-
核桃Julgans regia是胡桃科Juglandaceae核桃属Juglans核桃组植物[1]。中国种植核桃的历史可追溯到3 000 a前,且核桃分布广泛[2],品种繁多,产量高。中国为核桃六大主产国之一[3]。有关研究显示,在城区内大面积种植核桃,并搭配其他园林植物,可有效减轻颗粒物污染浓度。核桃具有极高的生态价值[4]。核桃木材外观优美、质地坚硬、材性优良,可做家具、木地板等;核桃青皮中的萘醌类物质可作染料,黄酮类物质可作抗氧化药品[5]。核桃果实富含蛋白质、磷脂以及维生素B1等能增强细胞活力、提高脑神经功能的物质,具有极高的药用价值,被称为“21世纪的超级食品”[6−7]。
恶劣的生长环境会导致核桃停止发育甚至死亡,如重度干旱环境等[8]。中国干旱、半干旱区面积约占国土面积的1/3以上,干旱地区降水稀少,水资源匮乏,无法满足核桃生长需求[9−10]。目前研究发现,抗旱关键酶基因包括α糖类、脯氨酸合成酶、甜菜碱,抗旱相关联蛋白包括LEA (late embryogenesis abundant)家族蛋白、蛋白激酶SnRK2等,其中LEA 第二家族成员脱水素蛋白(dehydrin, DHN)最早于受水分胁迫的水稻Oryza sativa中被发现,后来被证实广泛存在于植物细胞中[11]。许多研究都表明:非生物逆境胁迫下,植物中DHN基因的表达和积累与植物适应逆境的能力相关。如刘慧春等[12]将牡丹Paeonia suffruticosa PsDHN1基因转入拟南芥Arabidopsis thaliana中,发现转基因拟南芥耐涝抗旱水平明显优于野生型。史学英等[13]将从小麦Triticum aestivum中获得的DHN14基因转入大肠埃希菌Escherichia coli,发现可以提高在低温、干旱、金属离子等非生物胁迫下大肠埃希菌的存活率。LIU等[14]将玉米Zea mays中提取的ZmDHN13转入烟草Nicotiana tabacum,发现可显著提升转基因烟草对氧化损伤的耐受性。DHN可以结合金属离子,从根本上减少活性氧(ROS)的产生;DHN还可以结合DNA,保护其不受外界环境压力带来的损害;DHN非特异性地与生物膜结合,从而维持生物膜结构的稳定性等[15]。
在全球干旱的大背景下,水资源短缺仍会是未来中国甚至全球面临的主要问题,因此,研究植物的抗旱机制及挖掘抗旱基因都具有重要意义[16]。本研究分析了核桃脱水素JrDHN基因对干旱逆境胁迫的响应机制,为该基因进一步应用于核桃抗旱分子标记辅助育种提供参考依据,为利用基因工程手段培育抗旱核桃新品种提供理论依据。
-
野生型核桃(WT)来自浙江农业大学省部共建亚热带森林培育国家重点实验室的奇异核桃J. hindsii×J. regia苗。前期已获得奇异核桃JrDHN过表达体胚,并萌发获得JrDHN过表达株系幼苗,后经扩繁并分为JrDHN1、JrDHN2、JrDHN3等3个株系,获得每株系50株生长健壮,长势一致的组培苗后,进行后续阳性鉴定及干旱胁迫试验(图1)。核桃苗于培养室中培养,培养室温度为(25±2) ℃,湿度为80%~85%,光照强度为15 000~20 001 lx,光照周期黑暗8 h/光照16 h。
-
选用北京天根生化科技有限公司的多糖多酚植物总 RNA 提取试剂盒提取核桃体胚的 RNA,基因克隆及后续的验证过程采用 TaKaRa 公司的 cDNA 反转录试剂盒。以反转录cDNA为模板进行荧光定量PCR (qPCR)表达分析,采用NCBI网站在线设计引物,引物见表1。qPCR反应体系为:TB Green 0.5 μL,F/R primer 0.2 μL,cDNA 0.4 μL,ddH2O 4.2 μL。反应程序为:95 ℃ 10 min,95 ℃ 10 s,60 ℃ 31 s,40个循环;95 ℃ 15 s,60 ℃ 1 min,95 ℃ 30 s,60 ℃ 15 s。
引物 序列(5′→3′) Actin-F ATGATGTCAAGGTTAAGGACTC Actin-R CACAATGATCTCAGCTCCG QJrDHN-F ATTCAGCTCACCGACGAACA QJrDHN-R CTCCTCATGCTGCTGCTTCT Table 1. qPCR primers
-
在体式荧光显微镜下观察再生JrDHN过表达植株茎和茎横切中绿色荧光标记蛋白(GFP)荧光激发情况,散发绿色荧光的植株为阳性植株。
-
配置质量分数为5%PEG
8000 的DKW固体培养基,模拟干旱胁迫处理,分别设置模拟干旱胁迫时间分别为7、14、21、28 d的处理组,以培养于DKW正常培养基(001)中的核桃体苗为对照组,每组设置3个生物学重复。 -
在模拟干旱环境中处理0和14 d后,分别剪取同一部位WT和JrDHN过表达植株的叶片,在叶片下表面涂抹1层指甲油,风干后撕下盖上盖玻片在生物显微镜下观察并拍照。取0 d叶片加石英砂研磨,在显微镜下观察并记录叶绿体形态。
-
分别剪取干旱胁迫处理7、14、21、28 d的JrDHN过表达株系和WT叶片,于大试管中加入20 mL配置好的DAB (1.0 g·L−1)、NBT (0.5 g·L−1)染液。室温下避光染色1~3 h,加入30 mL 体积分数为95%的乙醇,将大试管置于100 ℃沸水中水浴加热15 min,将叶绿素完全洗脱干净,之后固定拍照。
-
分别取WT及JrDHN1干旱胁迫7、14、21、28 d的叶片0.1 g,加入10 mL 体积分数为 95%的乙醇溶液。黑暗条件下浸提48 h。以体积分数为95%乙醇为空白对照,测定波长663和646 nm处的吸光度,按叶绿素总质量分数=[20.2D(645)+8.2D(663)]×[V/(1 000W)]公式计算,其中V表示叶绿素提取液总体积,W表示所用叶片鲜质量。超氧化物歧化酶(SOD)、过氧化酶(POD)、过氧化氢酶(CAT)活性及超氧阴离子自由基(O2·−)、过氧化氢(H2O2)、丙二醛(MDA)均使用苏州科铭生物技术有限公司试剂盒进行测定。具体方法见使用说明书。
-
Ca2+与 CaM形成的复合物可以与线粒体膜上的 NADKc结合,驱动RBOH蛋白产生细胞外ROS[17],从而促进胞外ROS向胞内转移[18]。通过这种方式,可以改变细胞质中信号成分的氧化还原状态,从而调节多种蛋白质和转录因子[19],后续可以调节一系列抗旱相关基因的表达,如ADH、SOD、POD基因等[20]。因此,对WT及JrDHN1在干旱胁迫28 d下核桃基因组中的ABA信号转导通路途径中的关键节点基因MYB、ADH、CAM表达量进行qPCR检测。测定方法同上。
-
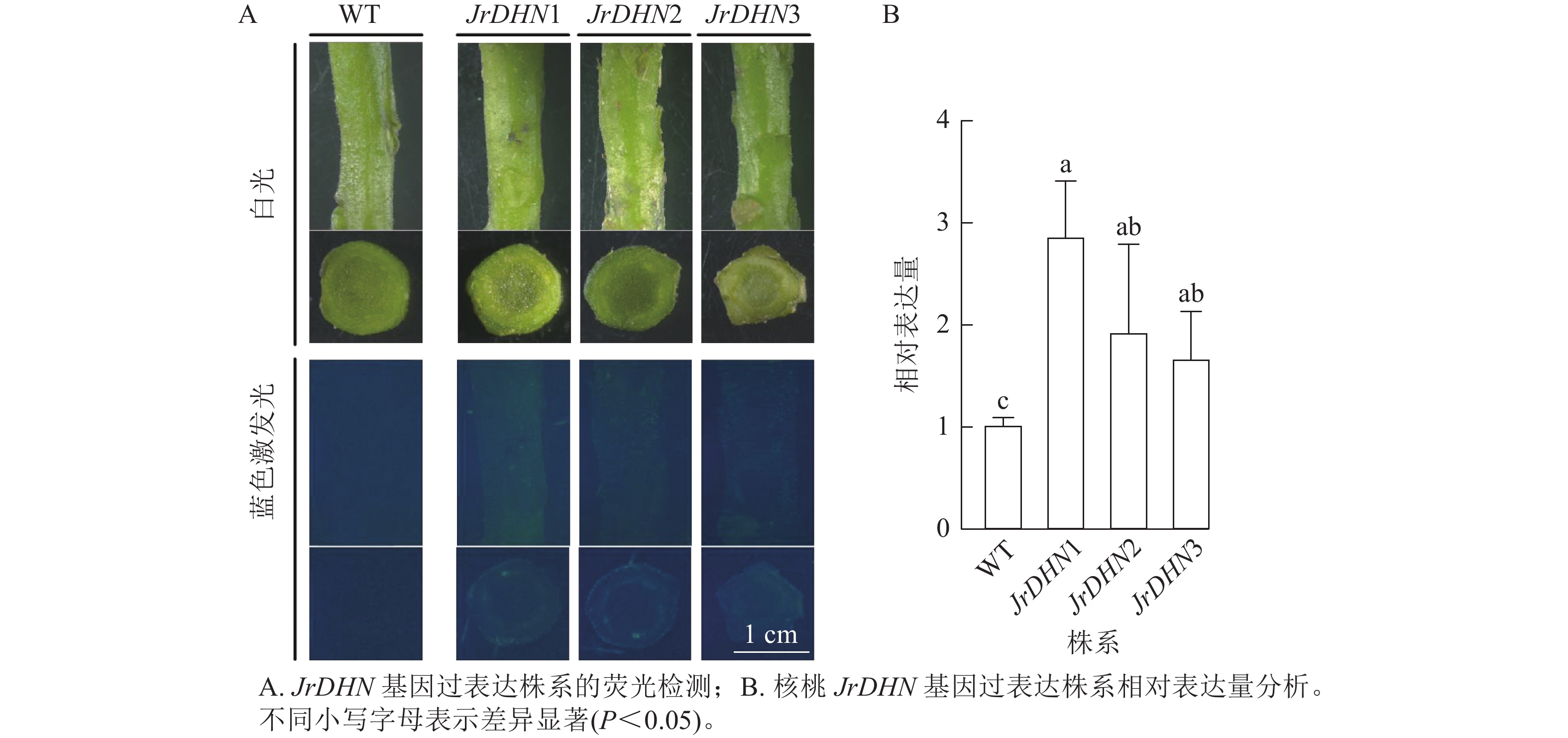
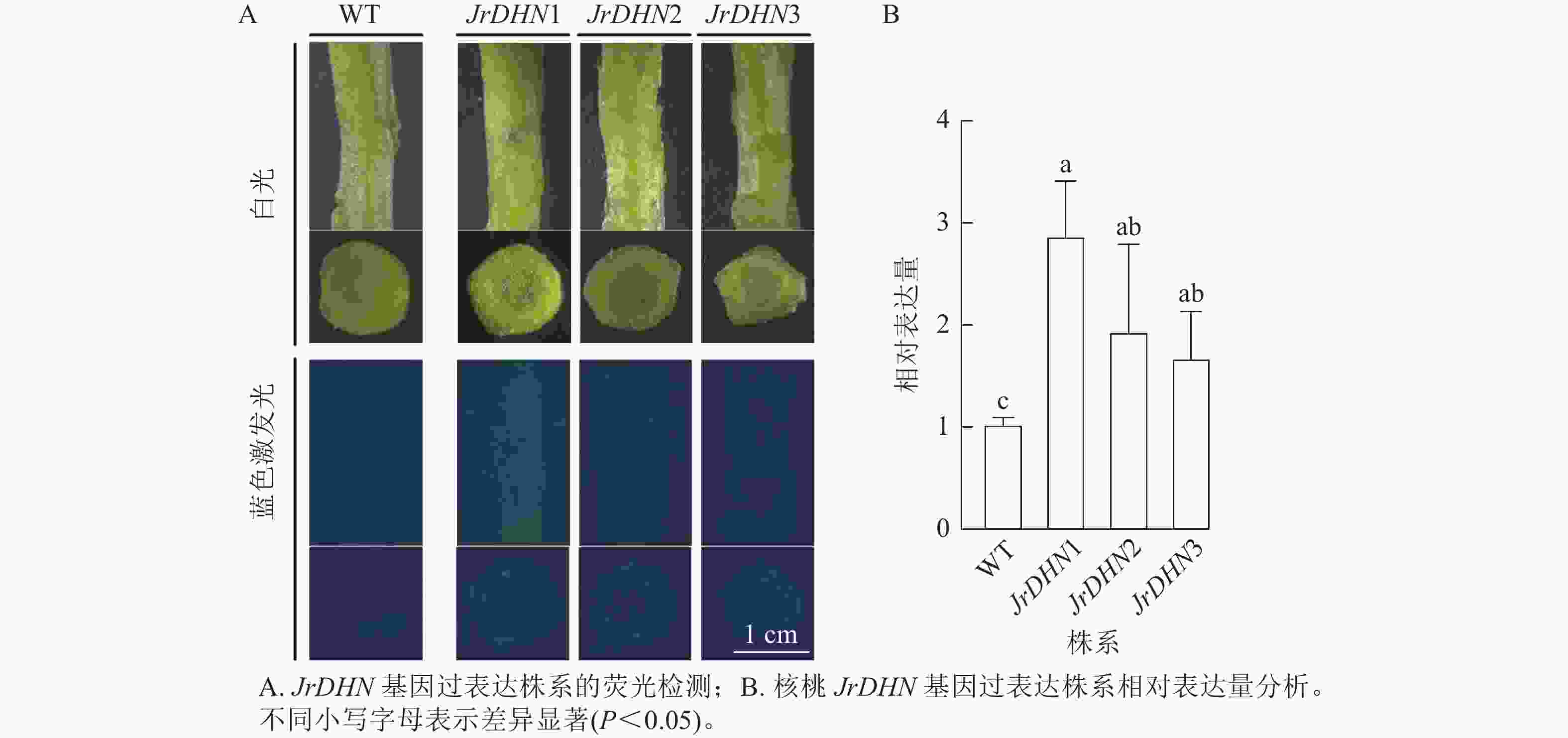
从图2A可以看出:在白光视野下,WT和JrDHN1、2、3过表达株系的形态相似;在蓝色激发光(488 nm)下,WT的茎及横切面无荧光激发,JrDHN1、2、3过表达株系在蓝色激发光下呈现绿色荧光。以上表明JrDHN1、2、3过表达株系体内均表达了GFP荧光蛋白,为阳性植株。
实时荧光定量PCR结果(图2B)显示:核桃JrDHN过表达再生株系JrDHN基因相对表达量均显著高于野生型(P<0.05),JrDHN1株系的相对表达量为野生型的2.55倍,JrDHN2株系的相对表达量为野生型的1.72倍,JrDHN3株系的相对表达量为野生型的1.49倍,差异均显著。JrDHN1、2、3之间基因表达量差异不显著,但JrDHN1株系过表达量最高。
-
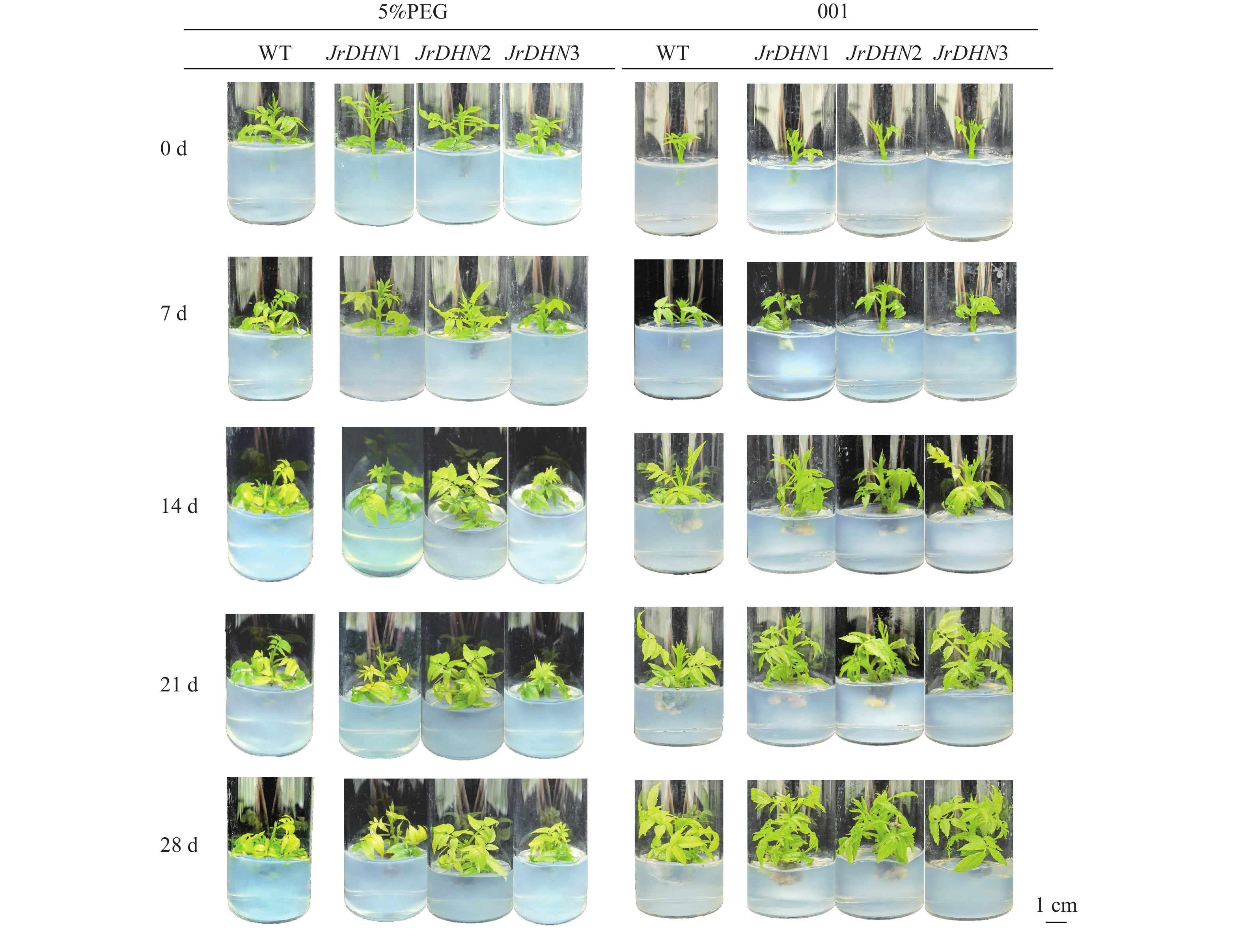
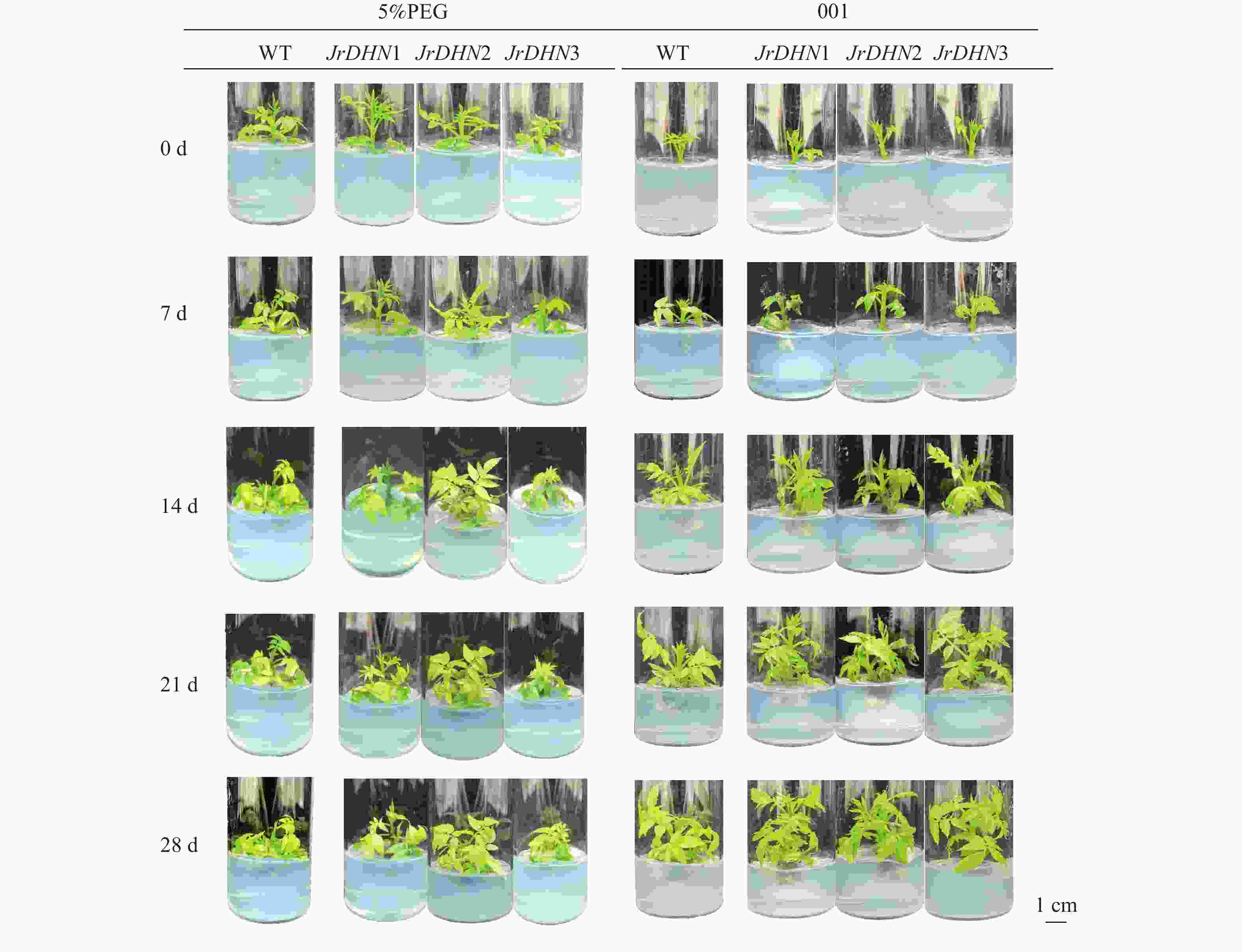
由图3可见:WT和3个JrDHN过表达株系在001培养条件下均能正常生长,28 d时其表型无明显差异。在PEG处理下,随着胁迫时间延长,WT和过表达株系叶片逐渐黄化脱落。干旱胁迫处理14 d时,WT叶片大部分已经脱落,而JrDHN过表达株系叶片脱落较少,初步说明JrDHN过表达株系的抗旱能力强于WT。
-
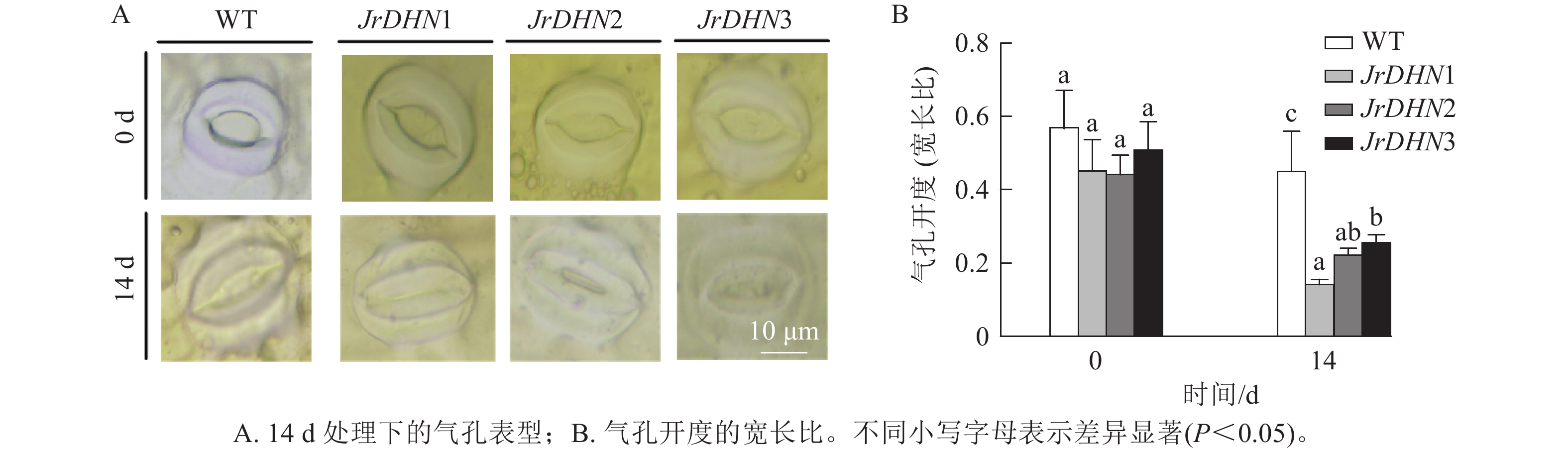
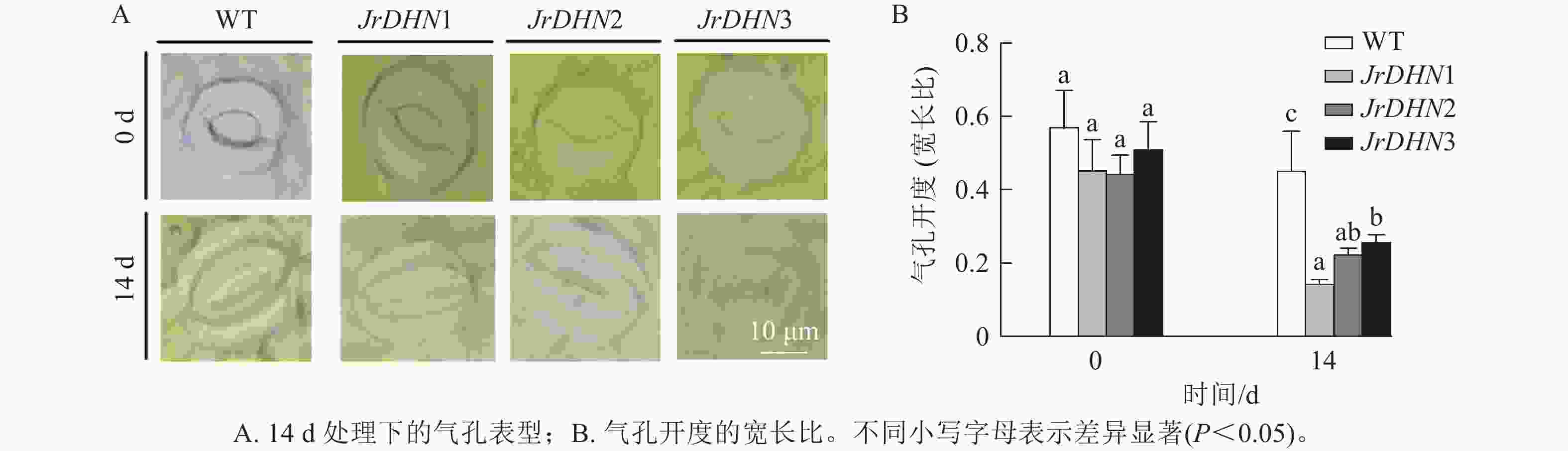
如图4所示:在0 d时,WT及JrDHN1、2、3的气孔开度(宽长比)分别为0.57、0.46、0.45、0.51。3种JrDHN过表达株系的气孔开度与WT差异不显著。而在PEG胁迫14 d时,WT和JrDHN 过表达株系气孔开度均缩小,其中WT气孔开度为0.46,JrDHN1、2、3气孔开度分别为0.15、0.23、0.26,JrDHN过表达株系的气孔开度显著低于WT (P<0.05),其中,JrDHN1的气孔开度显著低于JrDHN3 (P<0.05)。说明过表达JrDHN基因可降低植株在干旱胁迫下的气孔开度,从而提高核桃对干旱的耐受能力。
-
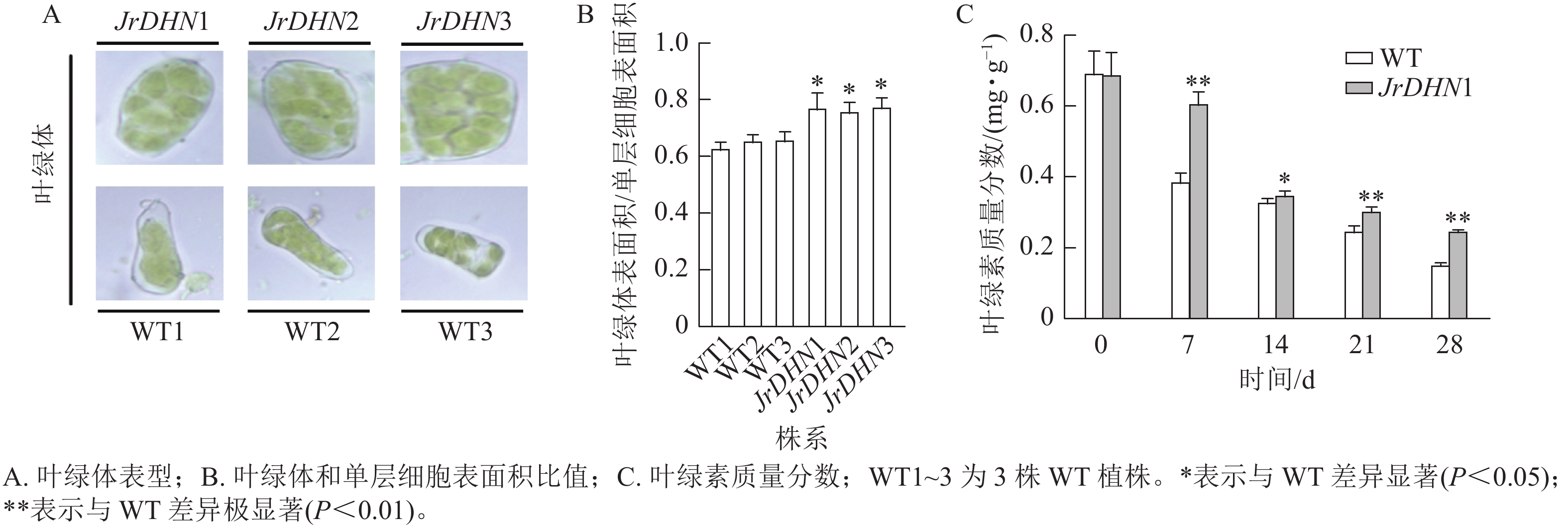
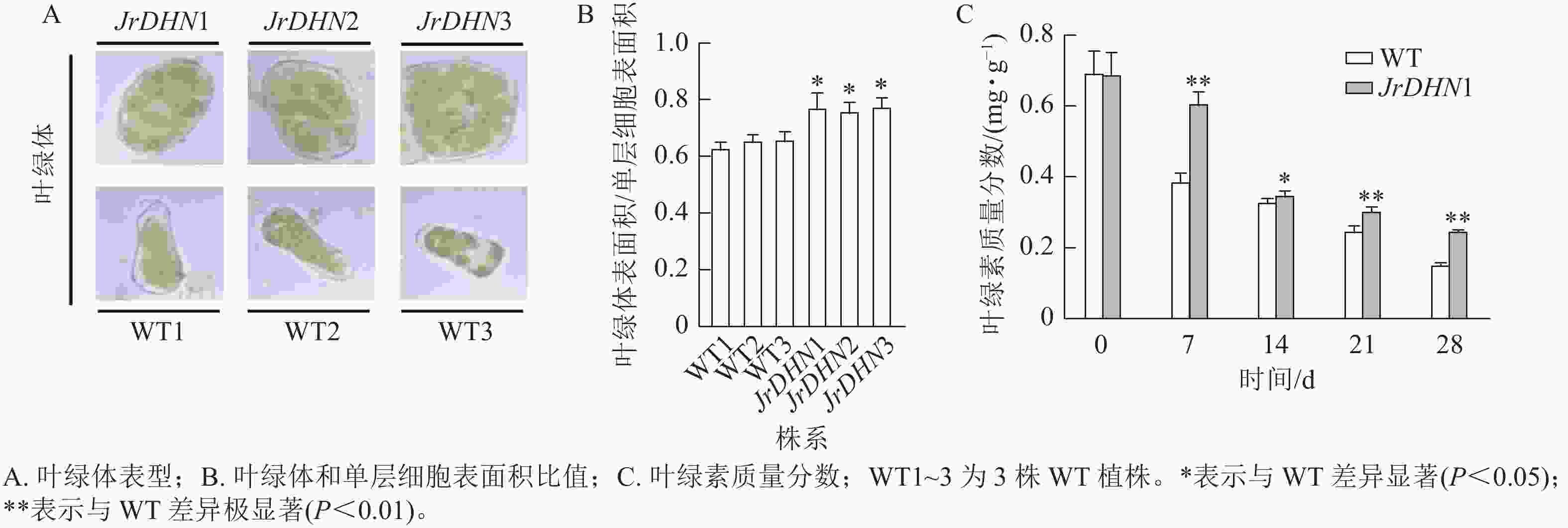
在显微镜下观察发现:3个核桃 JrDHN 过表达株系叶肉细胞中的叶绿体更致密(图5A)。JrDHN过表达株系中叶绿体表面积与单层细胞表面积的比率显著高于WT (P<0.05),WT叶绿体表面积与单层细胞表面积的平均比率为0.65,3个JrDHN过表达株系叶绿体表面积与单层细胞表面积的比率分别为0.77、0.76、0.78,与WT相比分别增加了18.5%、17%、20%(图5B)。进一步分析发现,在胁迫0 d时, WT及JrDHN过表达株系的叶绿素质量分数无显著差异(图5C)。随着干旱胁迫时间的延长,WT及JrDHN1中的叶绿素质量分数均出现下降趋势。但在处理7 d后,WT中的叶绿素质量分数迅速下降到0.39 mg·g−1,而此时JrDHN1中的叶绿素质量分数为0.61 mg·g−1,显著高于WT。在此后的14~28 d,WT叶绿素质量分数下降渐缓,分别为0.33、0.25、0.15 mg·g−1,JrDHN1中的绿叶素质量分数分别为0.35、0.31、0.25 mg·g−1,JrDHN1在整个干旱胁迫处理中叶绿素的质量分数均高于WT。综上所述,JrDHN 基因过表达提高了叶绿体表面积与单层细胞表面积的比率及核桃叶肉细胞内叶绿素的积累。
-
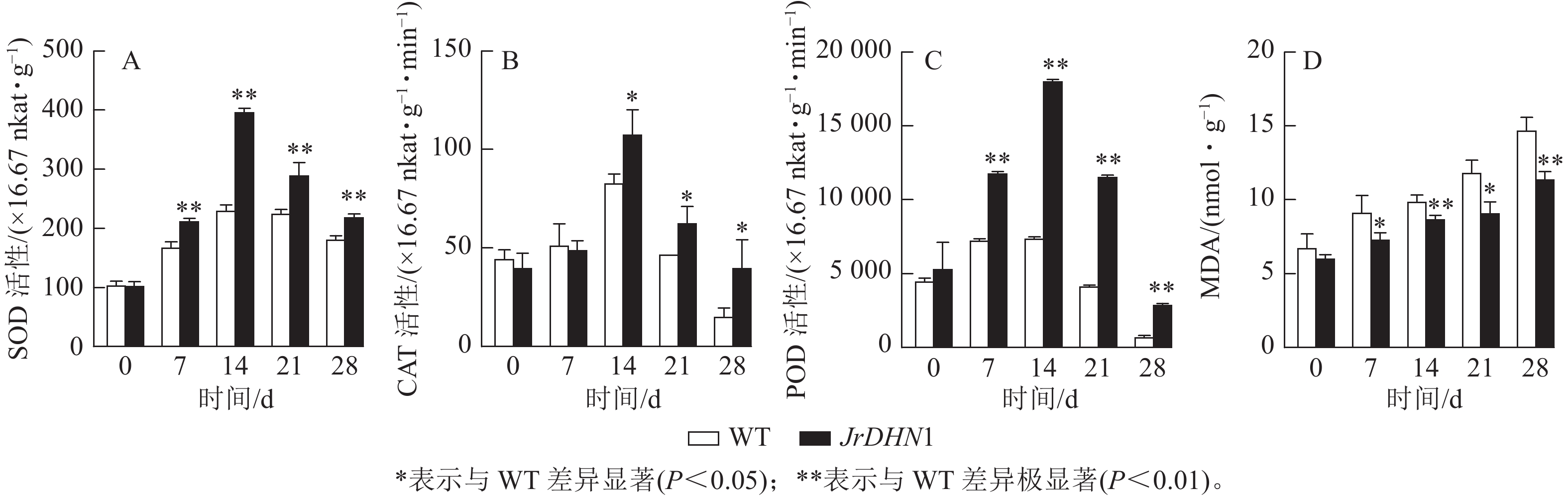
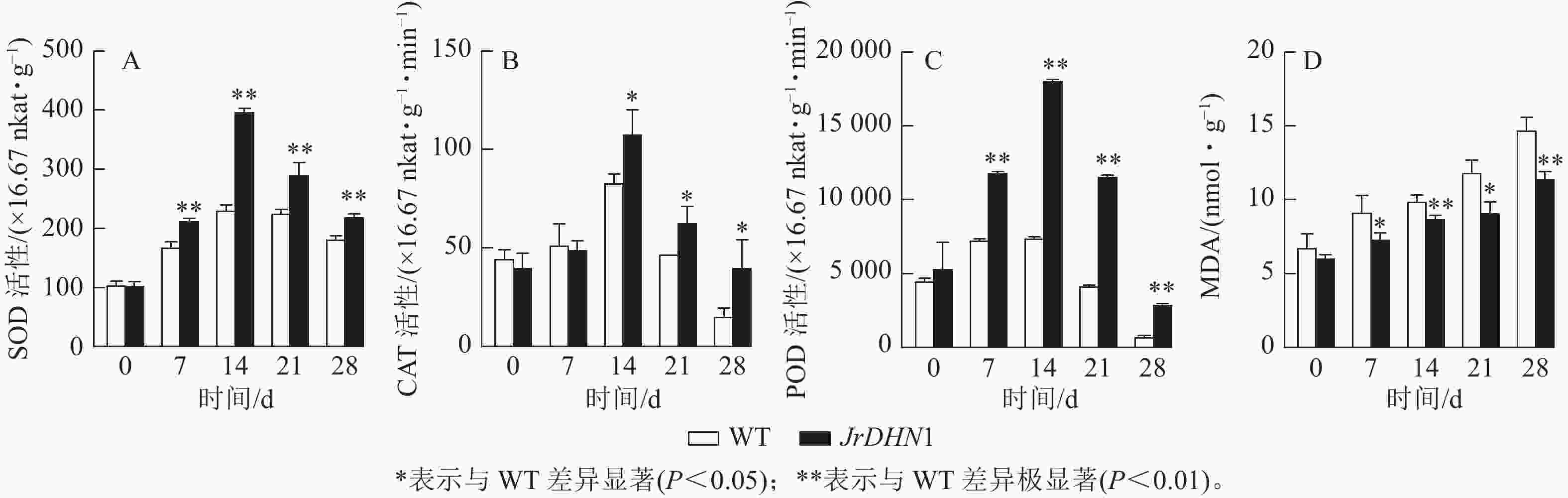
由图6A~C显示:在干旱胁迫条件下,3种抗氧化酶活性伴随着胁迫时间延长均呈现先上升后下降的趋势。0 d时,WT 和JrDHN1 的SOD、POD、CAT活性无显著性差异;在14 d时酶活性达到最大值,其中 JrDHN1与WT的SOD及POD活性差异极显著(P<0.01),CAT活性差异显著(P<0.05);JrDHN1的抗氧化酶活性在干旱胁迫7~28 d均显著高于WT (P<0.05)。由图6D显示:随着 干旱 胁迫处理时间的增加,各株系的MDA 质量摩尔浓度逐渐上升。0 d时,WT 和 JrDHN1 植株内的MDA质量摩尔浓度没有显著差异;在14和28 d时 JrDHN1的 MDA质量摩尔浓度极显著低于WT (P<0.01);7和21 d时, JrDHN1 MDA质量摩尔浓度均显著低于WT。综上表明,过表达JrDHN 基因可使植株的抗氧化酶活性显著升高,减少植物体内脯氨酸的积累,从而提高核桃的抗干旱胁迫能力。
-
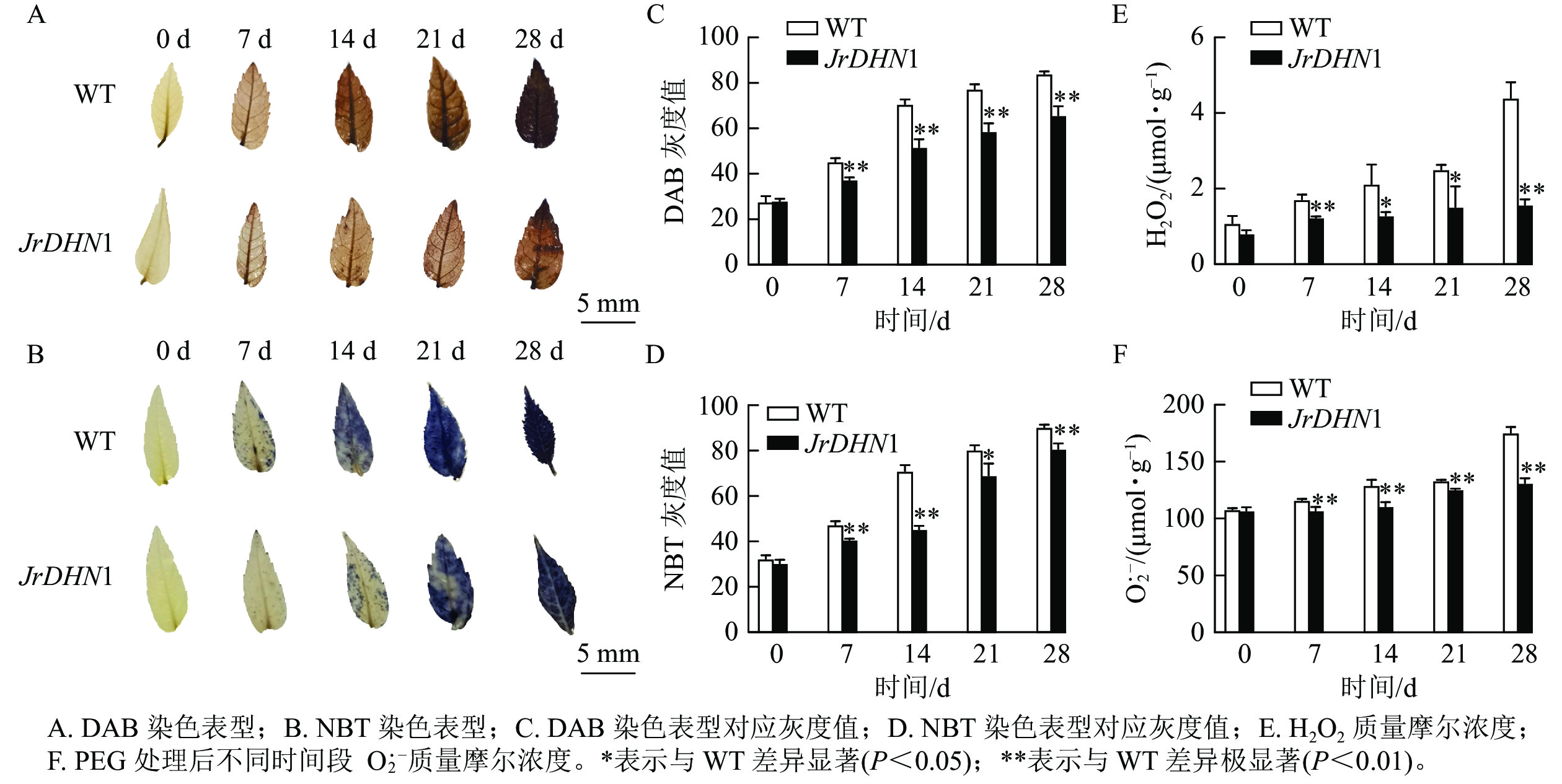
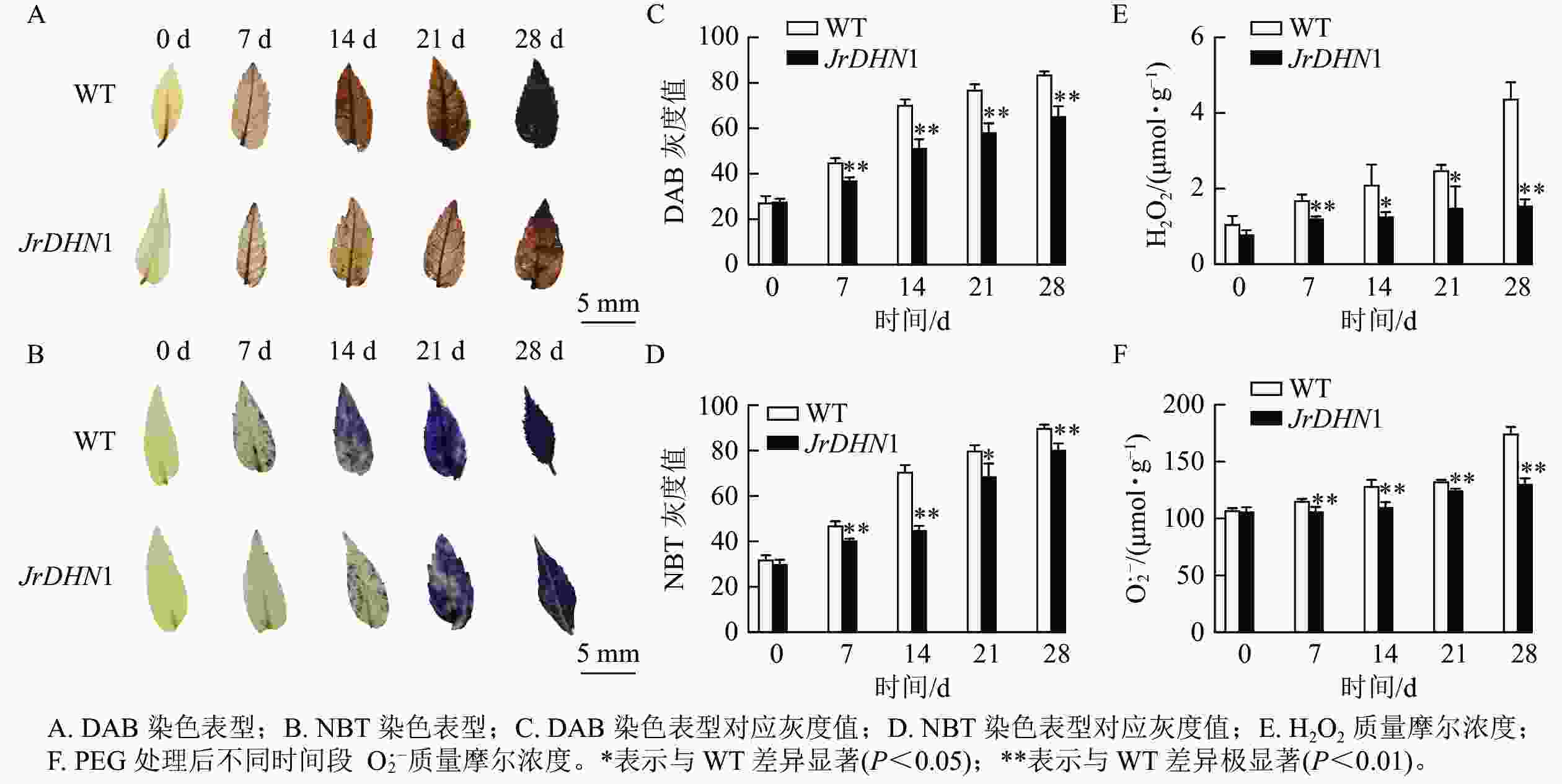
由图7A和图7B可见:0 d时,核桃 WT及JrDHN1的 NBT染色后叶片颜色均为浅黄色,无差异;随着干旱胁迫时间的增加,DAB染色表型为叶片的黄色逐渐加深,在28 d时 WT 叶片完全为棕褐色,而 JrDHN1染色比 WT 浅;NBT染色表型为叶片的蓝色逐渐加深,WT 叶片的颜色加深程度比JrDHN1 突变体株系叶片的颜色深,且至28 d被完全染为深蓝色,JrDHN1仍有部分区域为浅蓝色或无色。通过量化的方式对颜色的灰度值进行测定,灰度值越大代表颜色越深,发现干旱处理7~28 d,JrDHN1灰度值均显著低于WT,与肉眼观察的结果相符(图7C和图7D)。进一步分析发现:随着干旱胁迫时间的增加,WT及JrDHN1中的H2O2 和O2·−质量摩尔浓度也逐渐增多,在第28 天达到最大值。0 d时,WT和JrDHN1中的 H2O2 和O2·−质量摩尔浓度无显著差异;28 d时,WT中的 H2O2 和O2·−质量摩尔浓度均显著低于WT (P<0.05);在7 和28 d时,JrDHN1中的H2O2质量摩尔浓度与WT差异极显著(P<0.01),在14和21 d时H2O2质量摩尔浓度则显著低于WT (P<0.05),而7 ~28 d时,JrDHN1与WT的O2·−质量摩尔浓度均差异极显著(P<0.01)(图7E和图7F)。综上所述,过表达JrDHN 基因显著降低核桃植株中的H2O2和O2·−的积累,从而提高其抗旱能力。
-
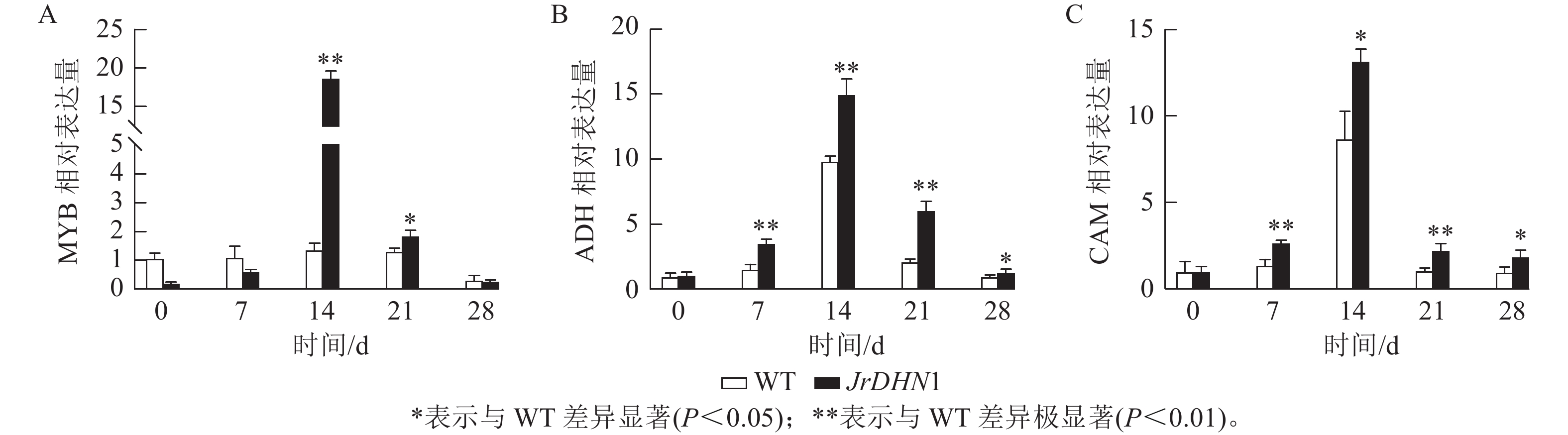
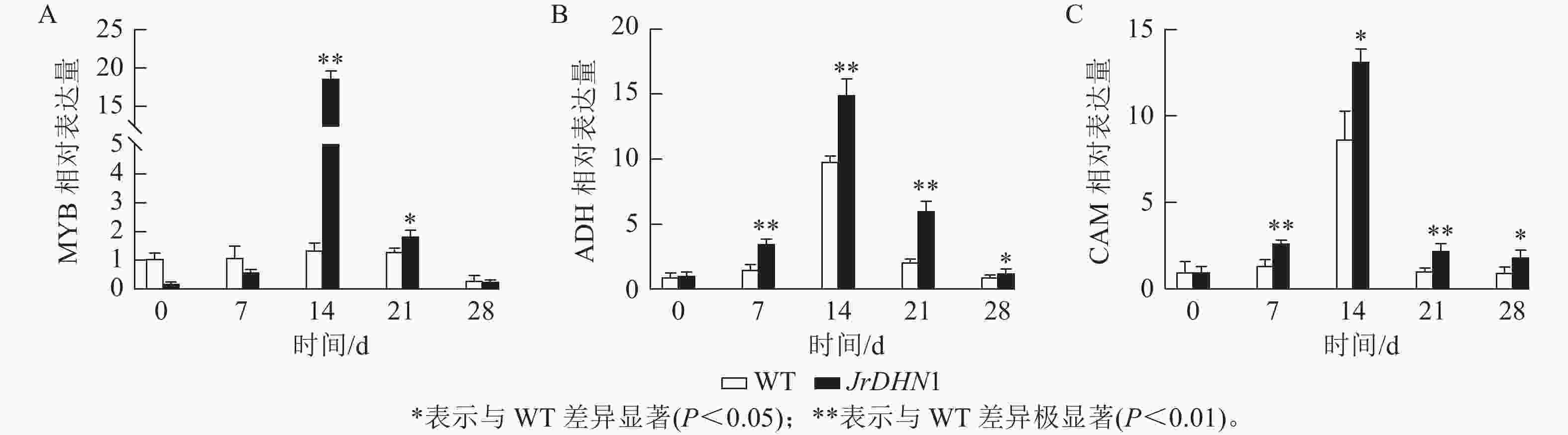
荧光定量qPCR 检测结果(图8)显示:各株系MYB、ADH、CAM的表达量均呈先上升后下降的趋势,且各基因的表达量均在14 d时明显增高,这与抗旱表型出现的时间一致。14 d时,MYB的表达量最高,为WT的18.8倍,ADH和CAM基因分别为WT的15.0和13.2倍。综合分析表明,过表达JrDHN基因可以提高MYB、ADH,CAM基因的表达量,提升脱落酸(ABA)信号转导通路对干旱胁迫的响应,从而增强植株抗旱性。
-
脱水素(dehydrins, DHNs)为晚期胚胎丰富(late embryogenesis abundant, LEA)蛋白的一个分支,广泛存在于高等植物体内,并当植物处于干旱等胁迫环境时可以在细胞中迅速积累,引起植物的一系列变化,抵御外界胁迫环境[21]。研究发现:脱水素可以通过提高叶绿素水平来提高干旱胁迫下的光合作用,从而保护植物细胞[22]。本研究结果显示:干旱胁迫0 d时,JrDHN过表达株系和WT叶绿素质量分数差异不显著;随着胁迫时间的增加,过表达株系叶绿素质量分数始终显著高于WT,说明过表达JrDHN基因可以提高干旱胁迫过程中核桃叶片中的叶绿素水平,从而提高光合作用,增强植株抗旱性。将从早花白子莲Agapanthus praecox中提取到的脱水素基因ApY2SK2 和ApSK3转入拟南芥,转基因拟南芥相比野生型在干旱胁迫下的光合能力显著提升[23],与本研究的结果相似。在铁皮石斛Dendrobium officinale中的研究也发现:高DHN表达量可以增加干旱胁迫条件下光合作用相关基因的表达量,从而提高植株光合作用水平来抵御干旱[24]。
抗氧化酶系统是植物遭受环境胁迫时重要的防御体系。当植物遭受干旱胁迫时,ROS的积累会触发该防御系统,从而保护植物减少胁迫环境带来的危害[25]。用体积分数为15%的PEG 6000溶液模拟干旱胁迫处理4个不同的葡萄Vitis vinifera品种,结果表明,随着干旱时间的增加,4个不同葡萄品种的叶绿素水平均逐渐降低,MDA含量上升,4个品种的SOD、POD、CAT以不同的变化幅度呈现先增长后降低的变化趋势[26]。用体积分数5%的PEGDKW培养基模拟干旱胁迫环境,随着干旱时间的延长,与WT相比,沉默JrGA20ox1基因表达能使核桃株系的抗氧化酶SOD、POD、CAT活性呈先升高后降低的趋势,同时减少MDA和ROS的积累,从而提高植株抗旱能力[27]。本研究通过对JrDHN过表达株系生理指标测定及化学组织染色得出:随着干旱时间的延长,WT及JrDHN过表达株系的抗氧化酶活性呈先升高后降低的趋势,这与前文的研究结果类似;JrDHN过表达植株的抗氧化酶活性始终显著高于WT,ROS及MDA水平始终显著低于WT。这表明过表达JrDHN可以提高核桃体内的抗氧化酶活性,减少ROS及MDA的积累,从而提高植株的抗旱能力。之前的研究也显示了类似的结果,以体积分数10% PEG处理烟草时,脱水素可以降低ROS在植物细胞中的积累,减少MDA的产生,同时增强抗氧化酶活性,从而提升植株对干旱胁迫的耐受性[28]。CHIAPPETTA等[29]研究发现:将野生油橄榄Olea europaea中提取到的OesDHN基因转入烟草后提高了烟草对干旱胁迫的耐受性。干旱胁迫下,小麦中TabHLH49转录因子可提高WYZ2DHN基因的表达量,从而提高小麦的耐受性[30]。在拟南芥中过表达CdDHN 4-L和CdDHN4-S 均可以导致两者清除活性氧的能力升高,从而更好地抵御干旱胁迫[31]。在甘蓝型油菜Brassica napus中的研究发现BnaMYB11、BnaMYB26、BnaMYB30和BnaMYB4基因在干旱处理后显著上调,通过木质素合成,从而响应干旱胁迫[32]。褪黑素能通过调节抗氧化剂和氧化还原相关成分ADH的mRNA水平,从而调节渗透保护,增强植株耐旱性[33]。EcCaM基因的过表达使拟南芥能够耐受PEG诱导的干旱和盐胁迫[34]。本研究结果也表明:过表达JrDHN基因可以提高MYB、ADH、CAM基因的表达量,从而增强植株抗旱性。
核桃作为营养价值较高的经济作物,其种质资源的优化及品种的选育备受关注。在全球干旱的大背景下,培育抗旱耐旱的核桃品种具有重要意义,因此,通过探究JrDHN 过表达核桃植株对干旱的响应机制,可为选育抗旱核桃品种提供理论价值和实践意义。
-
本研究发现:过表达JrDHN转基因核桃苗在PEG模拟干旱胁迫下的表型、光合能力和抗氧化能力均强于WT,过表达JrDHN基因在模拟干旱胁迫下可以有效提升抗氧化酶系统活力,清除活性氧,减少细胞受到的损伤,从而提高植株的抗旱性。综上所述,过表达JrDHN基因核桃的抗旱性要强于非转基因核桃。
Response of dehydrin JrDHN gene in walnut to drought stress
doi: 10.11833/j.issn.2095-0756.20240282
- Received Date: 2024-04-08
- Accepted Date: 2024-06-11
- Rev Recd Date: 2024-06-11
- Publish Date: 2024-11-20
-
Key words:
- walnut /
- drought /
- JrDHN /
- overexpression /
- functional analysis
Abstract:
| Citation: | ZHANG Yuhang, ZHANG Manman, MA Yuhang, et al. Response of dehydrin JrDHN gene in walnut to drought stress[J]. Journal of Zhejiang A&F University, 2024, 41(6): 1150-1159. DOI: 10.11833/j.issn.2095-0756.20240282 |










 DownLoad:
DownLoad: