-
中国小麦花叶病毒(Chinese wheat mosaic virus,CWMV)是引起小麦Triticum aestivum黄花叶病的重要病原体,严重威胁小麦的生产安全[1]。CWMV病毒直径约为20 nm,长度为80~360 nm[2],包含2条正义RNA(ssRNA)链,根据大小分别命名为RNA1和RNA2。CWMV-RNA1全长7 147 nt,编码甲基转移酶、RNA聚合酶蛋白(RNA-dependent RNA polymerase,RdRp)和运动蛋白(movement protein,MP)等3个完整蛋白质,分子量分别是153、212和37 kDa[3]。CWMV-RNA2全长3 563 nt,编码病毒外壳蛋白(coat protein,CP)、N-CP蛋白(cystein rich protein,CRP)、CP-RT蛋白和1个富含半胱氨酸蛋白等4个蛋白质,分子量分别是19、25、84及18~19 kDa。CWMV以根部专性寄生的禾谷多黏菌Polymyxa graminis为介体传播[4-5]。当带毒的禾谷多黏菌侵染植株后,病毒会在寄主体内不断复制使植株发病;未带毒的禾谷多黏菌通过侵染带毒植株后获得病毒,成为新的病源侵染其他植株[6],且常与小麦黄花叶病毒(wheat yellow mosaic virus,WYMV)复合侵染[7];由于禾谷多黏菌的休眠孢子具有极强的抗逆性,小麦黄花叶病毒病的防治难度大大增加[6],患病小麦出现花叶、黄化、分蘖增生等症状[2]。国内外大量实践证明,培育并推广抗病品种是防治小麦黄花叶病毒病最为经济有效的措施。目前,小麦抗病毒研究仅得到少量的抗病毒病相关基因,亟需挖掘新的基因资源。利用病毒基因对植物进行遗传改良是近年来新出现的病害防治方法,原理包括利用病毒外壳蛋白、病毒复制酶、病毒运动蛋白介导的抗性途径等来增强植物的抗病性,以病毒外壳蛋白介导的抗病性应用最为广泛[8]。自ABEL等[9]报道获得携带烟草Nicotiana tabacum花叶病毒(tobacco mosaic virus, TMV)的外壳蛋白转基因植株后,黄瓜Cucumis sativus花叶病毒(cucumber mosaic virus, CMV)[10]、马铃薯Solanum tuberosum Y属病毒(potato virus Y, PVY)[11]、玉米Zea mays矮花叶病毒(maize dwarf mosaic virus, MDMV)[12]等重组外壳蛋白的转基因植株相继出现。该抗病性的机制目前存在3种假说:一是认为转基因植物细胞中形成的外壳蛋白一定程度上抑制了病毒外壳蛋白的脱壳,二是认为转基因植株在RNA水平上通过依赖同源序列的酶降解mRNA从而获得抗性,三是认为当病毒的核酸进入细胞后,立即被细胞中的自由外壳蛋白重新包裹,从而抑制了病毒的侵染[13-16]。此外,有研究表明:病毒外壳蛋白介导的抗性途径不仅对该种病毒存在抗性,在某些情况下对该病毒的不同菌株以及近缘病毒也存在抗性[13]。本研究拟利用农杆菌介导的转基因方法将CWMV的外壳蛋白基因导入烟草,获得阳性转基因植株后,通过抗病性鉴定证实转基因烟草对CWMV的抗病性,以期提高寄主植物的抗病性,并为利用病毒基因培育小麦抗病材料奠定基础。
-
根据美国国立生物技术信息中心(National Center for Biotechnology Information, NCBI)数据库中CWMV的外壳蛋白基因序列(登录号:NP_059483.1)设计上游引物CP-F(5'-CGCGGATCCATGGCCGTGAAATCTGGTTAT-3',下划线部分为BamHⅠ酶切位点)和下游引物CP-R(5'-ACGCGTCGACACTCGAACCTTCCCACTTAAG-3',下划线部分为SalⅠ酶切位点),扩增得到外壳蛋白基因全长。聚合酶链式反应(PCR)参数为94 ℃ 5 min;94 ℃ 30 s,58 ℃ 30 s,72 ℃ 60 s,循环35次;72 ℃ 10 min。PCR扩增产物经质量分数1.0%的琼脂糖凝胶电泳分离。将胶回收产物连接至PCV-GFP载体上,构建含有外壳蛋白基因的表达载体PCV-CP-GFP。
-
利用RNA提取试剂盒(HiPure Plant RNA Mini Kit)提取烟草叶片总RNA,利用分光光度计检测RNA的浓度和纯度,D(260)/D(280)读数在1.8~2.1表明提取的RNA符合要求。
-
将RNA定量到1 μg,按照RevertAidTM First Strand cDNA Synthesis Kit说明书完成cDNA的合成。用双蒸水将得到的cDNA产物稀释10倍并作为模板,以CP-1F(5'-ATGGCCGTGAAATCTGGTTAT-3')和CP-2R(5'-CTCGAACCTTCCCACTTAAG-3')为引物进行PCR检测。PCR反应体系为:94 ℃预变性5 min,94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸1 min,35次循环。
-
根据NCBI数据库核苷酸序列信息,通过Prime 5.0软件,设计检测小麦的实时定量PCR引物,引物为qRTMP-F(5'-TGAAGCGGTTGGTGCAAATG-3')和qRTMP-R(5'-GCCCGAATCGAGCAGTGATA-3'),由杭州擎科梓熙生物技术有限公司合成。使用SYBR premix ExTaqⅡ试剂盒(TaKaRa)在荧光定量PCR-7900(ABI)上进行实时荧光定量PCR(RT-PCR)反应,反应程序为:95 ℃预变性10 min;95 ℃变性15 s,65 ℃退火30 s,72 ℃延伸30 s,35次循环。
-
称取0.2 g处理后的烟草叶片放入2.0 mL离心管中震荡研磨,加入200 μL蛋白裂解液,剧烈震荡混匀3 min,冰上静置5 min,4 ℃下5 000 r·min-1离心5 min。吸取上清液200 μL至新的1.5 mL离心管并加入48 μL的5×SDS缓冲液,沸水浴中煮沸10 min,冰上放置5 min。量取10 μL经ExpressPlusTM PAGE Gels跑胶检测(140 V,50 min)。
-
利用半干转膜仪,将跑胶后得到的蛋白质转至硝酸纤维膜(NC膜),并利用封闭液(50 g·L-1脱脂牛奶)封闭1 h。取V[绿色荧光蛋白(GFP)特异性抗体]:V(封闭缓冲液)=1:5 000混合,放入NC膜,室温孵育1 h后用1×PBS洗脱3次,15 min·次-1。将NC膜置于封闭缓冲液中(与HRP标记的兔抗按10 000:1比例混合)室温孵育1 h,1×PBS洗3次,15 min·次-1。加入Novex ECL HRP Chemiluminescent Substrate Reagent Kit显色液,并用Amersham imager 600成像系统成像。
-
利用DNA提取试剂盒(HiPure SF Plant DNA Mini Kit)提取烟草叶片DNA,利用分光光度计检测DNA浓度和纯度,D(260)/D(280)读数在1.8~2.0表明提取的DNA符合要求。
-
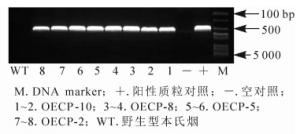
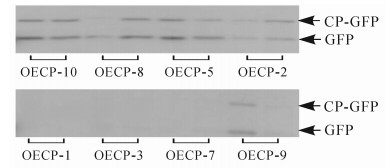
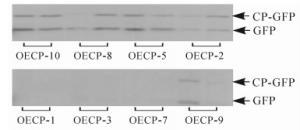
利用农杆菌介导基因转化法[11]将1.1中构建的表达载体(PCV-CP-GFP)转至本氏烟中。通过组织培养技术获得表达外壳蛋白的转基因烟草(OECP),共获得10个株系,20株·株系-1,移苗并栽培至4叶期,其中OECP-4和OECP-6株系的烟草没有存活。随机挑选存活10株·株系-1转基因苗,提取总蛋白质进行免疫分析。结果发现:OECP-1、OECP-3、OECP-7、OECP-9表达量较低,而OECP-10、OECP-8、OECP-5、OECP-2这4个株系表达量较高,条带大小为43 kDa,与CP-GFP蛋白大小一致(图 1)。为进一步证实得到的转基因植株为阳性植株,提取样株DNA进行PCR检测,结果发现:OECP-10、OECP-8、OECP-5、OECP-2均扩增出约530 bp的片段,与CWMV外壳蛋白基因大小一致(图 2)。表明外壳蛋白在转基因烟草中能够正常表达。
-
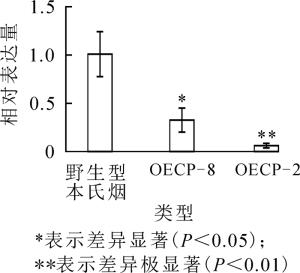
筛选到的OECP阳性植株放置于25 ℃的恒温培养室中培养2个月,观察发现:与野生型本氏烟草相比,OECP-2和OECP-8的阳性烟草植株出现了矮化表型(表 1)。OECP-8与野生型的平均株高比为1.00:0.65,OECP-2与野生型的平均株高比为1.00:0.43,表明外壳蛋白干扰了烟草植株的正常生长。
表 1 转基因烟草的株高
Table 1. Plant height of transgenic tobaccos
不同转基因烟草的株高/cm OECP-10 OECP-8 OECP-5 OECP-2 MOCK 34.9 ± 0.4 a 23.1 ± 1.5 b 34.2 ± 1.0 a 15.3 ± 6.0 c 35.6 ± 1.0 a 说明:不同小写字母表示差异显著(P<0.05) -
对OECP-2及OECP-8接种CWMV并栽植7 d,之后提取系统叶RNA,并检测其植株中运动蛋白(move protein,MP)基因的表达量。由图 3可知:转基因植株中CWMV的运动蛋白基因表达量显著低于对照植株,表明表达外壳蛋白基因提高了烟草对CWMV的抗病性。
-
近年来,外壳蛋白介导的抗病毒途径是应用最为成熟的提高寄主抗病性的方法之一,获得的抗病性也更为高效[13]。本研究共获得了OECP-10、OECP-8、OECP-5和OECP-2等4个高表达外壳蛋白基因的转基因烟草株系,对OECP-8和OECP-2株系接种CWMV,植株对CWMV的抗病性显著提高。说明CWMV外壳蛋白介导的抗病毒途径可被用作培育寄主的抗病材料。
外壳蛋白介导的抗病性分别从蛋白质水平和RNA水平发挥功能。当病毒入侵进入植物体后,细胞内的外壳蛋白会立刻包裹病毒核酸从而阻止病毒的翻译与复制或直接引起病毒的脱壳;此外,植物还会通过依赖同源序列的酶降解病毒mRNA[14-16]。将病毒外壳蛋白基因融入植株中从而使转基因植株获得抗性已应用于多种病毒与植株互作体系中[9-12]。本研究利用烟草与CWMV互作体系,成功将CWMV的外壳蛋白基因导入至烟草,显著提高了烟草对CWMV的抗病性,进一步验证了病毒外壳蛋白基因融入植株后会使转基因植株获得抗性。但本研究也发现转基因烟草出现矮化现象,说明融入外源基因影响烟草的正常生长,降低了转基因烟草的品质。与先前研究表明抗病性转基因植株会出现生长发育异常,器官变异进而影响品质[17]的结论一致。今后研究不仅要致力于培育具高抗的转基因植株,还要在提高技术水平时尽量保证其产品的优良品质,更好地满足人类生活所需。
Transgenetic expression coat protein of Chinese wheat mosaic virus(CWMV) enhances resistance of Nicotiana benthamiana to CWMV
-
摘要:
目的 中国小麦花叶病毒(Chinese wheat mosaic virus,CWMV)是引起小麦黄花叶病的重要病原之一。获得表达CWMV外壳蛋白(coat protein,CP)的转基因烟草Nicotiana benthamiana(OECP)并分析其抗病性,为培育小麦抗病材料奠定工作基础。 方法 利用体外重组DNA技术构建含有CWMV外壳蛋白基因的表达载体,并通过农杆菌介导的转化方法,获得OECP。进一步利用Western Blot和PCR检测明确CWMV的外壳蛋白在OECP中能够正常的表达。 结果 部分转基因阳性植株出现矮化表型。此外,OECP阳性植株接种CWMV后7 d的定量分析表明,OECP中CWMV运动蛋白的表达量显著受到抑制。 结论 在烟草中表达CWMV的CP蛋白基因可显著增强烟草对CWMV的抗病性。 Abstract:Objective Chinese wheat mosaic virus (CWMV) is an important virus causing wheat mosaic virus disease. The objective of this study is to obtain expression transgenic tobacco (OECP) of CWMV coat protein (CP) gene and analyze its disease resistance, which lays a foundation for the cultivation of wheat disease resistance materials. Method The expression vector containing the coat protein gene of CWMV was constructed by in vitro recombinant DNA technology and OECP was obtained by Agrobacterium-mediated transformation assay. Western Blot and PCR analysis showed that the CP gene of CWMV was correctly expressed in OECP. Result Some transgenic positive plants displayed dwarf phenotype. The quantitative analysis in OECP showed that expression level of CWMV motor protein gene was significantly reduced 7 days after inoculation with CWMV. Conclusion Expressing CP protein gene could significantly enhance resistance of Nicotiana benthamiana to CWMV. -
Key words:
- plant protection /
- Chinese wheat mosaic virus (CWMV) /
- coat protein /
- expression /
- Nicotiana benthamiana /
- resistance
-
表 1 转基因烟草的株高
Table 1. Plant height of transgenic tobaccos
不同转基因烟草的株高/cm OECP-10 OECP-8 OECP-5 OECP-2 MOCK 34.9 ± 0.4 a 23.1 ± 1.5 b 34.2 ± 1.0 a 15.3 ± 6.0 c 35.6 ± 1.0 a 说明:不同小写字母表示差异显著(P<0.05) -
[1] DIAO Aipo, CHEN Jianping, YE Rong, et al. Complete sequence and genome properties of Chinese wheat mosaic virus, a new furovirus from China[J]. J Gen Virol, 1999, 80(5):1141-1145. [2] 张巧艳, 陈剑平.中国小麦花叶病毒(CWMV)生物学特性初探[J].浙江农业学报, 2005, 17(3):155-157. ZHANG Qiaoyan, CHENG Jianping. Biological properties of Chinese wheat mosaic virus (CWMV)[J]. Acta Agric Zhejiang, 2005, 17(3):155-157. [3] ANDIKA I B, ZHENG Shiling, TAN Zilong. Endoplasmic reticulum export and vesicle formation of the movement protein of Chinese wheat mosaic virus are regulated by two transmembrane domains and depend on the secretory pathway[J]. Virology, 2013, 435(2):493-503. [4] 徐磊, 陈剑平.中国小麦花叶病毒RNA2外壳蛋白通读区及19 ku富半胱氨酸基因的克隆、表达及编码蛋白定位[C]//中国植物病理学会.中国植物病理学会第7届代表大会暨学术研讨会论文集.北京: [s.n.], 2002. [5] ADAMS M J. Transmission of plant viruses by fungi[J]. Ann Appl Biol, 2010, 118(2):479-492. [6] MCKINNEY H H. Amosaic of wheat transmissible to all rereal species in the tribe hordear[J]. J Agric Res, 1930, 40(6):547-556. [7] 阮义理, 陈剑平, 洪健.小麦梭条斑病毒(WSSMV)和小麦黄花叶病毒(WYMV)的细胞质内含体的电镜观察[J].植物病理学报, 1991, 21(3):165-171. RUAN Yili, CHEN Jianping, HONG Jian. Electron microscopical comparision of cytoplasmic inclusions associated with wheat spindle streak mosaic or wheat yellow mosaic virus[J]. Acta Phytopathol Sin, 1991, 21(3):165-171. [8] 王平安, 吴刘记, 杨艳坤, 等.转基因植物抗病毒策略及其风险分析[J].河南农业科学, 2011, 40(2):19-24. WANG Ping'an, WU Liuji, YANG Yankun, et al. The antivirus strategies and potential risks of virus-resistant transgenic plants[J]. J Henan Agric Sci, 2011, 40(2):19-24. [9] ABEL P P, NELSON R S, DE B, et al. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene[J]. Science, 1986, 232(4751):738-743. [10] 杨荣昌, 徐鹤林, 龙明生, 等.表达黄瓜花叶病毒外壳蛋白的转基因番茄及其对CMV的抗性[J].江苏农业学报, 1995, 11(1):40-44. YANG Rongchang, XU Helin, LONG Mingsheng, et al. Transgenic tomato plants expressing cucumber mosaic virus coat protein and their resistance to CMV[J]. Jiangsu J Agric Sci, 1995, 11(1):40-44. [11] 郭兴启, 李菡, 张杰道, 等.表达马铃薯Y病毒外壳蛋白基因的转基因烟草抗病机制[J].应用与环境生物学报, 2003, 9(4):372-376. GUO Xingqi, LI Han, ZHANG Jiedao, et al. Evidence for RNA-mediated resistance to PVYN in tobacco plants transformed with the viral coat protein gene[J]. Chin J Appl Environ Biol, 2003, 9(4):372-376. [12] MURRY L E, ELLIOTT L G, CAPITANT S A, et al. Transgenic corn plants expressing MDMV strain B coat protein are resistant to mixed infectious of maize dwarf mosaic virus and maize chlorotic mottle virus[J]. BioTechnology, 1993, 11(13):1559-1564. [13] 叶英林.植物转基因抗性策略研究进展[J].湖南农业科学, 2016(10):126-130. YE Yinglin. Research advances on transgenic resistance strategies in plants[J]. Hunan Agric Sci, 2016(10):126-130. [14] LOESCH-FRIES L S, MERLO D, ZINNEN T, et al. Expression of alfalfa mosaic virus RNA 4 in transgenic plants confers virus resistance[J]. EMBO J, 1987, 6(7):1845-1851. [15] OKUNO T, NAKAYAMA M, YOSHIDA S, et al. Comparative susceptibility of transgenic tobacco plants and protoplasts expressing the coat protein gene of cucumber mosaic virus to infection with virions and RNA[J]. Phytopathology, 1993, 83(5):542-547. [16] van DUN C M P, BOL J F, van VLOTEN-DOTING L. Expression of alfalfa mosaic virus and tobacco rattle virus coat protein genes in transgenic tobacco plants[J]. Virology, 1987, 159(2):299-305. [17] 吕靖, 蒿若超, 张文英, 等.烟草转基因研究进展[J].黑龙江农业科学, 2012(7):148-152. LÜ Jing, HAO Ruochao, ZHANG Wenying, et al. Progress in transgenic tobacco[J]. Heilongjiang Agric Sci, 2012(7):148-152. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2020.02.013






 下载:
下载: