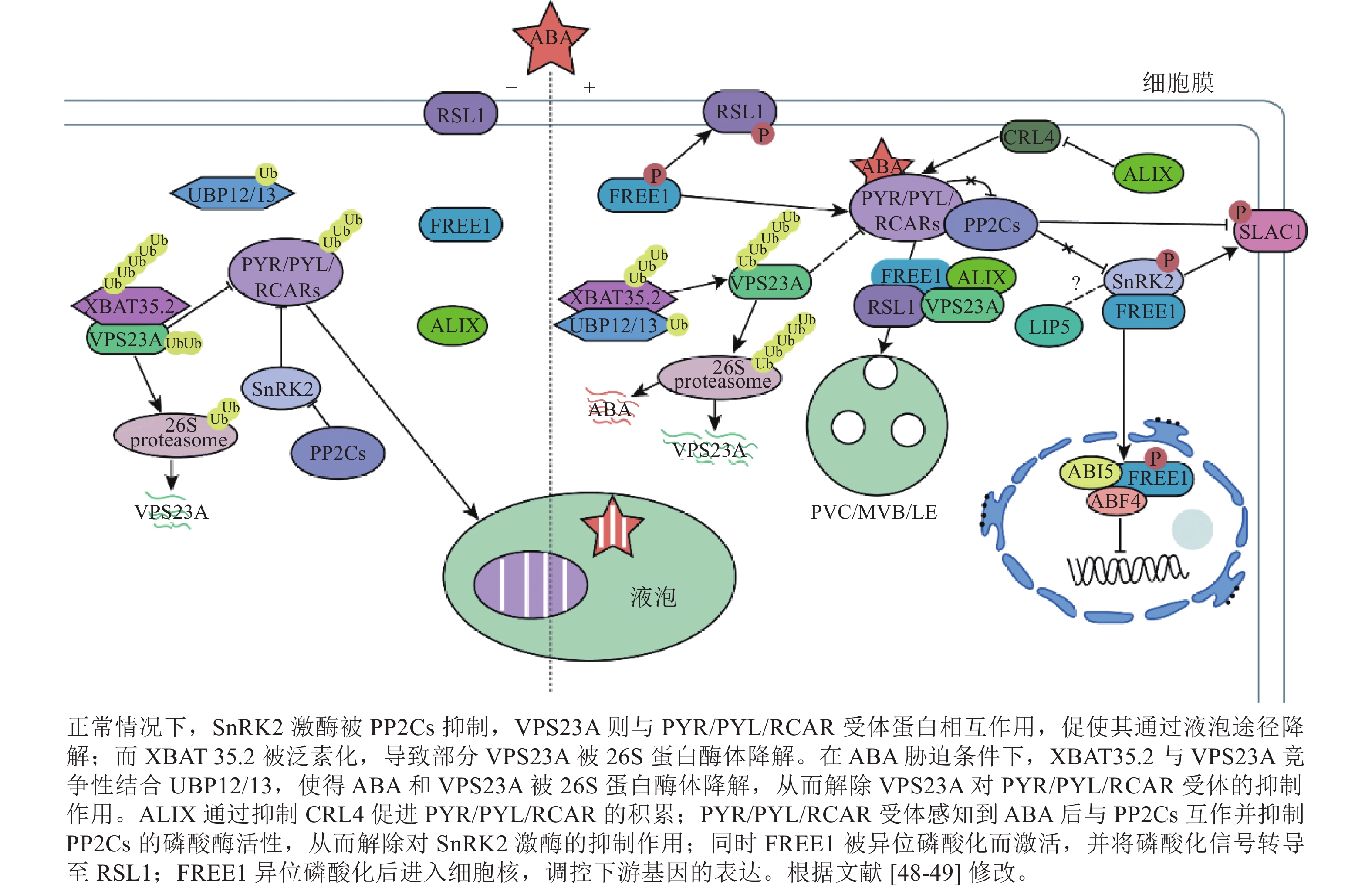
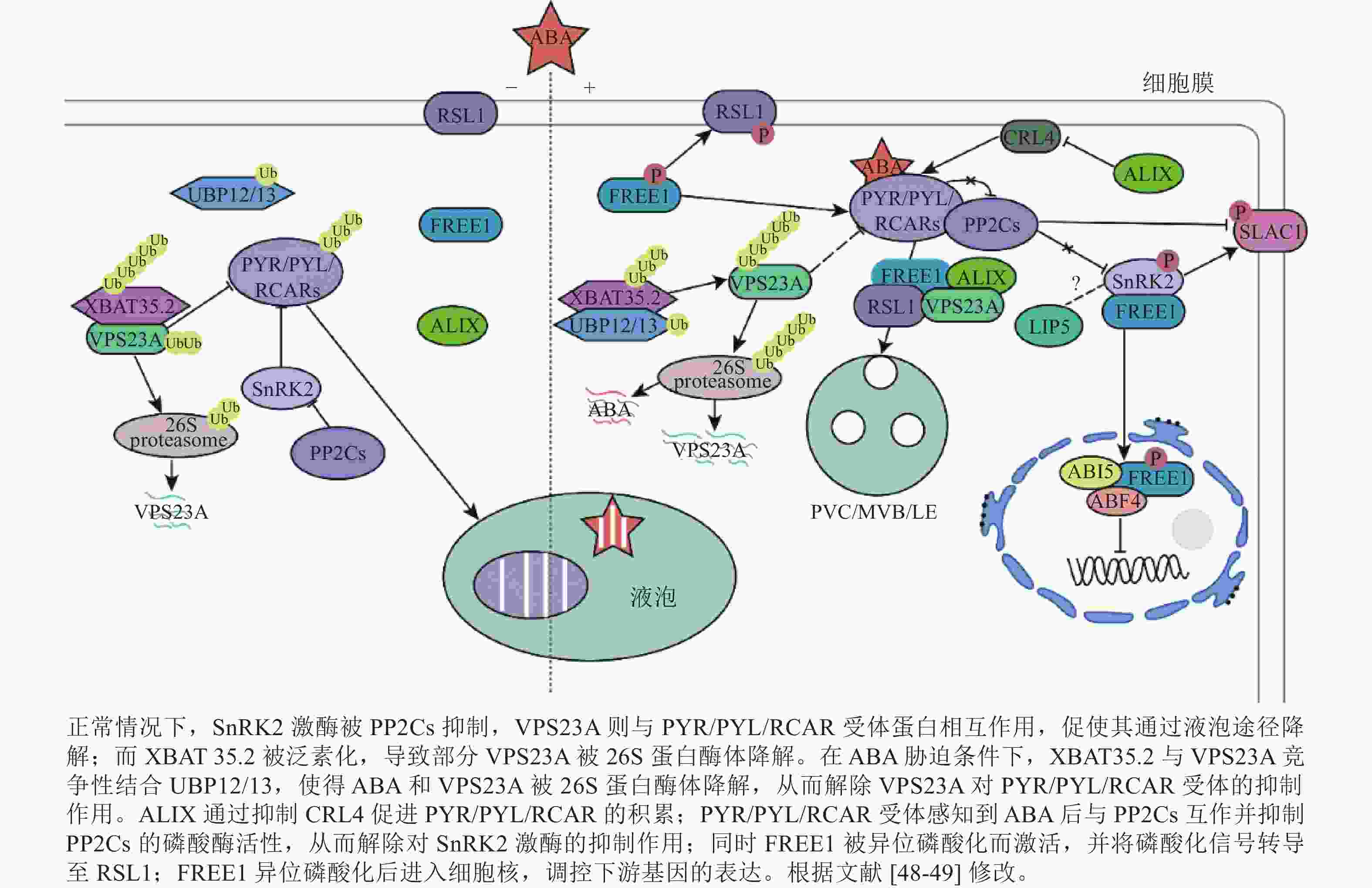
干旱是影响植物正常生长发育的非生物胁迫之一。干旱能破坏渗透调节系统,导致细胞脱水并合成ABA,进而导致气孔闭合、活性氧(ROS)积累和光合作用抑制[10]。ABA信号通路的核心成分包括可溶性ABA受体PYR/PYL/RCAR、PP2C和SnRK2。 PYR/PYL/RCAR受体通过PP2C依赖性SnRK2激活调节ABA转录反应[43−44]。在干旱条件下,ABA浓度的升高导致ABA与PYR/PYL/RCAR受体直接结合,形成ABA-PYR/PYL/RCAR复合物。该复合物抑制PP2C,将SnRK2维持在磷酸化的活性状态,随后激活下游转录因子AREB (ABA responsive element binding protein)/ABF(ABRE binding factor)和阴离子通道SLAC1 (Slow anion channel 1)[45],正向调节ABA响应基因的表达[10, 46]。
PYR/PYL/RCAR受体(PYR1、PYL4、PYL8和PYL9)的泛素化发生在质膜上,由CRL4 (Cullin4-RING E3 ubiquitin ligase)和 RSL1(E3 ubiquitin ligase RING finger of seed longevity 1)触发网格蛋白介导的内吞作用,进而促使了PYR1和PYL4进入囊泡递送至液泡降解,导致ABA信号衰减。ABA受体从质膜到内吞体的转运依赖于ESCRT组分(FYVE1、VPS23A和ALIX)(图1)。这些ESCRT组分直接与未修饰的PYL相互作用[35, 41, 47],随后ABA受体通过26S蛋白酶体或液泡途径降解。
-
VPS23A具有一个泛素偶联酶变体UEV结构域,其中缺乏泛素偶联(ubiquitin-conjugating, UBC)结构域中保守的半胱氨酸,暗示其具有泛素结合能力[48]。作为ESCRT-Ⅰ复合物的组分,VPS23A主要参与质膜货物分子的运输和降解,负调控ABA 信号转导[48]。敲除VPS23A能增强ABA信号通路中关键激酶OST1 (open stomata 1)的活性。VPS23A通过调节OST1的上游关键调控因子,进而在转录和翻译水平上影响关键转录因子的表达。这一过程中,VPS23A不仅识别PYL4,还能识别K63连接的泛素链。此外,VPS23A影响PYR1的亚细胞定位和PYL4的稳定性。说明VPS23A可能识别植物中泛素化和非泛素化的PYLs,从而促进PYLs与PP2C的结合并影响ABA受体的稳定性,从而调节ABA信号转导和抗旱性[47]。此外,有研究表明:去泛素化酶 UBP12/UBP13与E3泛素连接酶XBAT35.2协同调控VPS23A的去泛素化[10]。然而,ubp12和ubp13突变体表现出与vps23a突变体类似的ABA敏感和耐旱性的表型。过表达VPS23A能恢复这些表型。但是VPS23A蛋白的稳定性随着ABA诱导 UBP12/UBP13 蛋白水平的升高而降低。UBP12/UBP13的积累增强了VPS23A的E3连接酶XBAT35.2的去泛素化。XBAT35.2微妙地调节了VPS23A的蛋白质水平,对ABA反应起到了正调节作用[10, 48]。总而言之,UBP12/UBP13通过对VPS23A进行精确的泛素化修饰,间接调控ABA信号转导。这对ABA通路和ESCRT复合体之间的相互作用提供了新的思路。
-
FREE1蛋白N端(含有1个IDR)以不依赖ABA的方式与ABA受体相互作用[35],随后RSL1蛋白将PYL4募集到内吞体表面。在干旱条件下,FREE1可通过异位磷酸化激活,与RSL1形成复合物,并将磷酸化信号转导至RSL1。从而启动钙信号、ROS通路等关键胁迫信号通路,激活靶基因,增强抵御外界环境胁迫的能力。有研究表明:free1突变体表现出对ABA的超敏反应[50]。free1突变体中ABA受体的液泡运输受阻,导致PYL4积累和对ABA的反应性增强。这项研究证明了FREE1在ABA受体从质膜转运到内吞体/液泡降解中的功能。此外,FREE1还在细胞核中转录抑制ABA信号转导作用[50−51]。ABA处理后,SnRK2激酶与FREE1相互作用,磷酸化C端CC结构域中的丝氨酸残基。随后FREE1向细胞核中迁移。进入细胞核后,FREE1就会与碱性亮氨酸拉链转录因子(basic leucine zipper transcription factor, bZIP) ABA反应元件结合因子4 (ABA-responsive element binding factor 4, ABF4)和ABA不敏感因子5 (ABA-insensitive 5, ABI5)相互作用,减弱它们与顺式调节序列下游基因的结合,从而抑制转录活性,削弱ABA反应并减轻ABA对植物生长的抑制[11]。具有C端部分缺失或磷酸化丝氨酸位点突变的free1-c端突变体植物,避免了free1功能缺失突变体的胚胎致死性,并在正常生长条件下表现出正常的液泡分选和植物发育。这些研究证明了FREE1磷酸化的缺失和入核受阻会增强植物对ABA的响应,说明了内膜转运和ABA信号在转录水平上相互关联。暗示了植物ESCRT蛋白FREE1在细胞质和细胞核中具有双重功能[50]。
-
有研究表明:ALIX参与调节植物气孔开闭和ABA受体的降解。ALIX能直接与 PYR/PYL/RCAR受体相互作用,通过抑制CRL4和E3泛素连接酶来促进PYL8的积累[52−53]。ALIX与PYL的相互作用可能依赖于C端的IDR区域。ALIX的Bro1结构域中260位的甘氨酸突变为天冬氨酸能破坏其与PYL和VPS23A的相互作用,暗示了这个在维持货物与受体复合物完整性中的作用,并为alix-1突变体对ABA的超敏反应提供了分子基础[41, 54]。ALIX功能受损会导致亚细胞定位发生改变,进而过度积累PYR/PYL/RCAR受体,这意味着ALIX作为负调节因子参与ABA信号通路的调控[41]。
-
LIP5 (lyst-interacting protein 5)作为SKD1的正调节因子,激活SDK1的ATP酶活性[55]。LIP5属于Vtal家族,是一种参与各种生物过程的ESCRT-Ⅲ相关蛋白。它在ABA介导的信号转导中发挥关键作用来正向调节抗旱性[56]。在正常条件下,LIP5蛋白不稳定,细胞中丰度较低[57]。LIP5的表达受到ABA和干旱的诱导,其过表达会导致植物的ABA超敏反应,造成气孔过度闭合,减少水分流失,从而提高耐旱性。相反,lip5突变体表现出ABA不敏感的表型,这个表型进一步说明了LIP5在抗旱中的作用[56]。
-
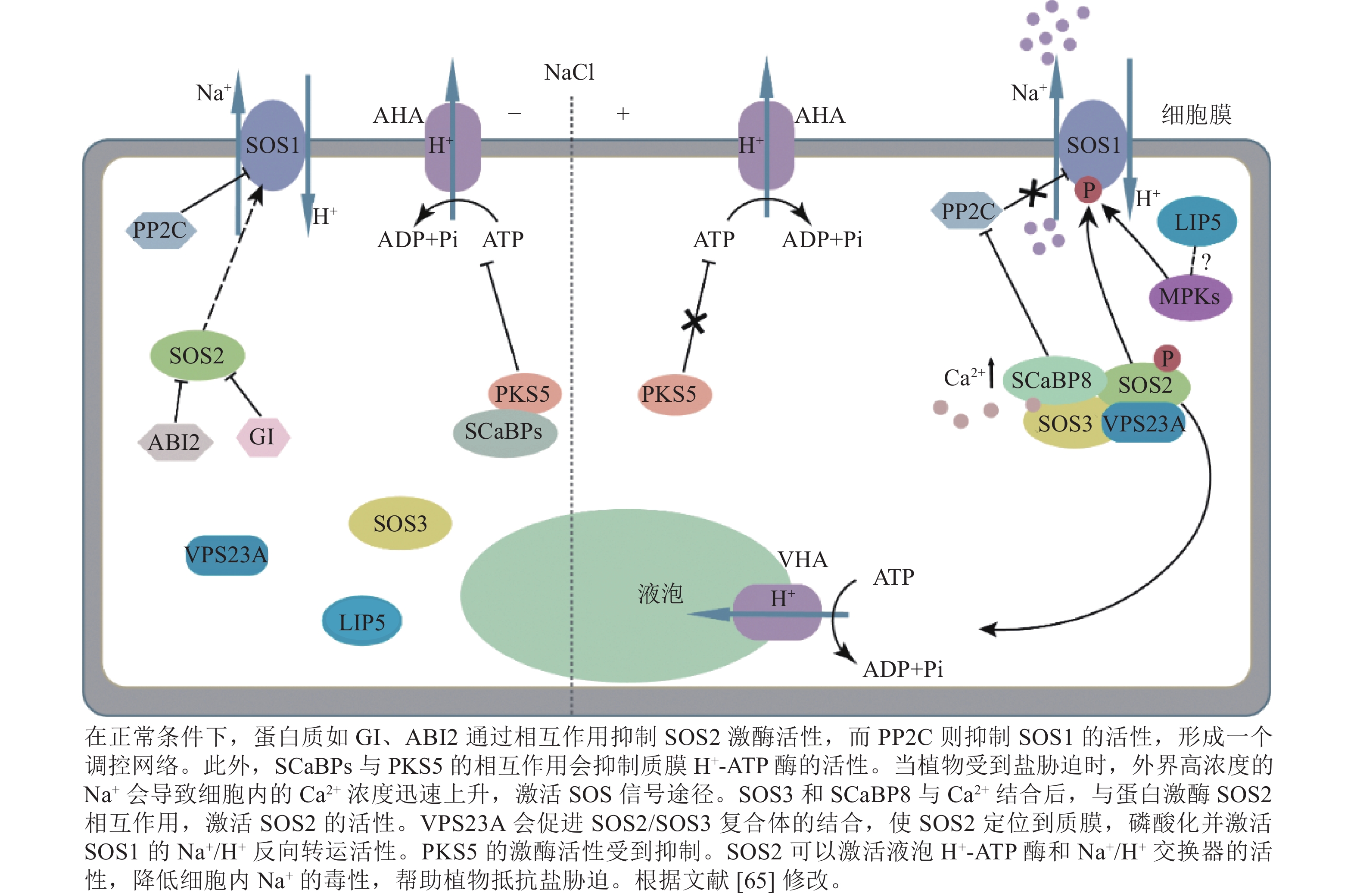
土壤盐碱化是严重影响植物生长发育的非生物胁迫之一,在世界范围内给作物生产造成了巨大损失[58−59]。盐胁迫会升高细胞渗透压,导致钠积累。遭受盐胁迫后,植物可通过调节离子稳态、激活渗透胁迫途径、激活激素信号通路,以及调节细胞骨架动力学和细胞壁组成等多种机制进行应对[60]。作为保守的盐胁迫响应信号途径,SOS信号通路由钠转运蛋白SOS1、调节蛋白SOS2和SOS3,以及SOS3钙结合蛋白8 (SOS3-like calcium-binding protein 8/ calcineurin b-like protein10, SCaBP8/CBL10)组成。受到盐胁迫后,植物细胞通过SOS3/SCaBP8-SOS2-SOS1的顺序激活而将胞内过量 Na+ 排出,以维持 K+/Na+平衡,保护植物免受盐胁迫[9, 11]。
-
SOS途径通过调节盐胁迫下Na+/H+逆向转运蛋白活性来维持离子稳态。SOS1是植物耐盐能力的关键Na+/H+反向转运蛋白,能利用H+梯度来驱动Na+排出细胞,其由定位于质膜的具有12个跨膜结构域的N端和定位于细胞质C端自抑制区域组成[61]。过表达SOS1能维持较高的K+/Na+,因而增强了植物的耐盐性[62]。SOS2被NaCl激活,随后磷酸化SOS3/SCaBP8。被招募到质膜后,SOS2能激活质膜H+-ATP酶,为SOS1的Na+转运活性提供能量[63−64]。SOS3/SCaBP8可以感知盐胁迫诱导的Ca2+升高,在结合Ca2+时与SOS2相互作用,并磷酸化SOS2。SOS3蛋白的中间区域能通过肉豆蔻酰化与质膜相连,并因此将 SOS3/SCaBP8-SOS2复合物招募到质膜,最终磷酸化SOS1增强其Na+/H+逆向转运活性[61]。
盐胁迫可以刺激植物细胞ESCRT复合物的活性和组装,从而促进质膜定位的转运蛋白的内吞作用和降解。因此,盐胁迫信号通路与ESCRT复合体之间的联系对于理解植物响应盐胁迫的机制尤为重要(图2)。
-
ESCRT-Ⅰ组分VPS23A通过增强SOS途径调节植物耐盐性[11]。VPS23A与SOS2、SOS3之间均存在相互作用,但不与SOS1互作。VPS23A与SOS2互作促进其泛素化,从而调控SOS2蛋白向质膜的循环再利用。而VPS23A功能缺失突变体对盐胁迫敏感,由于SOS2细胞膜定位减少,导致SOS1的磷酸化水平降低,SOS1活性受到抑制,使得Na+外排能力降低,从而影响植物对盐胁迫的响应。因此,VPS23A的功能是促进SOS2/SOS3复合体的结合来驱动SOS2定位到细胞膜[11]。
-
LIP5调控的内吞体分选途径是植物响应非生物胁迫的关键细胞过程之一[55]。LIP5与SKD1相互作用提高植物的盐耐受性。LIP5是盐诱导的细胞内活性氧增加所必需的,与盐胁迫反应的信号转导有关。盐胁迫在很大程度上依赖于LIP5刺激植物细胞内吞作用和囊泡运输。在缺乏功能性丝裂原活化蛋白激酶(mitogen-activated protein kinase 3/6, MAPK3/6)的情况下,LIP5蛋白会被快速降解[36]。在盐胁迫下,LIP5被MAPK磷酸化的水平增加,同时稳定性也增加,因而蛋白水平升高。然而LIP5中MAPK磷酸化位点的突变降低了LIP5的稳定性,并破坏了LIP5突变体盐敏感表型回补的可能性[36, 55]。
-
植物在自然生境中会遭受各种生物胁迫,包括各种微生物、病原菌的侵染和植食性昆虫的取食等。植物免疫系统是植物在自然生态系统中生存和生产的基础。植物为抵抗病原菌的入侵,进化出2层先天免疫系统来检测和应对各种生物攻击,分别由细胞表面定位的模式识别受体(pattern-recognition receptors, PRRs)和细胞内核苷酸结合域富含亮氨酸重复序列的受体(nucleotide-binding leucine-rich repeat proteins, NLRs)启动[66]。
-
第1层免疫系统通过PRRs直接识别病原体相关分子模式(pathogen-associated molecular patterns, PAMPs)或损伤相关分子模式(damage-associated molecular patterns, DAMPs),触发PTI [13]。PTI具有较低的病原体识别特异性,早期不诱导强烈的免疫反应。PTI通过细菌鞭毛蛋白等PAMPs激活MAPK依赖和MAPK不依赖的信号通路[12]。PTI在抑制病原体入侵和维持植物叶片微生物群落的动态平衡方面发挥着重要作用。为了抑制PTI,病原体通常会向植物细胞传递效应物,这些效应物可能被NLR受体蛋白直接或间接识别并激活第2层更强的免疫信号ETI [13],ETI具有较高的病原体识别特异性,通常表现为与快速程序性细胞死亡相关的超敏反应[12]。PRRs和NLRs发起的信号传导导致了大量重叠的下游细胞反应,包括防御基因表达、ROS产生和胼胝质沉积[13, 67]。在免疫反应早期,NADPH氧化酶RBOHD介导的ROS产生是连接PRR和NLR介导的免疫反应的关键。此外,受体样细胞质激酶BIK1 (Botrytis-induced kinase 1)是ETI期间RBOHD、基因表达和细菌抗性充分激活所必需的[66−67]。flg22是细菌鞭毛蛋白中的一个保守的小肽,它通过识别PRR蛋白FLS2 (flagellin sensing 2)及其共受体BAK1引发免疫反应。flg22是研究最多的PAMP,其诱导的免疫是研究植物与微生物相互作用的最常使用的模式系统之一。
-
ESCRT复合体亚基及其相关蛋白的突变通常会导致严重的发育缺陷,然而,vps37-1和vps28-2突变体没有明显的发育缺陷,但都对病原菌感染更敏感,可见VPS37-1和 VPS28-2调控了FLS2的MVB分选,在flg22激活的气孔防御和植物免疫中起着至关重要的作用[68]。
-
拟南芥LIP5是植物基础防御中病原响应MAPK级联反应的关键靶点,正向调节MVB的生物发生。正常情况下LIP5表达水平较低, 但病原菌诱导后LIP5与MAPK3/6相互作用并磷酸化,LIP5蛋白稳定性和含量得到提升,这对病原体诱导的囊泡运输和植物基础免疫力都非常重要[36]。如盐胁迫响应通路一样,LIP5在植物免疫系统中的关键作用也依赖于其与SKD1的相互作用,进而刺激SKD1的ATP酶活性。虽然LIP5蛋白的MAPK磷酸化位点突变不会影响其与SKD1互作,但会降低其稳定性[36, 55, 69]。
-
酵母ESCRT蛋白Vps23、Vps24、Snf7和Vps4的表达抑制了病毒在植物宿主中的复制。对番茄丛状突病毒(tomato bushy stunt tombusvirus, TBSV)的全基因组筛选确定了7种参与病毒复制和复制酶复合物组装的ESCRT蛋白[70]。TBSV p33复制蛋白可直接与酵母ESCRT组分Vps23和Bro1相互作用,将Vps23招募到病毒复制相关的过氧化物酶体上。在缺乏ESCRT蛋白的情况下,病毒RNA更容易被核糖核酸酶降解,ESCRT蛋白在复制酶复合体中对病毒RNA起到了保护作用。并且复制酶复合体的精确组装可能依赖于ESCRT蛋白,这种依赖关系可能帮助病毒逃避宿主防御监视系统的识别,并防止基因沉默机制对其RNA的破坏[70]。
-
随着全球气候变化和生态压力的增加,植物如何应对各种逆境胁迫已经成为植物学和农学领域的重要研究课题。最新研究发现:ESCRT复合体在调控植物胁迫响应中起着关键作用。例如,在干旱胁迫条件下,ESCRT通过调节ABA的积累和运输来帮助植物适应水分不足的环境。在盐胁迫下,ESCRT复合体参与Na+的排出和细胞壁的修复,以减轻盐害对植物的影响。此外,ESCRT还在生物胁迫中发挥作用,如参与植物与病原菌之间的相互作用。本研究总结了ESCRT复合体和相关蛋白参与调控植物非生物和生物胁迫响应分子机制的最新研究(表1)。
ESCRT亚基 基因名(基因编号) 调控胁迫响应中发挥的功能 参考文献 ESCRT-Ⅰ VPS23A(AT3G12400) 与SOS2/SOS3复合物相互作用进而增强耐盐性 [11] 与ABA受体相互作用并介导其液泡降解 [10, 11, 47] VPS28-2(AT4G05000)
VPS37-1(AT3G53120)调节病毒的基因表达,参与病毒粒子的组装和释放 [68] ESCRT-Ⅲ Vps24(AT5G22950)
Snf7(AT2G19830)
Vps4(AT2G27600)保护病毒RNA不受核糖核酸酶的影响 [70] VPS4/SKD1 LIP5(AT4G26750) 调节离子平衡、渗透势和应激相关基因的表达 [36, 55] 促进ABA的合成和信号转导 [56] 影响植物免疫应答的其他信号转导途径 [36, 55, 69] ESCRT相关蛋白 FREE1(AT1G20110) 利用内吞体和非内吞体功能反向调节ABA信号 [35, 50−51] ALIX(AT1G15130) 控制ABA受体的积累 [41, 52−53] Table 1. ESCRT machineries regulating stress responses
尽管现有的研究已经揭示了诸如FREE1、VPS23A等ESCRT蛋白在植物应对逆境胁迫中的重要作用,但是鉴于ESCRT复合体的复杂性和多样性,其余ESCRT蛋白应对逆境胁迫的功能探索尚处于初级阶段。此外,植物ESCRT蛋白有着数量不等的同源基因,如ESCRT-Ⅰ蛋白VPS23A和VPS23B、ESCRT-Ⅲ蛋白SNF7-1和SNF7-2等,这些同源基因编码的蛋白在调控逆境胁迫响应过程中是发挥不同的功能还是功能冗余尚不清楚。因此有必要深入发掘ESCRT调控植物逆境胁迫响应的分子机制。随着研究技术的更新迭代,超分辨显微镜、3D电子断层扫描成像、光电联用显微镜等更高分辨率的成像技术逐渐应用到细胞生物学领域。在纳米级分辨率下解析不同细胞器的形态特征变化,可以更加透彻地了解ESCRT和PVC/MVB/LE在植物逆境胁迫响应中的功能。总之,对ESCRT介导的内膜运输在调控植物应对逆境胁迫中的研究为农业生产和环境保护提供更多创新的思路和方法。
Research advances on the plant ESCRT machinery regulation of stress responses
doi: 10.11833/j.issn.2095-0756.20240166
- Received Date: 2024-02-04
- Accepted Date: 2024-04-03
- Rev Recd Date: 2024-04-02
- Available Online: 2024-09-25
- Publish Date: 2024-09-25
-
Key words:
- endosomal sorting complex required for transport (ESCRT) /
- stress responses /
- endomembrane trafficking /
- PVC/MVB /
- abscisic acid (ABA) /
- SOS pathway
Abstract: Stress is one of the major reasons causing global crop yield decline. Under stress conditions, the intracellular protein trafficking needs to be adjusted rapidly to ensure the correct delivery of the associated cargo molecules via endomembrane system. The endomembrane system in eukaryotic cells contains diverse membrane-bound organelles, which are accurately and efficiently generated in a well-organized way. These organelles play essential roles in protein transport. The endosomal sorting complex required for transport (ESCRT) complex mediates the biogenesis of prevacuolar compartment/multivesicular body (PVC/MVB), facilitating the vacuolar trafficking of the ubiquitinated proteins. This review highlights the recent research on ESCRT machinery in plant stress responses, including the basic composition and function of ESCRT, and the regulatory role of ESCRT in plant abiotic stress (i.e. drought and salt stress) and innate immunity. To explore how ESCRT specifically recognizes and regulates stress response proteins, it will be helpful to construct a more precise ESCRT-mediated molecular regulatory network of stress responses. [Ch, 2 fig. 1 tab. 70 ref.]
| Citation: | LI Juan, CAO Yueyinglun, SHI Linjuan, et al. Research advances on the plant ESCRT machinery regulation of stress responses[J]. Journal of Zhejiang A&F University, 2024, 41(5): 1094-1104. DOI: 10.11833/j.issn.2095-0756.20240166 |










 DownLoad:
DownLoad: