-
气候变化导致降水格局改变,伴随严重且频繁的极端天气,使得大量植被退化甚至死亡[1]。在气候变化的驱动下,植物采取一系列的生存策略来适应环境,其功能性状是用于预测植物如何适应不断变化的环境的重要指标[2]。种内变异能决定植物适应环境的能力以及策略[3]。因此,探究沿降水梯度带植物功能性状的种内变异及适应策略,可为气候变化背景下预测植物分布与适应潜力提供参考。
叶片和枝条是植物适应外部环境变化的重要器官,反映了资源获取和水力运输功能。其中,叶片功能性状对气候变化敏感,可用于探索碳水收支相关功能的优化机制[4]。叶经济谱中,叶功能性状沿保守策略到获取策略轴广泛分布,资源保守型的植物比叶重较高而光合作用速率较低;资源获取型的植物则与之相反[5]。枝条木质部结构与水分运输效率和安全性密切相关[6]。植物功能性状是相互关联的,不同性状形成多个性状组合协调变化以适应不断变化的外部环境。近年来的研究考虑了广泛的功能特征套件,所提出的植物性状网络(plant trait networks, PTNs)被越来越多地用于分析多性状之间的复杂关系。研究表明:更紧密的性状网络(连通性)有利于植物高效地获取和利用资源;多个内部紧密外部松散的性状模块(复杂性)有利于植物灵活适应外部环境[7−8]。沿大尺度环境梯度研究性状网络的变异规律可以揭示不同气候区植物整体适应策略的调整。
不论是功能性状还是多性状间的关系都受到非生物因素(如气温、降水和光照等)的驱动[9−13]。例如,降水梯度带上榆树Ulmus pumila的13个功能性状均受到气候因子影响,影响最大的是年平均降水量(PMA)[9]。光环境控制叶片厚度,是驱动叶片功能性状变异的主要气候因子[10],而在西北荒漠地区,气温是影响琵琶柴Reaumuria soongarica木质部解剖性状变异的主要因素[11]。性状网络对环境变化的响应也存在争议。有研究发现:气候条件较为适宜、资源丰富的热带森林具有更紧密和复杂的网络,性状间内部协同性资源利用效率高;反之,则资源吸收和传递功能受限[7]。但由于选择物种集合以及性状集合的差异,有时甚至会得出完全相反的结论。例如,在资源匮乏的环境下性状间关系更为紧密[12−13]。因此,探究气候因子怎样影响功能性状及性状网络仍需要更多的实证结果支持。
旱柳Salix matsudana为杨柳科Salicaceae柳属Salix植物,起源于东北、华北平原以及黄土高原,具有很强的适应性和耐旱能力,在中国广泛分布[14],也是这条降水梯度带下的共有种。然而,对旱柳的研究大多用来揭示对盐胁迫的分子机制以及基因表达变化[15],对功能性状响应气候变化的研究相对不足。在降水量连续变化的大区域尺度上对共有种旱柳应对不同环境的适应能力和策略,以及驱动其功能性状变异的主要气候因子尚不明确。因此,本研究选取沿中国东南—西北的降水梯度带下的10个样点,以样带共有种旱柳为研究对象,测量16个功能性状,用以探究降水梯度带旱柳功能性状的区域分异规律及其适应策略,揭示驱动旱柳功能性状变异的主要气候因子,有助于为气候变化背景下旱柳功能性状的变异特征研究提供理论依据,加深对气候变化驱动植物功能性状变异的理解。
-
本研究沿中国东南—西北走向样带,选择10个国有林场进行野外采样。从东南—西北依次为:安徽宣城胡乐国有林场、河南信阳南湾实验林场、河南三门峡国有河西林场、陕西铜川阳湾国有生态林场、甘肃庆阳国有大山门林场、宁夏吴忠国有张家湾林场、甘肃金昌东大河林场、甘肃张掖五泉林场、甘肃酒泉西峰林场、新疆哈密哈铁林场。各样点空间位置跨度大,气候特征各不相同,30.33°~42.62°N,93.44°~118.77°E,年平均降水量(PMA)为44.2~1 394.6 mm,生长季(5—9月)平均气温(TGS)为15.8~25.3 ℃,生长季(5—9月)光合有效辐射(PAR)为38.4~54.5 mol·m−2·d−1(表1)。
区域 样点 纬度(N) 经度(E) 生长季平均气温/℃ 年平均降水量/mm 生长季光合有效辐射/(mol·m−2·d−1) 湿润区 宣城 30.33° 118.77° 25.3 1 394.6 38.4 湿润区 信阳 32.06° 113.85° 24.7 1 082.3 40.2 半湿润区 三门峡 34.48° 110.54° 23.8 555.6 41.1 半湿润区 铜川 35.40° 109.14° 20.3 584.4 44.2 半湿润区 庆阳 35.88° 108.37° 19.0 581.9 42.2 半干旱区 吴忠 36.45° 105.77° 20.5 235.6 49.8 半干旱区 金昌 38.33° 102.00° 15.8 226.5 51.3 干旱区 张掖 39.23° 100.17° 19.6 131.7 50.3 干旱区 酒泉 39.67° 98.63° 19.2 81.9 52.0 干旱区 哈密 42.62° 93.44° 23.4 44.2 54.5 说明:依据年平均降水量区间(上限排除法)划分成湿润区(≥800 mm)、半湿润区(400~800 mm)、半干旱区(200~400 mm)和干旱区(<200 mm)。 Table 1. Coordinates of 10 sites and their climatic characteristics
-
本研究开展于2021年7—9月。在保护良好的10个国有林场,分别设置100 m×100 m的样地,每个样地选取立地条件基本一致、生长良好的旱柳15株[树高(8.22±0.58) m、胸径(22.36±2.43) cm、冠幅(8.15±0.19) m],并剪取3根长50 cm以上的南冠层中上部成熟健康且基部直径接近的枝条。
-
离体叶片在1 h内具有较稳定的光合活力,基本可以满足相关的光合生理测定[16],因此,本研究用Li-6800便携式光合作用系统(Li-6800, Li-Cor, Lincoln)于8:30—11:30离体实地测量叶片光合生理性状。每株树选择向南冠层中上部3根枝条,每枝选2片成熟且大小相似呈交替排列并暴露于阳光下的叶片测定光饱和点。将采下的树枝立即插入水中,选取成熟无破损叶片放入30 mm×30 mm的红蓝光源叶室。光诱导时,先设光照强度为1 000 μmol·m−2·s−1,待叶片光合各项参数趋于稳定后再设光照强度为1 800 μmol·m−2·s−1,同时控制叶室温度为25 ℃,二氧化碳(CO2)摩尔分数为400 μmol·mol−1,气体流速为500 μmol·s−1。测定完成后,对于小于叶室的叶片,用记号笔标记测定时叶室内的叶片面积,将叶片带回实验室扫描,并用Image-Pro Plus软件测定真实叶面积后校准光合数据,获取最大净光合速率(Pn,µmol·m−2·s−1)、最大蒸腾速率(Tr,mmol·m−2·s−1)及气孔导度(GS,mol·m−2·s−1)。计算水分利用效率(EWU=Pn/Tr,µmol·mmol−1)。
-
测定光饱和点后,使用指甲油印迹法[17]获取气孔样本,带回实验室,用于气孔性状测量。测量时将气孔样本放置在载玻片上制成临时装片,并置于Leica DM 3000显微镜下,每张装片分别在200和400倍镜下随机选取5个叶表皮无污渍和破损且气孔清晰可见的视野,拍摄保存气孔印迹图像各5张。用Image-Pro Plus软件统计200倍镜下每张图像的气孔数量,气孔数量与图像面积(避开叶脉)的比值为气孔密度(Sd,个·mm−2)。每张400倍镜图像中量取10个气孔长度(LS)、气孔宽度(WS)以及保卫细胞宽度(w),并计算气孔大小(SS= π/4 ×LS ×WS,μm2)和气孔面积分数(Sf=Sd×SS×10−4,%) [18]。解剖学最大气孔导度(gsmax)指光饱和、土壤、水分以及营养条件均达到最佳时的气孔导度,代表了植物气体交换的解剖学潜力。对水蒸气的最大气孔导度(gwmax)可用以下公式计算[9]:
式(1)中: d为 25 ℃时水蒸气扩散系数(d = 0.000 024 9 m2·s−1),v 为空气的摩尔体积(v = 0.024 5 m3·mol−1),amax是气孔打开的最大孔隙面积与气孔大小的比值(本研究的amax= 0.12,0.12是气孔完全开放时孔隙面积/气孔大小比值范围的中间值[19]);w为保卫细胞宽度。gsmax是对CO2的解剖学最大气孔导度,gsmax=gwmax/1.6,并计算气孔打开比率(gratio=GS/gsmax) [9]。本研究植物功能性状的符号及单位见表2。
植物功能性状 符号 单位 植物功能性状 符号 单位 最大净光合速率 Pn µmol·m−2·s−1 比叶重 ALM g·m−2 单位质量最大净光合速率 Amass µmol·g−1·s−1 胡伯尔值 Hv ×103 mm2 · cm−2 气孔导度 GS mol·m−2·s−1 叶导管直径 Dleaf µm 气孔打开比率 gratio % 叶导管壁厚 Tleaf µm 水分利用效率 EWU mmol·mol−1 导管直径 D µm 气孔大小 SS µm2 导管密度 N 条·µm−2 气孔密度 Sd 个·mm−2 导管壁厚 T µm 气孔面积分数 Sf % 木质部密度 DW g·cm−3 Table 2. Symbols and units of functional traits
-
将测定光合参数的枝条编号带回实验室,在万分之一天平上称取每根枝条上所有叶片的鲜质量,而后用EPSON扫描仪和EPSON Scan软件扫描叶片以及去除树皮和髓心后的边材面积,并保存图像,用Image-Pro Plus软件计算叶面积(AL,m2)和边材面积(AS,cm2)。扫描完成后将叶片放入到105 ℃烘箱中,杀青30 min后,放入75 ℃烘箱内72 h,烘干至恒质量,称得叶干质量(ML,g)。计算比叶重(ALM=ML/AL,g·m−2)、胡伯尔值(Hv=AS/AL,×103 mm2·cm−2)、单位质量最大净光合速率(Amass=Pn/ALM,µmol·g−1·s−1)[5]。
-
选取采回枝条的底部段并剥皮,切成0.5 cm长的小枝样品。同时,从枝条上选择生长良好无破损的叶片,垂直叶片主脉剪取长1.0 cm、宽0.5 cm的叶片样品,固定软化后用常规石蜡切片法制成永久装片。用Leica DM 3000显微镜在50倍镜下拍摄木质部横切图像,选定不小于60°的扇形区域,用Image-Pro Plus软件测量扇形区域面积和所选区域内导管个数,以此计算木质部导管密度(N,条·µm−2)。在200倍镜下各拍摄5张木质部和叶片横切图像,每张选取不小于20条导管,用Image-Pro Plus软件计算测量其导管直径(D,µm)、导管壁厚(T,µm)、叶导管直径(Dleaf,µm)和叶导管壁厚(Tleaf,µm)。
用刀片去除小枝段样品树皮,依据阿基米德原理确定体积(V),再置于75 ℃烘箱烘48 h后,测得干质量(mW),计算木质部密度(DW= mW/V,g·cm−3)[20]。
-
降水梯度带旱柳功能性状的变异程度用变异系数(VC)来量化,采用单因素方差分析(one-way ANOVA)和Tukey检验比较功能性状在不同区域间的差异(P<0.05)。用植物功能性状网络(PTNs)分析方法对各区域旱柳性状间协作性以及资源利用效率进行评价。性状网络的构建:先用Origin 2024进行Pearson相关性分析,构建相关系数矩阵,再将数据导入Gephi 0.10.0制作性状网络图,以功能性状为节点,性状间的相关性表示为“边”,并得到网络整体参数,包括平均路径长度、平均聚类系数和边密度。平均路径长度为网络中所有最短路径的平均值;平均聚类系数为网络所有节点聚类系数的均值;边密度为性状网络中观测到的边的数量与最大潜在边数量的比值。采用rdacca.hp包进行层次分割(HP),测试各气候因子对旱柳整体功能性状以及16个功能性状变异的贡献率,分析驱动植物功能性状变异的主要气候因子[21]。用Excel、Minitab 2021和R 4.3.1分析数据,Origin 2024和Gephi 0.10.0作图。
-
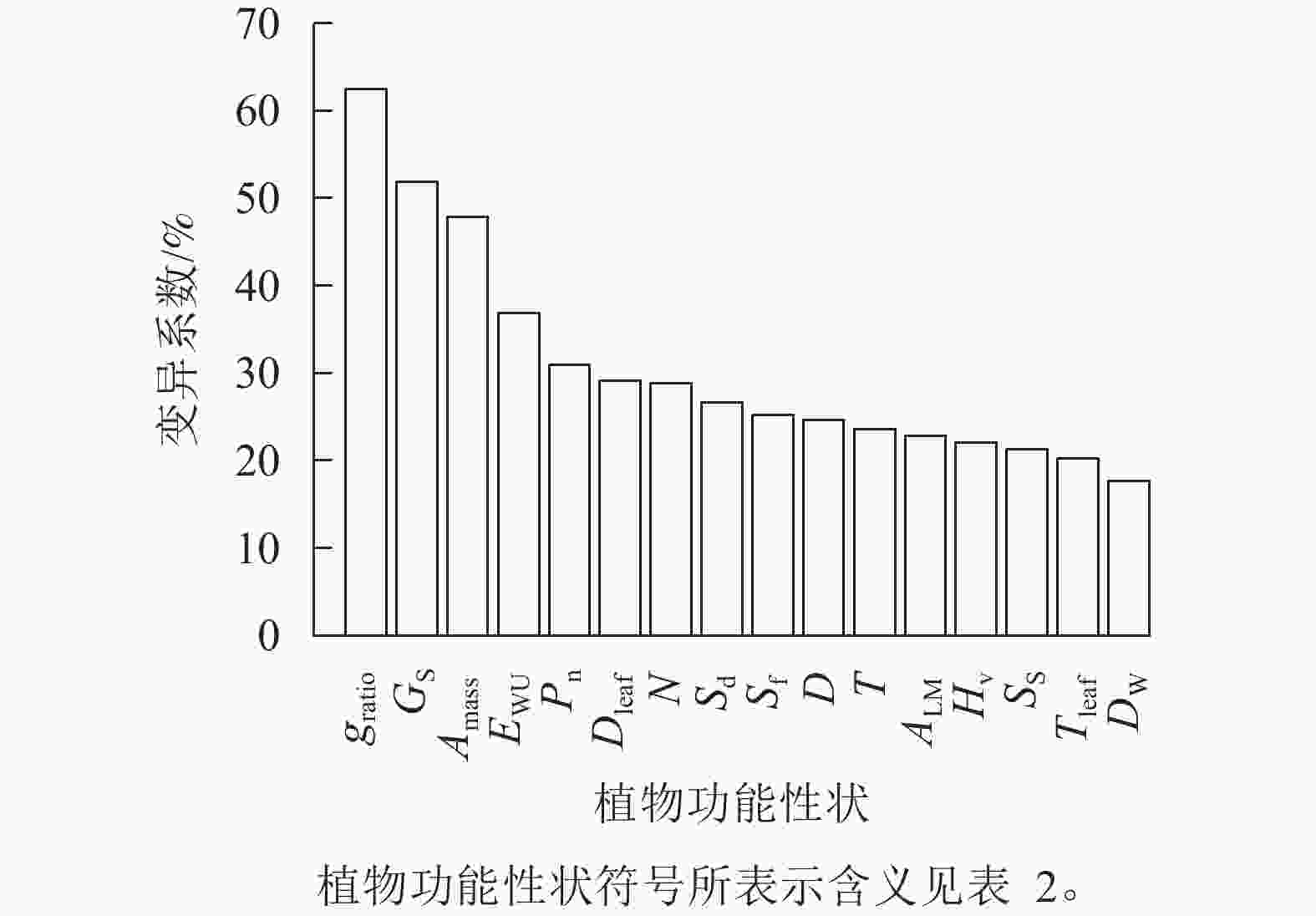
由图1可知:旱柳功能性状在降水梯度带上均存在变异,且变异程度差异较大。各功能性状的变异系数由高到低依次为气孔打开比率(62.4%)、气孔导度(51.8%)、单位质量最大净光合速率(47.9%)、水分利用效率 (36.9%)、最大净光合速率(30.9%)、叶导管直径(29.1%)、导管密度(28.8%)、气孔密度(26.7%)、气孔面积分数(25.2%)、导管直径(24.6%)、导管壁厚(23.6%)、比叶重(22.8%)、胡伯尔值(22.1%)、气孔大小(21.3%)、叶导管壁厚(20.2%)、木质部密度(17.7%),其中变异系数大于50%的功能性状为气孔打开比率、气孔导度,小于20%的是木质部密度。
-
如图2显示:单位质量最大净光合速率在半湿润区和半干旱区显著大于湿润区和干旱区(P<0.05)。半干旱区最大净光合速率最高且显著大于其余气候区(P<0.05)。气孔打开比率在半湿润区和半干旱区更接近20%。胡伯尔值在半湿润区和半干旱区显著大于湿润区和干旱区(P<0.05)。导管直径在半湿润区和半干旱区显著大于湿润区(P<0.05),大于干旱区。木质部密度在干旱区显著大于其余气候区(P<0.05)。比叶重在湿润区和干旱区显著大于半湿润区和半干旱区(P<0.05)。
-
网络分析表明:半湿润区和半干旱区功能性状间联系相比湿润区和干旱区更紧密(图3)。4个气候区性状网络的平均聚类系数、平均路径长度、边密度均存在差异(表3)。结果显示:平均聚类系数、边密度在半湿润区半干旱区较大,湿润区和干旱区较低;平均路径长度则与之相反。
区域 平均路径长度 平均聚类系数 边密度 区域 平均路径长度 平均聚类系数 边密度 湿润区 2.013 0.505 0.250 半干旱区 1.681 0.750 0.325 半湿润区 1.838 0.700 0.400 干旱区 2.124 0.576 0.250 Table 3. Variation of the overall parameters of the trait network in the four climatic regions
-
由图4可知:旱柳功能性状整体变异受到年平均降水量(21.57%)、生长季光合有效辐射(19.73%)、生长季平均气温(11.94%)的影响,总和达53.24%。旱柳功能性状变异主要受年平均降水量影响的有最大净光合速率、单位质量最大净光合速率、气孔导度、气孔打开比率、气孔密度、气孔面积分数、叶导管壁厚、导管壁厚;主要受生长季平均气温影响的有水分利用效率、胡伯尔值、叶导管直径、导管直径、导管密度;比叶重、气孔大小、木质部密度主要由生长季光合有效辐射驱动。气候因子对气孔大小、气孔密度的单独效应最大,分别为73.00%和61.00%。此外,降水梯度带旱柳16个功能性状的单独效应均不到100.00%。
-
3个气候因子对功能性状的总贡献度达53.24%,其中年平均降水量是驱动降水梯度带旱柳整体功能性状变异的主要气候因子(21.57%)。这些因子共同塑造了植物功能性状的区域分异规律。单位质量最大净光合速率、胡伯尔值和比叶重在中等降水量下均达到极值,其中前2个性状在半湿润区和半干旱区达到最大值,比叶重在半湿润区和半干旱区达到最小值。这种性状组合有利于旱柳在半湿润区和半干旱区获取碳资源。
-
气孔功能有关的性状对环境变化的敏感度最高,包括气孔的发育(GS)及运动控制(gratio),体现了气孔在植物适应策略中的重要作用。植物所处环境变化时,常先改变与气体交换有关的性状。这可能是由于气孔作为植物与其周围环境的“阀门”,控制叶片中碳和水的通量,来优化和平衡光合作用性能和水利用效率[22−23]。包括气孔性状在内,旱柳多个性状在梯度带上表现出显著的区域分异规律。从气孔发育来看,从湿润区到干旱区,旱柳气孔密度减少而气孔大小增加,通过两者间的权衡维持气孔的总面积以保证资源的获取,这一结果与之前的相关研究相符[23]。从气孔运动来看,半湿润区和半干旱区旱柳的气孔打开比率更接近20%“甜蜜点”,此时气孔开闭最为灵敏[24]。这一气孔特性使得旱柳在此区域拥有最大的水分利用效率、单位质量最大净光合速率。同时,在半湿润区和半干旱区比叶重较低。这些结果表明:旱柳在半湿润区和半干旱区对碳资源的利用策略为获取型。在半湿润区和半干旱区,旱柳较大的导管直径提高了水分运输效率[25],同时较大的胡伯尔值,导致单位叶面积得到的水力支持更大,使旱柳在此区域生长期内获得充足的水分供给,从而实现在生长季内快速生长发育[26]。
相比于水热资源平衡的半湿润区和半干旱区,旱柳在低资源供应的干旱区表现出更低的单位质量最大净光合速率以及最高的比叶重和木质部密度,体现了保守的碳资源利用策略。这种策略降低了碳在植物体内的周转速率,但有利于资源保护与储存(资源持久性)。尤其是在干旱区旱柳有最大生长季光合有效辐射,且旱柳在此区域表现出少而大的气孔(气孔密度最小,气孔大小和气孔打开比率最大)以及最低的胡伯尔值,这需要更大的比叶重来提高叶片的保水性[27],以弥补激进的气孔行为所带来的安全隐患。通常来说,保持较高的木质部密度,可以增强木质部栓塞抗性[28],干旱环境下高胡伯尔值对植物有利[29]。本研究中,旱柳在干旱区虽然胡伯尔值低,但同时也表现出最高的木质部密度,这表明较高的木质部栓塞抗性可能在较低的胡伯尔值下得到补偿。湿润区旱柳表现出最小的气孔打开比率,这可能是高湿高雨的环境给细菌和病毒的滋生创造了有利条件,细菌通过鞭毛运动向气孔周围靠拢,此时植物为抵御侵害降低气孔打开比率[9],这种功能的需要导致了较低的单位质量最大净光合速率。湿润区比叶重较半湿润区和半干旱区大,这可能是由于高降水下植物通过增加叶片厚度,提高机械抗性和适应不良环境的能力[30],以此来应对缺氧和高水含量的挑战,间接导致了较高的比叶重。
旱柳的性状网络在不同区域间也表现出差异。相较于湿润区和干旱区,资源充足且气候条件适宜(水分、温度以及光照)的半湿润区和半干旱区塑造了最紧密的性状连通性,有利于性状间合作以及提高资源获取效率[31]。例如,在相同降水梯度带对榆树Ulmus pumila的研究发现:气候条件较为适宜的半湿润区具有更紧密和复杂的网络,性状间协同性高,资源利用效率高[9]。
-
在本研究降水梯度上旱柳功能性状变异均受生长季平均气温、年平均降水量、生长季光合有效辐射驱动。气温、降水量以及光合有效辐射是影响植物功能性状变异的主要因素[32],与本研究结论相同。在这些气候因子中,年平均降水量对降水梯度带上旱柳功能性状变异的驱动力最强,体现了水分条件对植物生长的关键作用。这与HUANG等[33]的年平均降水量是大部分性状变异的主要气候因子的研究结果相同。其中,年平均降水量对气孔密度变异的贡献最大,可能是水分供应改变了细胞的大小,从而控制叶片的伸展,最终影响了气孔密度[34]。
生长季光合有效辐射对旱柳功能性状变异的贡献率仅次于年平均降水量,其原因可能是光照强度对植物生物量分配和碳水化合物积累有很强的影响[35]。本研究发现:气候因子对比叶重的贡献度较低,表明比叶重可能与除气候因子(生长季平均气温、年平均降水量、生长季光合有效辐射)之外的土壤资源可利用性密切相关[36]。此外,生长季光合有效辐射相比生长季平均气温和年平均降水量对比叶重变异的贡献率较大,这可能是由于光照能够影响比叶面积,进而改变光截获能力,以使比叶重发生变化[37−38]。生长季平均气温对旱柳的解剖性状变异的贡献率高,其中导管直径的变异主要受生长季平均气温驱动,表明气温能够影响木质部的导管特征。以往研究表明:气温可以通过细胞分裂与分化以及生长周期直接影响导管大小等特征,同时能通过影响植物光合和呼吸作用来调控物质的积累与分配,进而影响木质部导管特征[39]。这可能是造成这一结果的原因。
此外,气候因子对旱柳16个功能性状的贡献率均小于100%,表明驱动功能性状变异的不只是气候因子,可能还受土壤条件[25]、小气候[40]等影响。因此,在未来的实测或理论模型研究中还需要考虑更多环境因素以及物种自身的遗传特性对植物功能性状沿降水梯度带变异的多重影响。
-
年平均降水量是驱动旱柳功能性状沿降水梯度带变异最主要的气候因子,其中气孔的发育以及运动控制对年平均降水量均高度敏感。在气候因子的驱动下,半湿润区和半干旱区旱柳的单位质量最大净光合速率、胡伯尔值达到最大值,比叶重则最小,塑造了旱柳在半湿润区和半干旱区最有利于资源获取的适应策略。同时,旱柳在半湿润区和半干旱区的网络性状关联更加紧密,性状间高度的连通性有利于促进资源在植物内部的共享与利用。
Regional differentiation patterns of common functional traits of Salix matsudana in precipitation gradient zones
doi: 10.11833/j.issn.2095-0756.20240505
- Received Date: 2024-08-26
- Accepted Date: 2024-12-01
- Rev Recd Date: 2024-12-01
- Available Online: 2025-04-01
- Publish Date: 2025-04-01
-
Key words:
- plant functional traits /
- precipitation gradient zone /
- climatic factor /
- Salix matsudana
Abstract:
| Citation: | GUO Meimiao, ZHANG Bona, TANG Luyao, et al. Regional differentiation patterns of common functional traits of Salix matsudana in precipitation gradient zones[J]. Journal of Zhejiang A&F University, 2025, 42(2): 281−290 doi: 10.11833/j.issn.2095-0756.20240505 |













 DownLoad:
DownLoad:


