-
菊花Chrysanthemum × morifolium是菊科Asteraceae菊属Chrysanthemum植物,是观赏植物中变异类型最丰富的花卉之一,在中国已有3 000多年的栽培历史[1−2] ,品种资源丰富,文化底蕴深厚。菊花是药食兼用功能型花卉[3−4],其药用价值最早记载于《神农本草经》[5],并被《中国药典》收录为散热清风、平肝名目的药材,也是国家卫生健康委员会认定的8种花源性食用药之一。此外,菊花还具有多种人体必需的氨基酸[6],广泛应用于功能性食品和饮料中[2, 7]。近年来的研究揭示了菊花具有抗氧化、抗炎抑菌等生物活性,其活性成分的鉴定与功能研究不断深入。鉴于其天然来源的安全性,解析菊花活性物质的合成调控机制并开发绿色合成技术具有重要的科学意义和应用价值。本研究系统总结了菊花中活性物质的种类、合成途径及其调控机制的研究进展,旨在为菊花活性成分开发、绿色高效合成和多功能新品种培育提供理论支持,推动菊花在观赏、食用和药用领域的多元化发展,提升其经济与社会效益。
-
类黄酮、酚酸和萜类化合物是菊花中主要的活性物质类型,也是评价其质量的关键指标。随着高效液相色谱法(HPLC)、液相色谱-质谱联用技术(LC-MS)和核磁共振波谱法(NMR)等高灵敏度、高准确性植物代谢组学分析技术的广泛应用,菊花中活性物质的种类得以更全面且精确地解析 [8]。目前已知的菊花活性物质见表1。
表 1 菊花中已知活性物质
Table 1. Known bioactive compounds in C. morifilium
分类 名称 测定方法 测定部位 参考文献 类黄酮 芹菜素-7-葡萄糖苷apigenin-7-O-glucoside UF-LC-MS、UPLC 干花序 [18−21] 木犀草素luteolin NMR、UPLC-MS/MS、HPLC-MIPSPE 干花序 [22−25] 木犀草苷luteoloside UPLC-ESI-Q-TOF-MS/MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 23, 26] 木犀草素-7-葡萄糖醛酸苷luteolin-7-O-glucuronide LC-MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 26] 橙皮素hesperetin LC-MS、NMR 鲜花序、干花序 [8, 24] 山柰酚kaempferol HPLC-MIPSPE 干花序 [24] 杨梅素myricetin HPLC-MIPSPE 干花序 [24] 芹菜素apigenin LC-MS、NMR、UF-LC-MS 鲜花序、茎、
干花序[8, 22−23] 槲皮素quercetin UF-LC-MS、NMR 干花序、茎 [8, 22] 芦丁quercetin 3-O-rutinoside LC-MS 鲜花序 [8] 金合欢素acacetin UF-LC-MS、NMR 干花序、茎 [8, 22] 圣草酚eriodictyol NMR 茎 [22] 异鼠李素isorhamnetin HPLC-MIPSPE 干花序 [25] 樱黄素prunetin HPLC-Q-TOF-MS/MS 干花序 [21] 酚酸 1,4-O-二咖啡酰奎宁酸1,4-dicaffeoylquinic acid LC-MS、NMR、UPLC 干花序、茎 [8, 19, 22] 1,3-O-二咖啡酰奎宁酸1,3-dicaffeoylquinic acid UPLC-MS/MS 干花序 [26] 1,5-O-二咖啡酰奎宁酸1,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS 干花序、茎 [8, 20, 22] 3,5-O-二咖啡酰奎宁酸3,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS、UF-LC-MS 干花序、茎 [8, 20, 22−23] 4,5-O-二咖啡酰奎宁酸4,5-dicaffeoylquinic acid LC-MS、UPLC 干花序 [8, 19] 绿原酸chlorogenic acid LC-MS、NMR、UF-LC-MS、UPLC-MS/MS 干花序序、茎 [8, 20, 22−23, 26] 咖啡酸affeic acid LC-MS 干花序 [8] 没食子酸gallic acid NMR 茎 [24] 萜类 β-榄香烯(–)-β-elemene GC-MS 干花序 [17] (E)-β-金合欢烯cis-β-farnesene GC-MS 干花序 [17] β-半水芹烯(–)-β-sesquiphellandrene GC-MS 干花序 [17] (+)-喇叭烯(+)-ledene GC-MS 干花序 [17] 桉叶油素1,8-cineole GC-MS 鲜花序 [16] 樟脑camphor GC-MS 鲜花序 [16] 丁香酚eugenol GC-MS 鲜花序 [16] β-石竹烯β-caryophyllene GC-MS 鲜花序 [16] α-姜烯α-zingiberene GC-MS 鲜花序 [16] β-蒎烯β-curcumene GC-MS 鲜花序 [16] 石竹烯氧化物caryophyllene oxide GC-MS 鲜花序 [16] α-没药醇α-bisabolol GC-MS 鲜花序 [16] 香芹酮carvone NMR 茎 [24] 说明:UF-LC-MS. 超滤液相色谱-质谱联用技术;UPLC. 超高效液相色谱法;NMR.核磁共振波谱法;UPLC-MS/MS. 超高效液相色谱-串联质谱联用技术;HPLC-MIPSPE. 高效液相色谱-分子印迹固相萃取在线联用技术;UPLC-ESI-Q-TOF-MS/MS. 超高效液相色谱-电喷雾离子源-四级杆-飞行时间串联质谱联用技术。LC-MS. 液相色谱-质谱联用技术;GC-MS.气相色谱-质谱联用技术。 -
类黄酮是一类由植物次级代谢产生的多酚类化合物,广泛分布于水果、蔬菜和其他粮食作物中,对植物抵御生物和非生物胁迫至关重要[9] 。在菊花中,类黄酮是活性物质的主要成分,已鉴定出黄烷酮、黄酮类、黄酮醇及其苷类和衍生物等类黄酮不同亚类[8, 10] ,包括木犀草素、芹菜素、金合欢素、异鼠李素、橙皮苷、香叶木素、山柰酚及其衍生物等代表性化合物[11−14]。
-
菊花中酚酸类物质含量较低,却是其药理作用的关键成分。绿原酸(CGA)、3,5-O-二咖啡酰奎宁酸被《中国药典》列为质量控制指标,而4,5-O-二咖啡酰基奎宁酸则是菊花和野菊中常见的酚酸类物质[7, 15]。此外,‘杭菊’‘Hangju’、‘贡菊’‘Gongju’、‘怀菊’‘Huaiju’、‘福白菊’‘Fubaiju’等品种均含多种绿原酸异构体[14]。L-(−)-3-苯基乳酸和L-苹果酸等有机酸类是‘皇菊’‘Huangju’、‘金丝皇菊’‘Jinshihuangju’等菊花茶呈现苦味和鲜味的原因[13]。
-
萜类化合物是菊花中的主要挥发性成分,也是茶菊、精油风味的关键因素。菊花中萜类成分以倍半萜类为主,单萜较少,主要化合物包括樟脑、桉油精、α-姜黄烯、α-姜烯、β-榄香烯、红没药醇、β-倍半水芹烯、(+)-喇叭烯、(E)-β-金合欢烯等[16−17]。除花序外,菊花地下部位也富含萜类物质,通过气相色谱-质谱联用技术(GC-MS)对26个菊花品种根提取物的鉴定发现:‘中阳菊’‘Zhongyangju’等茶菊品种根系的总萜含量普遍高于‘中山梦庄’‘Zhongshanmengzhuang’等地被菊和‘白乒乓’‘Baipingpang’等切花菊品种 [3]。
-
菊花作为传统草药,源于天然植物,具有低成瘾性和高安全性[27],在抗炎抑菌、抗肿瘤、降血脂、增强免疫活性等方面表现出显著功效[28−36]。菊花花序中的多种黄酮类化合物,如圣草酚-7-O-葡萄糖苷、黄酮苷、芹菜素-7-O-葡萄糖苷、香叶木素-7-O-葡萄糖苷等被证实是透明质酸酶(HAase)的强效抑制剂,它们能显著抑制小鼠和人类巨噬细胞中诱导型一氧化氮合成酶(iNOS)的活性以及白细胞介素-1β (IL-1β)的表达,进而显著降低一氧化氮(NO)和白细胞介素-6 (IL-6)的水平[19]。此外,菊花中山奈酚、4-羟基苯甲酸和芹菜素与黄嘌呤氧化酶(XO)抑制活性密切相关,且这3种成分之间存在相加作用[24]。
菊花精油富含活性物质,具有优异的抗氧化活性和感官改善性能[37−39],是天然防腐剂的丰富来源[40],可广泛应用于食品添加剂和医药领域[17]。如添加菊花精油可显著抑制葵花Helianthus annuus籽油中油脂变质指标的上升[41]。此外,菊花与其他天然中草药混合时能产生协同作用,如菊花与黄芩Scutellaria baicalensis的热水提取物表现出更强的抗氧化活性[12]。
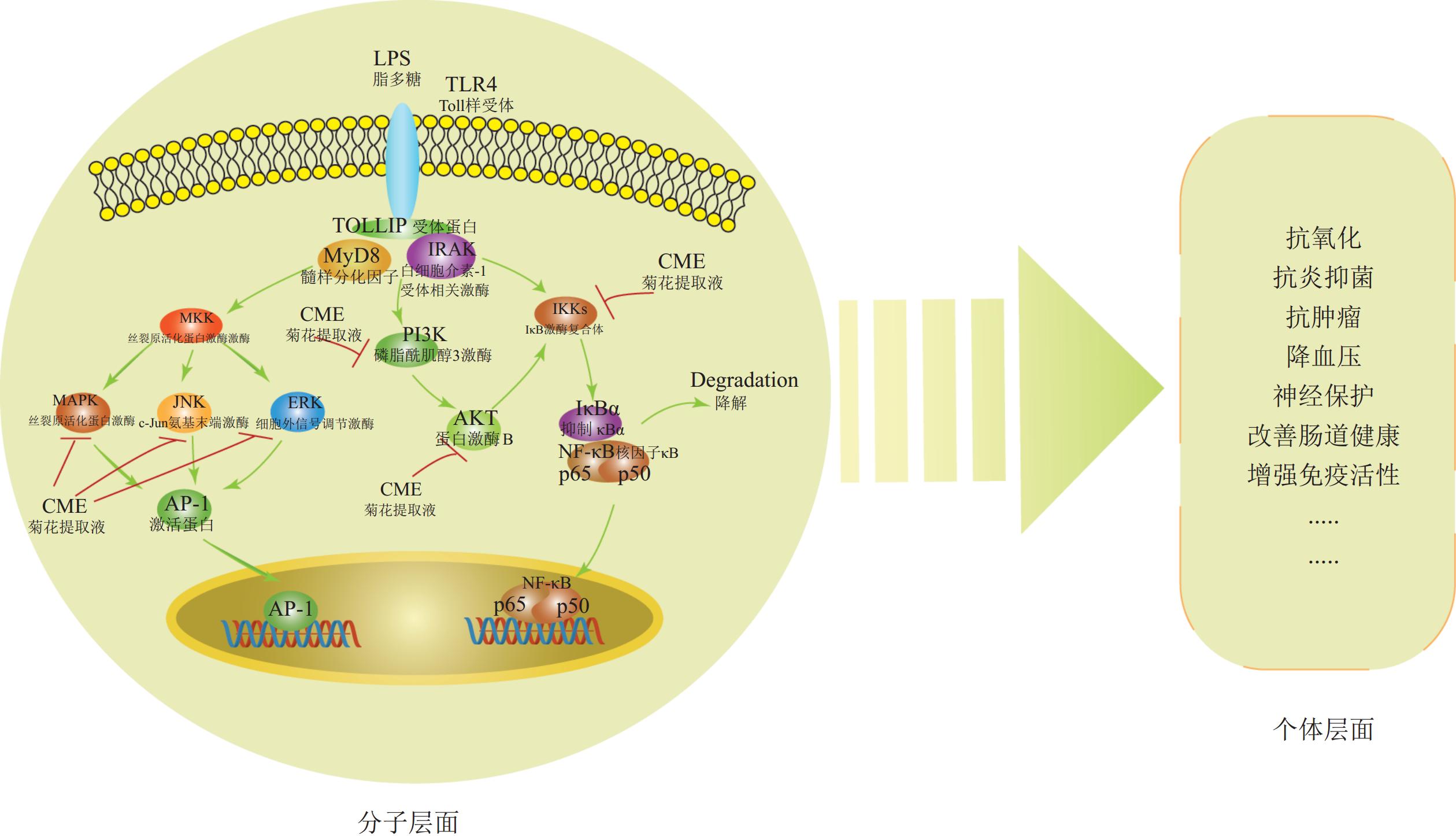
菊花活性物质的功效分子机制已部分阐明。这些物质通过调控丝裂原活化蛋白激酶(MAPK)、磷脂酰肌醇-3-激酶(PI3K/ Akt)和核因子-κB (NF-κB)信号通路,减少NO生成,抑制iNOS、肿瘤坏死因子-α(TNF-α)、IL-1β和IL-6的转录活性,同时抑制IκB激酶(IKK)、磷脂酰肌醇3-激酶(PI3K)的磷酸化,下调细胞外信号调节激酶(ERK)、 c-Jun氨基末端激酶(JNK)、p38丝裂原活化蛋白激酶(p38)、p50蛋白(p50)和p65蛋白(p65)的表达,从而发挥抗炎抗氧化等作用[26](图1)。此外,菊花的酪氨酸酶活性抑制能力(即抗氧化能力)与类黄酮和绿原酸等酚酸含量呈显著正相关[42]。
-
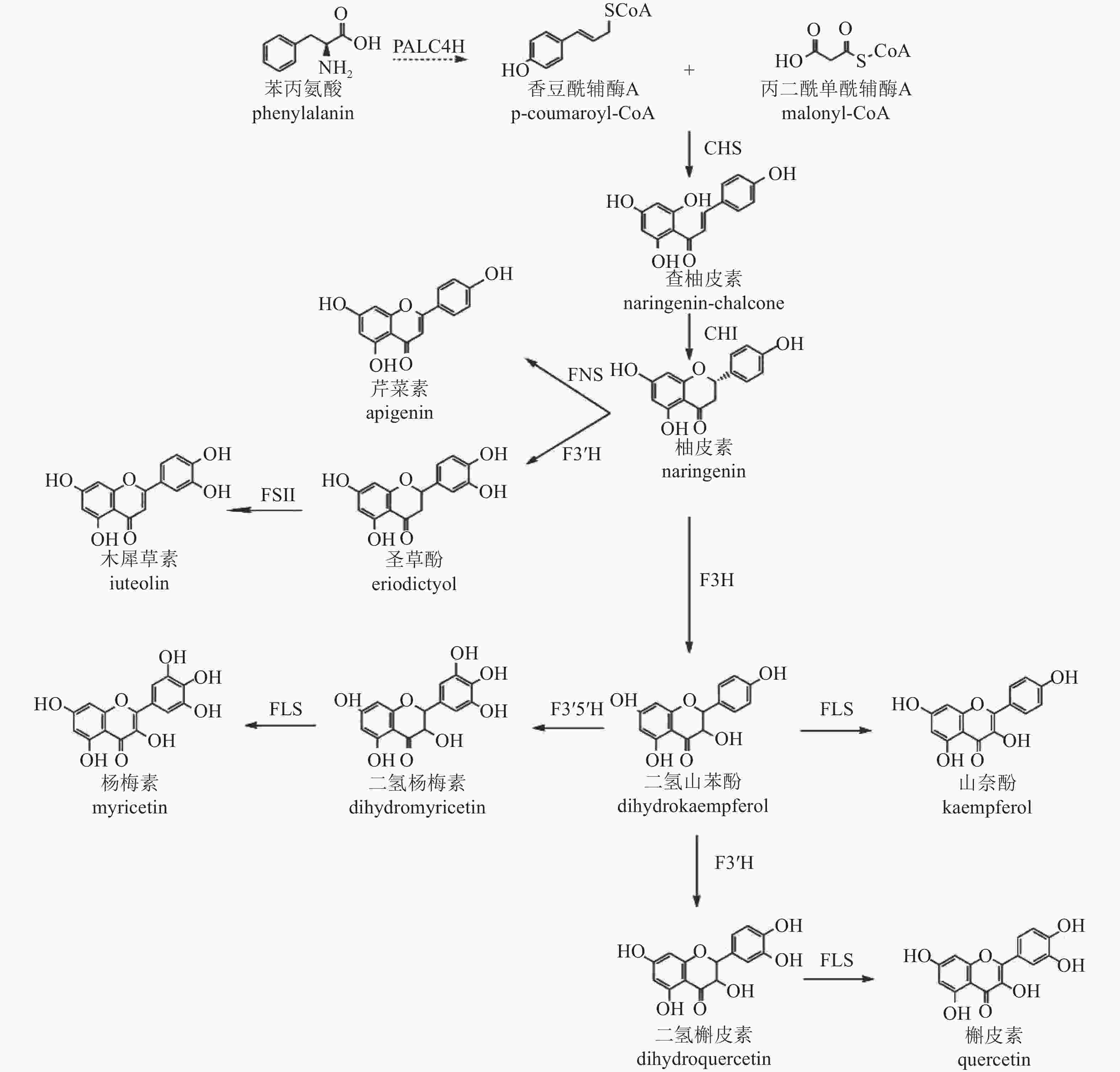
类黄酮化合物是植物中重要的次生代谢产物,其生物合成途径已较为明确[43],以香豆酰辅酶A (pCoA)为起始,在丙二酰单酰辅酶A (malonyl-CoA)参与下,经查尔酮酶(CHS)催化形成查柚皮素,并在查尔酮异构酶(CHI)进一步催化变构下形成类黄酮亚群常见的中间体柚皮素,再以柚皮素为基本骨架形成黄酮、黄烷酮、黄酮醇等其他类型化合物(图2)。根据基因在合成途径的位置,可将相关基因分为早期和晚期生物合成基因。早期生物合成基因(EBGs),包括查尔酮合成酶(CHS)、查尔酮异构酶(CHI)、二氢黄酮-3-氢化酶(F3H)、黄酮类-3'-氢化酶(F3'H)、黄酮醇合成酶(FLS),催化黄酮醇的生物合成,而晚期生物合成基因(LBGs)、二氢黄酮醇4-还原酶(DFR)、花青素合成酶(ANS)和花青素还原酶(ANR)等则促进花青素和原花青素的生物合成[44]。此外,其他基因如黄酮合成酶(FNS)和异黄酮合成酶(IFS)也分别催化黄酮和异黄酮的合成[45−46]。
转录组学分析表明:差异表达基因显著富集在类黄酮、不饱和脂肪酸、苯丙素和类胡萝卜素的生物合成途径[47],菊花中参与类黄酮生物合成的关键基因有苯丙氨酸裂解酶(PAL)、肉桂酸-4-氢化酶(C4H)、4-香豆酸辅酶A连接酶(4CL)、CHS、CHI、F3H、F3'H、FLS、FNS Ⅱ、二氢黄酮醇还原酶(DFR)和ANS。对木犀草素和槲皮素合成相关基因家族分析显示,存在5个CmCHS、3个CmCHI、1个CmF3H、1个CmF3'H、1个CmFNS Ⅱ和2个CmFLS基因异构体。在药用菊花中未发现与催化蓝花青素底物黄酮类-3'-5'-氢化酶(F3'5'H)相似的序列,这与缺乏蓝色药用菊花品种的现象一致。此外,ANR和无色花青素还原酶基因(LAR)也未在数据库中被鉴定,进一步解释了药用菊花中原花青素的缺失[48]。
菊花中CHS、CHI、F3'H等类黄酮合成基因已被成功克隆并鉴定功能。CHS、CHI作为植物聚酮合酶超家族成员,在类黄酮合成途径中起关键作用,影响黄酮类代谢产物的积累[49−50],且表达具有显著组织特异性[48]。F3'H基因编码的酶催化黄酮类化合物B环3-O位置的羟基化[51−52],是下游化合物合成的关键酶。基因结构分析显示:‘贡菊’和‘滁菊’‘Chuju’的F3'H基因第2内含子中含有与转录密切相关的基序,表明内含子可能参与了药菊F3'H基因的表达调控[53]。
-
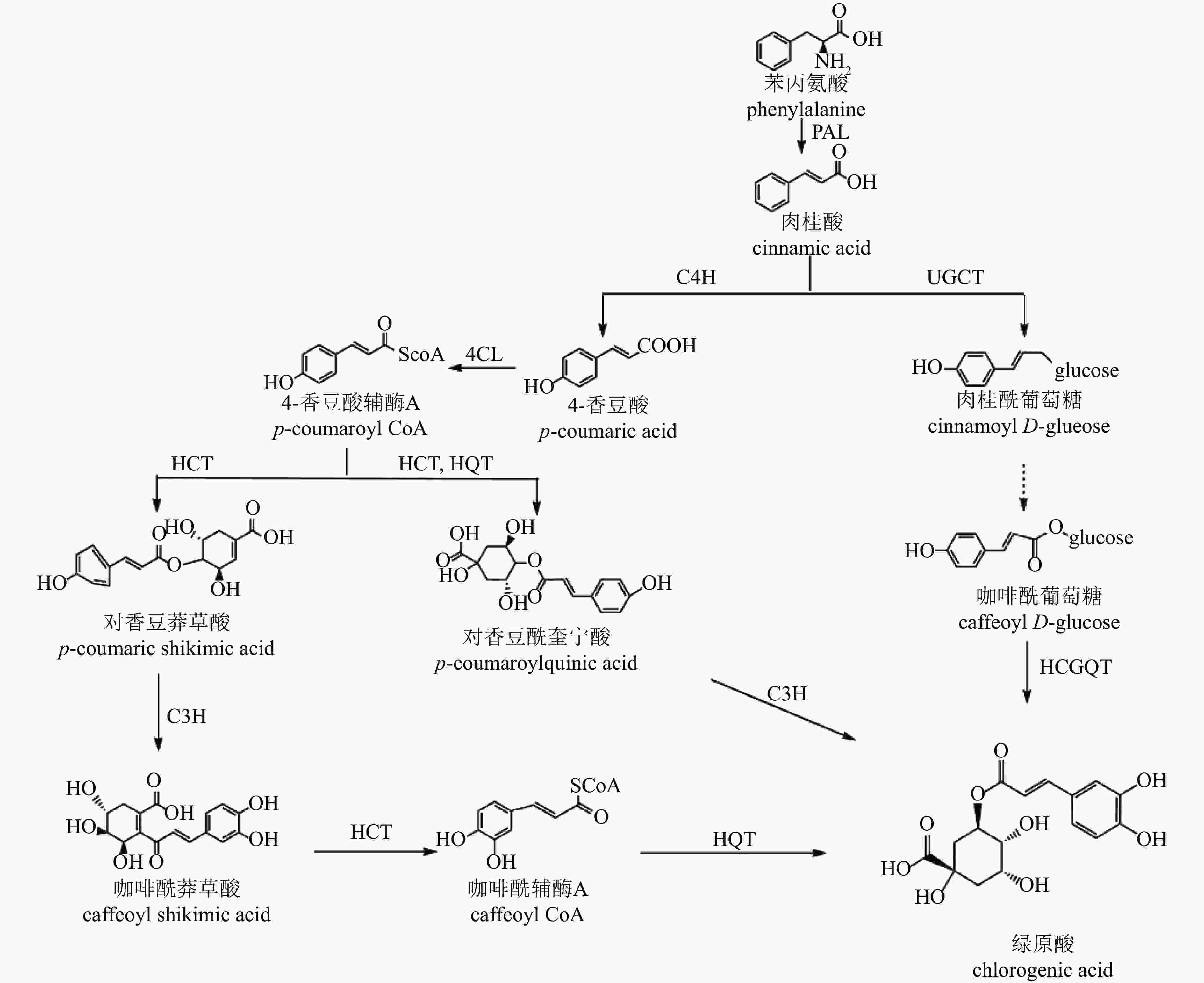
CGA的生物合成发生在植物次级代谢的苯丙素途径下游,目前已提出3种生物合成途径[54](图3):肉桂酸经肉桂酸4-羟化酶(C4H)催化生成中间产物4-香豆酸辅酶A(4-CL),在途径1中,该中间产物经奎宁酸羟基肉桂酰转移酶(HCT)、对香豆酸-3-羟化酶(C3H)、羟基肉桂酰辅酶A-奎宁酸羟基肉桂酰转移酶(HQT)等酶的连续作用转化为CGA;在途径2中,中间产物先形成对香豆酰奎宁酸(pCoQA),再由C3H催化生成CGA;在途径3中,肉桂酸经肉桂酸葡萄糖转移酶(UGCT)作用形成肉桂酰葡萄糖,进一步转化为咖啡酰葡萄糖,最终在奎宁酸羟基肉桂酰基转移酶(HCGQT)催化下形成CGA。
菊花中CGA合成研究较少。目前已有研究鉴定出13个HCT、2个HQT、2个C3H和3个UGCT基因与CGA合成有关[47]。
-
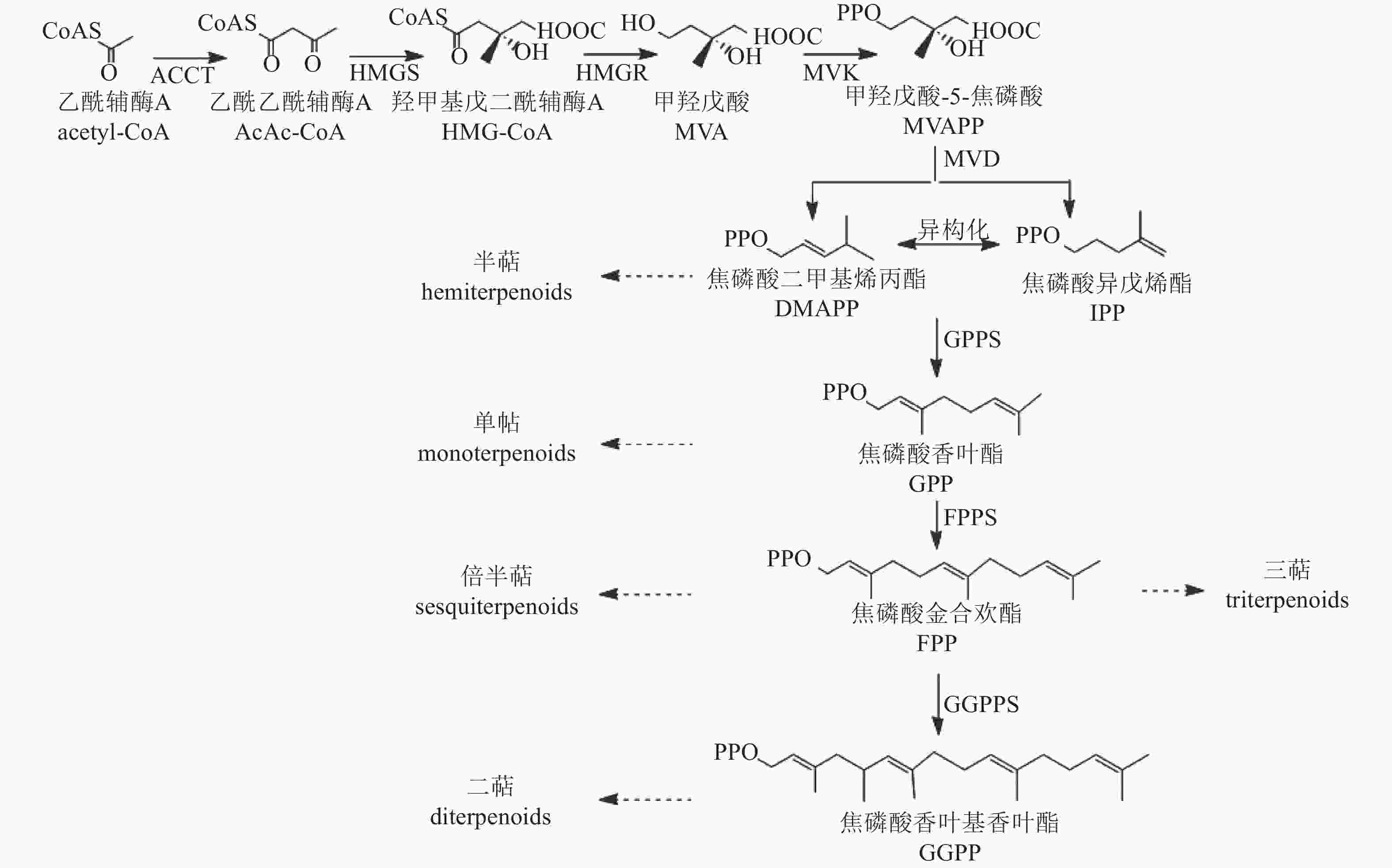
植物中萜类化合物主要经甲羟戊酸途径(MVA)合成[55](图4)。该途径从乙酰辅酶A缩合生成乙酰乙酰辅酶A开始,经羟醛缩合反应形成β-羟基-β-甲基戊二酸单酰辅酶A(HMG-GoA),随后生成中间体甲羟戊酸(MVA)。MVA经激酶催化形成甲羟戊酸-5-焦磷酸(MVAPP),再转化为焦磷酸异戊烯酯(IPP),IPP可异构化为焦磷酸二甲基烯丙酯(DMAPP)。最终,IPP和DMAPP在酶的作用下形成单萜、半倍萜、倍半萜、三萜等萜类化合物。
萜类化合物的多样性主要由萜类合成酶基因(TSs)驱动,包括TIDSs、SQSs、TPSs和TCCs。研究发现:TPS基因在菊属菊花脑C. nankingense基因组中显著扩增[56]。在‘滁菊’中鉴定出8个TPS基因,其编码蛋白均含有保守的二价阳离子结合域(DDXXD)和第二金属离子结合基序(NSE/DTE),这些结构是辅助因子Mg2+或Mn2+结合所必需的,其中,CmCJTPS5和CmCJTPS8被认为是菊花产生单半萜和倍半萜的关键基因。此外,CmCJTPS基因的表达水平与花香萜类挥发物的积累模式密切相关,萜类物质主要在完全开花前的盘状小花和叶根花托中合成[57]。
-
转录因子是一类能与基因5′端上游特定序列特异性结合、调控下游靶基因表达水平的蛋白质,在植物次生代谢中起关键作用[58]。目前已鉴定出多个转录因子家族参与植物次生代谢调控,其中MYB和bHLH家族研究最为深入。
MYB是植物转录因子家族的重要成员,其结构包含2个区域:N端高度保守,C端可变。这种特征赋予MYB蛋白多样化的功能[59]。MYB有3个亚类:MYB1R、R2R3-MYB和MYB3R,其中R2R3s是最大的亚类[60],主要来自SG4、SG5、SG6和SG7亚家族[61]。部分MYB参与调控开花时间、花药和花粉发育[62−63],例如在菊花‘金巴’‘Jinba’中,CmMYB2过表达的植株开花早于野生型,而CmMYB2表达受抑制的植株则开花延迟[64]。此外,一些MYB参与类黄酮的生物合成调控[65],转录分析显示菊花中CHS、CHI、F3H、F3'H和DRF的表达均受CmMYB8转基因抑制[66],在菊花‘粉色安娜斯塔’‘Anastasia Pink’中,CmMYB9a正向调节CmCHS、CmDFR和CmFNS,同时抑制CmFLS的表达,促进花青素和其他类黄酮积累,影响花色形成[67]。
bHLH转录因子的N端含有DNA结合的基本区域,C端含有HLH结构域,可调控植物的生长发育、次生代谢、抗逆性和信号转导[68],在活性物质合成尤其是花青素代谢中发挥重要作用。如丹参Salvia miltiorrhiza基因组中共鉴定出127个bHLH转录因子基因,其中7种可能参与丹参酮生物合成调控 [69],通过RNA干扰(RNAi)抑制丹参毛状根中SmbHLH92的表达,可显著增加酚酸和丹参酮积累[68]。在油菜Brassica napus籽中,BnbHLH92a通过抑制花青素和原花青素生物合成基因的表达,降低花青素积累,影响种子颜色[70]。酵母杂交实验表明:菊花中的CmbHLH2可直接结合CmDFR启动子,并与CmbHLH2和CmMYB6之间发生蛋白-蛋白相互作用,共同调控菊花花青素合成[71]。
AP2/ERF也是一个重要的转录因子大家族,通过结合靶基因启动子中的特定顺式作用元件调控基因表达,参与多种生理、发育和应激相关反应[72],包括AP2、ERF、RAV、Soloist等4个亚家族。染色质免疫沉淀(ChIP)和双萤光素酶(Dual-Luc)实验表明:烟草Nicotiana tabacum中的NtERF4a可结合NtPAL1和NtPAL2启动子中的GCC盒,激活其转录,促进烟草叶片中绿原酸和黄酮类化合物的积累[73]。HRE2-like作为AP2/ERF家族成员,在‘金巴’中调控类黄酮合成,CmCHS、CmCHI、CmF3'H在CmHRE2-like植株中下调,而在CmHRE2-like-SRDX植株上调,导致CmHRE2-like-SRDX系总黄酮含量显著增加[74]。
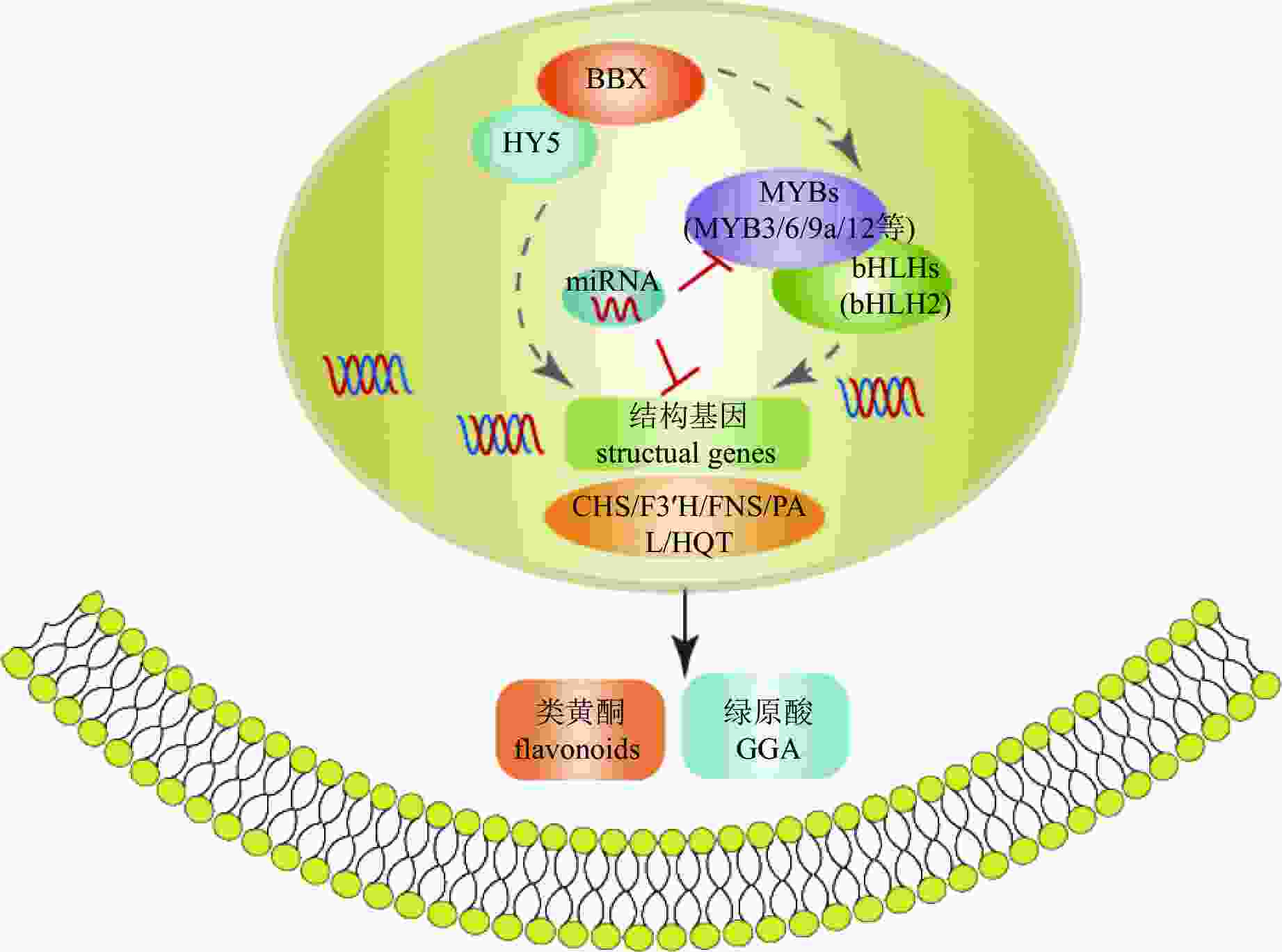
转录因子对活性物质合成的调控网络复杂且多层次,既可单独作用,也可通过互作调控。如菊花中BBX-HY5模块可能通过2种不同方式参与调控次生代谢(图5):一是直接调控CmHQT、CmCHS1/2、CmFNS、CmF3'H和CmPAL的转录水平;二是通过上调CmMYB3、CmMYB6和CmMYB16的表达水平,正向调节结构基因[75]。
-
miRNAs是一类广泛存在的内源性非编码小RNA分子,通过与下游靶向基因和转录因子结合参与调控多项生物学过程[76]。如芸薹Brassica rapa及其亲本miRNA表达谱分析发现有204个差异表达的miRNA,其中184个在芸薹中非加性表达,多组学数据表明miR858a和miR157a可能调控类黄酮合成[77]。有关菊花miRNA的研究相对有限,已鉴定出与HD-ZIP、DOF、SBF-like和Trihelix等转录因子家族相关的miRNA [78−81],并对其功能进行了初步分析。YANG等[47]检测到菊花中38个家族的169个miRNAs,其中118个miRNAs靶向1 954个基因,6种miRNA靶向5个活性成分生物合成调控相关基因,5个miRNAs通过靶向MYB-bHLH复合体相关转录因子,参与类黄酮和绿原酸的合成。
-
目前,植物活性物质合成过程中有关基因酶的克隆鉴定以及异源表达都取得了突破性进展。在天然次生代谢产物中,类黄酮化合物的结构及生物合成途径是目前研究最为清楚的途径之一,菊花中的活性物质研究也集中在类黄酮,包括木犀草素、芹菜素、金合欢素等,CmCHS、CmCHI、CmF3H、CmF3'H、CmFNS、CmFLS、Cm4CL、CmUGT是菊花类黄酮合成的关键基因,而TPS基因家族控制萜类合成酶(TSs)的合成,是菊花产生单半萜和倍半萜的决定因素,相比之下,绿原酸合成机制研究较少,有待进一步探索。
传统菊花活性物质提取是从植物中直接提取,受资源、季节、气候等影响较大,且天然含量低、化学合成困难,难以满足市场需求。因此,绿色高效的生物合成是未来获取菊花活性物质的重要途径,也是大健康领域研究热点。然而活性物质合成是一个多层次、立体的复杂网络,目前对其合成途径的总体调控以及次生代谢途径之间的协调机制等知之甚少,因此解析菊花活性物质的结构、合成途径及其调控网络,从不同侧面对活性物质合成过程及其调控加以整合分析和设计,是未来菊花活性物质利用的重点任务。
Research progress on the types, biosynthetic pathways, and regulatory mechanisms of bioactive compounds in Chrysanthemum × morifolium
-
摘要: 菊花 Chrysanthemum × morifolium是一种历史悠久的观赏、食用和药用植物,富含的活性物质(如类黄酮、酚酸、萜类等)具有显著的抗炎、抗氧化和抗肿瘤等功效。本研究综述了菊花主要活性物质的种类及功效机制,重点阐述了其生物合成途径及调控网络。研究表明:苯丙烷代谢途径中的8个关键酶基因(CHS、CHI、F3H、F3'H、FNS、FLS、4CL、UGT)在活性物质合成中发挥关键作用。活性物质的合成过程受到多层级调控,MYB、bHLH和AP2/ERF等转录因子家族通过特异性结合启动子元件调控结构基因表达,miRNA通过与结构基因及转录因子靶向结合影响活性物质的合成。菊花中类黄酮化合物的生物合成途径已较为明确,而绿原酸作为重要的药理成分,其合成机制研究较少,需要进一步探索。探明活性物质合成途径的总体调控以及次生代谢途径之间的协调机制,实现活性物质绿色高效的合成和品种改良,是菊花相关产业的重要方向。图5表1参81Abstract: Chrysanthemum × morifolium, a plant with a long history of ornamental, edible, and medicinal uses, contains bioactive compounds with multiple functions, such as anti-inflammatory, antioxidant, and anti-tumor properties. This article reviews the types and mechanisms of action of bioactive compounds in C. × morifolium, summarizes their biosynthetic pathways, and elucidates the regulatory mechanisms governing their synthesis. Chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3'-hydroxylase (F3'H), flavone synthase (FNS), flavonol synthase (FLS), 4-coumarate-CoA ligase (4CL), and UDP-glycosyltransferase (UGT) have been identified as key players in the synthesis of bioactive compounds. The biosynthesis is hierarchically regulated: transcription factor families such as MYB, bHLH, and AP2/ERF, modulate structural gene expression by binding to specific promoter elements. Additionally, miRNAs affect the synthesis of these compounds by targeting crucial structural genes and transcription factors. Although many researches have been conducted on flavonoid structure and biosynthesis in C. × morifolium, studies on the synthesis mechanism of chlorogenic acid, an important pharmacological component, remain limited and require further exploration. Clarifying the global regulatory network of bioactive compound biosynthesis and the molecular crosstalk between secondary metabolic pathways will be crucial for developing sustainable and efficient synthesis strategies for C. × morifolium active compounds, thereby advancing the development of the C. × morifolium industry. [Ch, 5 fig. 1 tab. 81 ref.]
-
表 1 菊花中已知活性物质
Table 1. Known bioactive compounds in C. morifilium
分类 名称 测定方法 测定部位 参考文献 类黄酮 芹菜素-7-葡萄糖苷apigenin-7-O-glucoside UF-LC-MS、UPLC 干花序 [18−21] 木犀草素luteolin NMR、UPLC-MS/MS、HPLC-MIPSPE 干花序 [22−25] 木犀草苷luteoloside UPLC-ESI-Q-TOF-MS/MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 23, 26] 木犀草素-7-葡萄糖醛酸苷luteolin-7-O-glucuronide LC-MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 26] 橙皮素hesperetin LC-MS、NMR 鲜花序、干花序 [8, 24] 山柰酚kaempferol HPLC-MIPSPE 干花序 [24] 杨梅素myricetin HPLC-MIPSPE 干花序 [24] 芹菜素apigenin LC-MS、NMR、UF-LC-MS 鲜花序、茎、
干花序[8, 22−23] 槲皮素quercetin UF-LC-MS、NMR 干花序、茎 [8, 22] 芦丁quercetin 3-O-rutinoside LC-MS 鲜花序 [8] 金合欢素acacetin UF-LC-MS、NMR 干花序、茎 [8, 22] 圣草酚eriodictyol NMR 茎 [22] 异鼠李素isorhamnetin HPLC-MIPSPE 干花序 [25] 樱黄素prunetin HPLC-Q-TOF-MS/MS 干花序 [21] 酚酸 1,4-O-二咖啡酰奎宁酸1,4-dicaffeoylquinic acid LC-MS、NMR、UPLC 干花序、茎 [8, 19, 22] 1,3-O-二咖啡酰奎宁酸1,3-dicaffeoylquinic acid UPLC-MS/MS 干花序 [26] 1,5-O-二咖啡酰奎宁酸1,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS 干花序、茎 [8, 20, 22] 3,5-O-二咖啡酰奎宁酸3,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS、UF-LC-MS 干花序、茎 [8, 20, 22−23] 4,5-O-二咖啡酰奎宁酸4,5-dicaffeoylquinic acid LC-MS、UPLC 干花序 [8, 19] 绿原酸chlorogenic acid LC-MS、NMR、UF-LC-MS、UPLC-MS/MS 干花序序、茎 [8, 20, 22−23, 26] 咖啡酸affeic acid LC-MS 干花序 [8] 没食子酸gallic acid NMR 茎 [24] 萜类 β-榄香烯(–)-β-elemene GC-MS 干花序 [17] (E)-β-金合欢烯cis-β-farnesene GC-MS 干花序 [17] β-半水芹烯(–)-β-sesquiphellandrene GC-MS 干花序 [17] (+)-喇叭烯(+)-ledene GC-MS 干花序 [17] 桉叶油素1,8-cineole GC-MS 鲜花序 [16] 樟脑camphor GC-MS 鲜花序 [16] 丁香酚eugenol GC-MS 鲜花序 [16] β-石竹烯β-caryophyllene GC-MS 鲜花序 [16] α-姜烯α-zingiberene GC-MS 鲜花序 [16] β-蒎烯β-curcumene GC-MS 鲜花序 [16] 石竹烯氧化物caryophyllene oxide GC-MS 鲜花序 [16] α-没药醇α-bisabolol GC-MS 鲜花序 [16] 香芹酮carvone NMR 茎 [24] 说明:UF-LC-MS. 超滤液相色谱-质谱联用技术;UPLC. 超高效液相色谱法;NMR.核磁共振波谱法;UPLC-MS/MS. 超高效液相色谱-串联质谱联用技术;HPLC-MIPSPE. 高效液相色谱-分子印迹固相萃取在线联用技术;UPLC-ESI-Q-TOF-MS/MS. 超高效液相色谱-电喷雾离子源-四级杆-飞行时间串联质谱联用技术。LC-MS. 液相色谱-质谱联用技术;GC-MS.气相色谱-质谱联用技术。 -
[1] 张树林, 戴思兰. 中国菊花全书[M]. 北京: 中国林业出版社, 2013. ZHANG Shulin, DAI Silan. Chinese Chrysanthemum Book[M]. Beijing: China Forestry Publishing House, 2013. [2] YUAN Hanwen, JIANG Sai, LIU Yingkai, et al. The flower head of Chrysanthemum morifolium Ramat. (Juhua): a paradigm of flowers serving as Chinese dietary herbal medicine[J/OL]. Journal of Ethnopharmacology, 2020, 261: 113043[2024-10-05]. DOI: 10.1016/j.jep.2020.113043. [3] WU Jiayi, LIU Jingjing, JIANG Li, et al. Diversity and dose-dependent allelopathic potential of volatile sesquiterpenes from root extracts of Chrysanthemum morifolium cultivars[J/OL]. Scientia Horticulturae, 2024, 327: 112830[2024-10-05]. DOI: 10.1016/j.scienta.2023.112830. [4] SCHMITT M, ALI AHMADI S, XU Yonghao, et al. There are No data like more data: datasets for deep learning in earth observation [J]. IEEE Geoscience and Remote Sensing Magazine, 2023, 11(3): 63−97. [5] CUI Luming, ZHANG Qian, ZHANG Yifan, et al. Anxiolytic effects of Chrysanthemum morifolium Ramat. Carbonisata-based carbon dots in mCPP-induced anxiety-like behavior in mice: a nature-inspired approach[J/OL]. Frontiers in Molecular Biosciences, 2023, 10: 1222415[2024-10-05]. DOI: 10.3389/fmolb.2023.1222415. [6] 金潇潇, 陈发棣, 陈素梅, 等. 20个菊花品种花瓣的营养品质分析[J]. 浙江林学院学报, 2010, 27(1): 22−29. JIN Xiaoxiao, CHEN Fadi, CHEN Sumei, et al. Nutrition in 20 cultivars of Chrysanthemum [J]. Journal of Zhejiang Forestry College, 2010, 27(1): 22−29. [7] CHU Qingcui, FU Liang, GUAN Yueqing, et al. Determination and differentiation of Flos Chrysanthemum based on characteristic electrochemical profiles by capillary electrophoresis with electrochemical detection [J]. Journal of Agricultural and Food Chemistry, 2004, 52(26): 7828−7833. [8] CHEN Sha, LIU Jing, DONG Gangqiang, et al. Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat. flowers: a potentially rich source of bioactive compounds[J/OL]. Food Chemistry, 2021, 344: 128733[2024-10-05]. DOI: 10.1016/j.foodchem.2020.128733. [9] SHEN Nan, WANG Tongfei, GAN Quan, et al. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity[J/OL]. Food Chemistry, 2022, 383: 132531[2024-10-05]. DOI: 10.1016/j.foodchem.2022.132531. [10] PANDEY J, BASTOLA T, DHAKAL B, et al. Chrysanthemum morifolium Ramat.: a medicinal plant with diverse traditional uses, bioactive constituents, and pharmacological activities [M]// DEVKOTA H P, AFTAB T. Medicinal Plants of the Asteraceae Family, Singapore: Springer, 2022: 125−143. [11] JANG H Y, LEE H S, NOH E M, et al. Aqueous extract of Chrysanthemum morifolium Ramat. inhibits RANKL-induced osteoclast differentiation by suppressing the c-fos/NFATc1 pathway[J/OL]. Archives of Oral Biology, 2021, 122: 105029[2024-10-05]. DOI: 10.1016/j.archoralbio.2020.105029. [12] SUH M G, CHOI H S, CHO K, et al. Anti-inflammatory action of herbal medicine comprised of Scutellaria baicalensis and Chrysanthemum morifolium [J]. Bioscience, Biotechnology, and Biochemistry, 2020, 84(9): 1799−1809. [13] TIAN Xing, WANG Haodong, CHEN Liang, et al. Distinct changes in metabolic profile and sensory quality with different varieties of Chrysanthemum (Juhua) tea measured by LC-MS-based untargeted metabolomics and electronic tongue[J/OL]. Foods, 2024, 13(7): 1080[2024-10-05]. DOI: 10.3390/foods13071080. [14] LI Yanfang, YANG Puyu, LUO Yinghua, et al. Chemical compositions of Chrysanthemum teas and their anti-inflammatory and antioxidant properties [J]. Food Chemistry, 2019, 286: 8−16. [15] URANISHI R, AEDLA R, ALSAADI D H M, et al. Evaluation of environmental factor effects on the polyphenol and flavonoid content in the leaves of Chrysanthemum indicum L. and its habitat suitability prediction mapping[J/OL]. Molecules, 2024, 29(5): 927[2024-10-05]. DOI: 10.3390/molecules29050927. [16] ZHAN Jianfeng, HE Feng, CAI Huimin, et al. Composition and antifungal mechanism of essential oil from Chrysanthemum morifolium cv. Fubaiju[J/OL]. Journal of Functional Foods, 2021, 87: 104746[2024-10-05]. DOI: 10.1016/j.jff.2021.104746. [17] JIANG Zhongrong, ZHANG Ting, JI Lingbo, et al. Chemical composition and bioactivities of the essential oil of Coreopsis tinctoria and Chrysanthemum morifolium [J]. International Journal of Food Properties, 2023, 26(1): 1036−1046. [18] WANG Yuxiao, XU Zhenzhen, HUANG Yuqi, et al. Extraction, purification, and hydrolysis behavior of apigenin-7-O-glucoside from Chrysanthemum morifolium tea[J/OL]. Molecules, 2018, 23(11): 2933[2024-10-05]. DOI: 10.3390/molecules23112933. [19] ZHOU Huiji, ZHANG Xue, LI Bo, et al. Fast and efficient identification of hyaluronidase specific inhibitors from Chrysanthemum morifolium Ramat. using UF-LC-MS technique and their anti-inflammation effect in macrophages[J/OL]. Heliyon, 2023, 9(2): e13709[2024-10-05]. DOI: 10.1016/j.heliyon.2023.e13709. [20] LEE M S, KIM Y. Chrysanthemum morifolium flower extract inhibits adipogenesis of 3T3-L1 cells via AMPK/SIRT1 pathway activation[J/OL]. Nutrients, 2020, 12(9): 2726[2024-10-05]. DOI: 10.3390/nu12092726. [21] NG T L, LOH K E, TAN S A, et al. Anti-hyperuricemic effect of ethyl acetate sub-fractions from Chrysanthemum morifolium Ramat. dried flowers on potassium oxonate-induced hyperuricemic rats[J/OL]. Applied Sciences, 2022, 12(7): 3487[2024-10-05]. DOI: 10.3390/app12073487. [22] QU Lu, RUAN Jingya, JIN Lijun, et al. Xanthine oxidase inhibitory effects of the constituents of Chrysanthemum morifolium stems [J]. Phytochemistry Letters, 2017, 19: 39−45. [23] YUAN Jun, HUANG Jun, WU Gang, et al. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. [J]. Industrial Crops and Products, 2015, 74: 192−199. [24] LOH K E, CHIN Y S, SAFINAR ISMAIL I, et al. Rapid characterisation of xanthine oxidase inhibitors from the flowers of Chrysanthemum morifolium Ramat. Using metabolomics approach [J]. Phytochemical Analysis, 2022, 33(1): 12−22. [25] GAO Die, YANG Fengqing, XIA Zhining, et al. Molecularly imprinted polymer for the selective extraction of luteolin from Chrysanthemum morifolium Ramat. [J]. Journal of Separation Science, 2016, 39(15): 3002−3010. [26] ZHANG Nan, HE Zhengjun, HE Siyu, et al. Insights into the importance of dietary Chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties [J]. Food Research International, 2019, 116: 810−818. [27] ONO M, SUNAGAWA Y, MOCHIZUKI S, et al. Chrysanthemum morifolium extract ameliorates doxorubicin-induced cardiotoxicity by decreasing apoptosis[J/OL]. Cancers, 2022, 14(3): 683[2024-10-05]. DOI: 10.3390/cancers14030683. [28] YANG Feng, WANG Tao, GUO Qiaosheng, et al. The CmMYB3 transcription factors isolated from the Chrysanthemum morifolium regulate flavonol biosynthesis in Arabidopsis thaliana [J]. Plant Cell Reports, 2023, 42(4): 791−803. [29] LIANG Fengjie, HU Changfeng, HE Zhengchun, et al. An Arabinogalactan from flowers of Chrysanthemum morifolium: structural and bioactivity studies [J]. Carbohydrate Research, 2014, 387: 37−41. [30] TAO Jinhua, DUAN Jinao, JIANG Shu, et al. Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat. by gas chromatography with flame ionization detector [J]. Journal of Chromatography B, 2016, 1029: 88−94. [31] LI Liping, GU Liqiang, CHEN Zhongjian, et al. Toxicity study of ethanolic extract of Chrysanthemum morifolium in rats [J]. Journal of Food Science, 2010, 75(6): T105−T109. [32] CHEN Liangmian, KOTANI A, KUSU F, et al. Quantitative comparison of caffeoylquinic acids and flavonoids in Chrysanthemum morifolium flowers and their sulfur-fumigated products by three-channel liquid chromatography with electrochemical detection [J]. Chemical & Pharmaceutical Bulletin, 63: 25−32. [33] HODAEI M, RAHIMMALEK M, ARZANI A. Variation in bioactive compounds, antioxidant and antibacterial activity of Iranian Chrysanthemum morifolium cultivars and determination of major polyphenolic compounds based on HPLC analysis [J]. Journal of Food Science and Technology, 2021, 58(4): 1538−1548. [34] YOUSSEF F S, EID S Y, ALSHAMMARI E, et al. Chrysanthemum indicum and Chrysanthemum morifolium: chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities[J/OL]. Foods, 2020, 9(10): 1460[2024-10-05]. DOI: 10.3390/foods9101460. [35] CHO B O, SHIN J Y, KANG H J, et al. Anti-inflammatory effect of Chrysanthemum zawadskii, peppermint, Glycyrrhiza glabra herbal mixture in lipopolysaccharide-stimulated RAW264.7 macrophages[J/OL]. Molecular Medicine Reports, 2021, 24(1): 532[2024-10-05]. DOI: 10.3892/mmr.2021.12171. [36] GUVEN H, ARICI A, SIMSEK O. Flavonoids in our foods: a short review[J/OL]. Journal of Basic and Clinical Health Sciences, 2019, 3(1): 555[2024-10-05]. DOI: 10.30621/jbachs.2019.555. [37] SAYYARI Z, FARAHMANDFAR R. Stabilization of sunflower oil with pussy willow (Salix aegyptiaca) extract and essential oil [J]. Food Science & Nutrition, 2017, 5(2): 266−272. [38] EMBUSCADO M E. Spices and herbs: natural sources of antioxidants: a mini review [J]. Journal of Functional Foods, 2015, 18: 811−819. [39] WANG Dongying, MENG Yudong, WANG Chenxin, et al. Antioxidant activity and sensory improvement of Angelica dahurica cv. Yubaizhi essential oil on sunflower oil during high-temperature storage[J/OL]. Processes, 2020, 8(4): 403[2024-10-05]. DOI: 10.3390/pr8040403. [40] ZHANG Xiaoxi, YU Xinfen, SHI Yueyue, et al. Chrysanthemum morifolium cv. Hang-ju leaves: an abundant source of preservatives for food industry[J]. European Food Research and Technology, 2020, 246(5): 939−946. [41] MENG Yudong, YANG Haoduo, WANG Dongying, et al. Improvement for oxidative stability and sensory properties of sunflower oil flavored by Huai Chrysanthemum × morifolium Ramat. essential oil during accelerated storage[J/OL]. Processes, 2021, 9(7): 1199[2024-10-05]. DOI: 10.3390/pr9071199. [42] CHEN Y H, YAN S L, WU J Y, et al. Analyses of the compositions, antioxidant capacities, and tyrosinase-inhibitory activities of extracts from two new varieties of Chrysanthemum morifolium Ramat. using four solvents[J/OL]. Applied Sciences, 2021, 11(16): 7631[2024-10-05]. DOI: 10.3390/app11167631. [43] DENG Yinai, YANG Peng, ZHANG Qianle, et al. Genomic insights into the evolution of flavonoid biosynthesis and O-methyltransferase and glucosyltransferase in Chrysanthemum indicum[J/OL]. Cell Reports, 2024, 43(2): 113725[2024-10-05]. DOI: 10.1016/j.celrep.2024.113725. [44] DENG Yuxing, LU Shanfa. Biosynthesis and regulation of phenylpropanoids in plants [J]. Critical Reviews in Plant Sciences, 2017, 36(4): 257−290. [45] RIGHINI S, RODRIGUEZ E J, BEROSICH C, et al. Apigenin produced by maize flavone synthase Ⅰ and Ⅱ protects plants against UV-B-induced damage [J]. Plant, Cell & Environment, 2019, 42(2): 495−508. [46] HU Ting, GAO Zhiqiang, HOU Jiaming, et al. Identification of biosynthetic pathways involved in flavonoid production in licorice by RNA-seq based transcriptome analysis [J]. Plant Growth Regulation, 2020, 92(1): 15−28. [47] YANG Yanjun, LIU Jie, YI Taiyao, et al. Integrated mRNA and miRNA omics reveal the regulatory role of UV-B radiation in active ingredient biosynthesis of Chrysanthemum morifolium Ramat. [J/OL]. Industrial Crops and Products, 2023, 197: 116657[2024-10-05]. DOI: 10.1016/j.indcrop.2023.116657. [48] WANG Tao, YANG Feng, GUO Qiaosheng, et al. Long-read sequencing of Chrysanthemum morifolium transcriptome reveals flavonoid biosynthesis and regulation [J]. Plant Growth Regulation, 2020, 92(3): 559−569. [49] WANG Lanlan, LIU Xiaomeng, MENG Xiangxiang, et al. Cloning and expression analysis of a chalcone isomerase (CnCHI) gene from Chamaemelum nobile [J]. Biotechnology (Faisalabad), 2017, 17(1): 19−25. [50] ZHAO Xiting, SONG Lingyu, MA Mengdan, et al. RNA-Seq for excavation of genes involved in the biosynthesis of primary active components and identification of new EST-SSR markers in medicinal chrysanthemum [J]. Archives of Biological Sciences, 2019, 71(3): 489−500. [51] TANAKA Y, BRUGLIERA F. Flower colour and cytochromes P450[J]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 2013, 368(1612): 20120432[2024-10-05]. DOI: 10.1098/rstb.2012.0432. [52] AYABE S I, AKASHI T. Cytochrome P450s in flavonoid metabolism [J]. Phytochemistry Reviews, 2006, 5(2/3): 271−282. [53] ZHAO Weiping, ZHAO Jun, HE Lan, et al. Molecular structure and the second introns variation of gene F3'H of two medicinal Chrysanthemum morifolium populations [J]. Biochemical Systematics and Ecology, 2013, 51: 251−258. [54] LU Chenfei, YAN Xiaoyun, ZHANG Haohao, et al. Integrated metabolomic and transcriptomic analysis reveals biosynthesis mechanism of flavone and caffeoylquinic acid in Chrysanthemum[J/OL]. BMC Genomics, 2024, 25(1): 759[2024-10-05]. DOI: 10.1186/s12864-024-10676-6. [55] XU Yi, HUANG Dongmei, MA Funing, et al. Identification of key genes involved in flavonoid and terpenoid biosynthesis and the pathway of triterpenoid biosynthesis in Passiflora edulis [J]. Journal of Integrative Agriculture, 2023, 22(5): 1412−1423. [56] SONG Chi, LIU Yifei, SONG Aiping, et al. The Chrysanthemum nankingense genome provides insights into the evolution and diversification of chrysanthemum flowers and medicinal traits [J]. Molecular Plant, 2018, 11(12): 1482−1491. [57] ZHANG Wanbo, JIANG Yifan, CHEN Fei, et al. Dynamic regulation of volatile terpenoid production and emission from Chrysanthemum morifolium capitula [J]. Plant Physiology and Biochemistry, 2022, 182: 11−21. [58] ZHAN Xiaori, CHEN Zhehao, CHEN Rong, et al. Environmental and genetic factors involved in plant protection-associated secondary metabolite biosynthesis pathways[J/OL]. Frontiers in Plant Science, 2022, 13: 877304[2024-10-05]. DOI: 10.3389/fpls.2022.877304. [59] WANG Xiangyuan, TIAN Lu, FENG Shijing, et al. Identifying potential flavonoid biosynthesis regulator in Zanthoxylum bungeanum Maxim. by genome-wide characterization of the MYB transcription factor gene family [J]. Journal of Integrative Agriculture, 2022, 21(7): 1997−2018. [60] DUBOS C, STRACKE R, GROTEWOLD E, et al. MYB transcription factors in Arabidopsis[J]. Trends in Plant Science, 2010, 15(10): 573−581. [61] WANG Yiguang, ZHOU Lijie, WANG Yuxi, et al. An R2R3-MYB transcription factor CmMYB21 represses anthocyanin biosynthesis in color fading petals of Chrysanthemum[J/OL]. Scientia Horticulturae, 2022, 293: 110674[2024-10-05]. DOI: 10.1016/j.scienta.2021.110674. [62] ZHANG Yunfei, CAO Guangyu, QU Lijia, et al. Characterization of Arabidopsis MYB transcription factor gene AtMYB17 and its possible regulation by LEAFY and AGL15[J/OL]. Journal of Genetics and Genomics, 2009, 36(2): 99−107. [63] PUNWANI J A, RABIGER D S, LLOYD A, et al. The MYB98 subcircuit of the synergid gene regulatory network includes genes directly and indirectly regulated by MYB98 [J]. The Plant Journal, 2008, 55(3): 406−414. [64] ZHU Lu, GUAN Yunxiao, LIU Yanan, et al. Regulation of flowering time in Chrysanthemum by the R2R3 MYB transcription factor CmMYB2 is associated with changes in gibberellin metabolism[J/OL]. Horticulture Research, 2020, 7: 96[2024-10-05]. DOI: 10.1038/s41438-020-0317-1. [65] AI Penghui, XUE Jundong, SHI Zhongya, et al. Genome-wide characterization and expression analysis of MYB transcription factors in Chrysanthemum nankingense[J/OL]. BMC Plant Biology, 2023, 23(1): 140[2024-10-05]. DOI: 10.1186/s12870-023-04137-7. [66] ZHU Lu, GUAN Yunxiao, ZHANG Zhaohe, et al. CmMYB8 encodes an R2R3 MYB transcription factor which represses lignin and flavonoid synthesis in Chrysanthemum [J]. Plant Physiology and Biochemistry, 2020, 149: 217−224. [67] WANG Yiguang, ZHOU Lijie, WANG Yuxi, et al. CmMYB9a activates floral coloration by positively regulating anthocyanin biosynthesis in Chrysanthemum [J]. Plant Molecular Biology, 2022, 108(1/2): 51−63. [68] ZHANG Jianhong, LÜ Haizhou, LIU Wanjing, et al. bHLH transcription factor SmbHLH92 negatively regulates biosynthesis of phenolic acids and tanshinones in Salvia miltiorrhiza [J]. Chinese Herbal Medicines, 2020, 12(3): 237−246. [69] ZHANG Xin, LUO Hongmei, XU Zhichao, et al. Genome-wide characterisation and analysis of bHLH transcription factors related to tanshinone biosynthesis in Salvia miltiorrhiza[J/OL]. Scientific Reports, 2015, 5: 11244[2024-10-05]. DOI: 10.1038/srep11244. [70] HU Ran, ZHU Meichen, CHEN Si, et al. BnbHLH92a negatively regulates anthocyanin and proanthocyanidin biosynthesis in Brassica napus [J]. The Crop Journal, 2023, 11(2): 374−385. [71] XIANG Lili, LIU Xiaofen, LI Xue, et al. A novel bHLH transcription factor involved in regulating anthocyanin biosynthesis in chrysanthemums (Chrysanthemum morifolium Ramat. )[J/OL]. PLoS One, 2015, 10(11): e0143892[2024-10-05]. DOI: 10.1371/journal.pone.0143892. [72] KLAY I, GOUIA S, LIU Mingchun, et al. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants [J]. Plant Science, 2018, 274: 137−145. [73] HE Shun, XU Xin, GAO Qian, et al. NtERF4 promotes the biosynthesis of chlorogenic acid and flavonoids by targeting PAL genes in Nicotiana tabacum[J/OL]. Planta, 2023, 259(2): 31[2024-10-05]. DOI: 10.1007/s00425-023-04301-1. [74] WANG You, ZHANG Wanwan, HONG Chaojun, et al. Chrysanthemum (Chrysanthemum morifolium) CmHRE2-like negatively regulates the resistance of chrysanthemum to the aphid (Macrosiphoniella sanborni)[J]. BMC Plant Biology, 2024, 24(1): 76[2024-10-05]. DOI: 10.1186/s12870-024-04758-6. [75] LU Chenfei, LIU Yuchen, YAN Xiaoyun, et al. Multiplex approach of metabolomic and transcriptomic reveals the biosynthetic mechanism of light-induced flavonoids and CGA in Chrysanthemum[J]. Industrial Crops and Products, 2024, 221: 119420[2024-10-05]. DOI: 10.1016/j.indcrop.2024.119420. [76] DONG Qingkun, HU Binbin, ZHANG Cui. microRNAs and their roles in plant development[J]. Frontiers in Plant Science, 2022, 13: 824240[2024-10-05]. DOI: 10.3389/fpls.2022.824240. [77] ZHANG Libin, XIA Heng, WU Jiangsheng, et al. MiRNA identification, characterization and integrated network analysis for flavonoid biosynthesis in Brassica coraphanus [J]. Horticultural Plant Journal, 2022, 8(3): 319−327. [78] SONG Aiping, GAO Tianwei, WU Dan, et al. Transcriptome-wide identification and expression analysis of chrysanthemum SBP-like transcription factors [J]. Plant Physiology and Biochemistry, 2016, 102: 10−16. [79] SONG Aiping, WU Dan, FAN Qingqing, et al. Transcriptome-wide identification and expression profiling analysis of Chrysanthemum trihelix transcription factors[J/OL]. International Journal of Molecular Sciences, 2016, 17(2): 198[2024-10-05]. DOI: 10.3390/ijms17020198. [80] SONG Aiping, GAO Tianwei, LI Peiling, et al. Transcriptome-wide identification and expression profiling of the DOF transcription factor gene family in Chrysanthemum morifolium[J/OL]. Frontiers in Plant Science, 2016, 7: 199[2024-10-05]. DOI: 10.3389/fpls.2016.00199. [81] SONG Aiping, LI Peiling, XIN Jingjing, et al. Transcriptome-wide survey and expression profile analysis of putative Chrysanthemum HD-zip Ⅰ and Ⅱ genes[J/OL]. Genes, 2016, 7(5): 19[2024-10-05]. DOI: 10.3390/genes7050019. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240567






 下载:
下载: