-
氧化亚氮(N2O)作为增温潜势极大的温室气体,对全球变化有着重要的影响,探究其源、汇关系意义十分重大。然而土壤N2O的源、汇具有很强的时空异质性,导致难以制定精准的N2O减排策略[1]。总的来看,土壤中N2O产生和排放过程复杂多样。目前,大多数研究认为羟胺氧化、硝化细菌反硝化和反硝化过程是N2O产生的三大主要途径[2-3],而关于N2O还原过程的认识也还存在较大争论,其中反硝化被认为是N2O还原的主导途径[3]。为了量化上述N2O过程,有针对性地提出N2O减排策略,同位素分析技术在N2O溯源方面提供了重要支撑。同位素自然丰度法中的同位素异位体法,是一种非侵入性方法,因其不受底物同位素影响[4-5],N2O同位素特征值δ15Nsp可被用作指示多种微生物产生N2O的作用过程[6-7]。N2O的其他同位素特征值,δ15Nbulk和δ18O也被用于指示N2O产生的微生物作用途径,但会受到N2O前体物[铵离子(NH4 +),硝酸根离子(NO3 −)和水(H2O)等]的同位素组成的影响[6, 8-9]。已有很多学者探索利用同位素特征值来准确分析N2O产生的微生物过程,综合研究δ15Nbulk、δ18O、δ15Nsp对揭示N2O产生机制更有重要的意义。而在N2O产生与排放过程中,不同功能微生物过程同位素分馏效应的差异构成了稳定同位素自然丰度技术分析微生物过程的基础[10],因此,同位素分馏是稳定同位素自然丰度技术应用的理论基础,在研究中的作用不可忽视。本研究梳理了土壤N2O产生和排放过程中氮同位素分馏的效应;阐述了环境因子及微生物对土壤N2O产生和排放过程的同位素分馏效应影响;总结了稳定同位素自然丰度技术在土壤N2O源解析中的应用及进展。
-
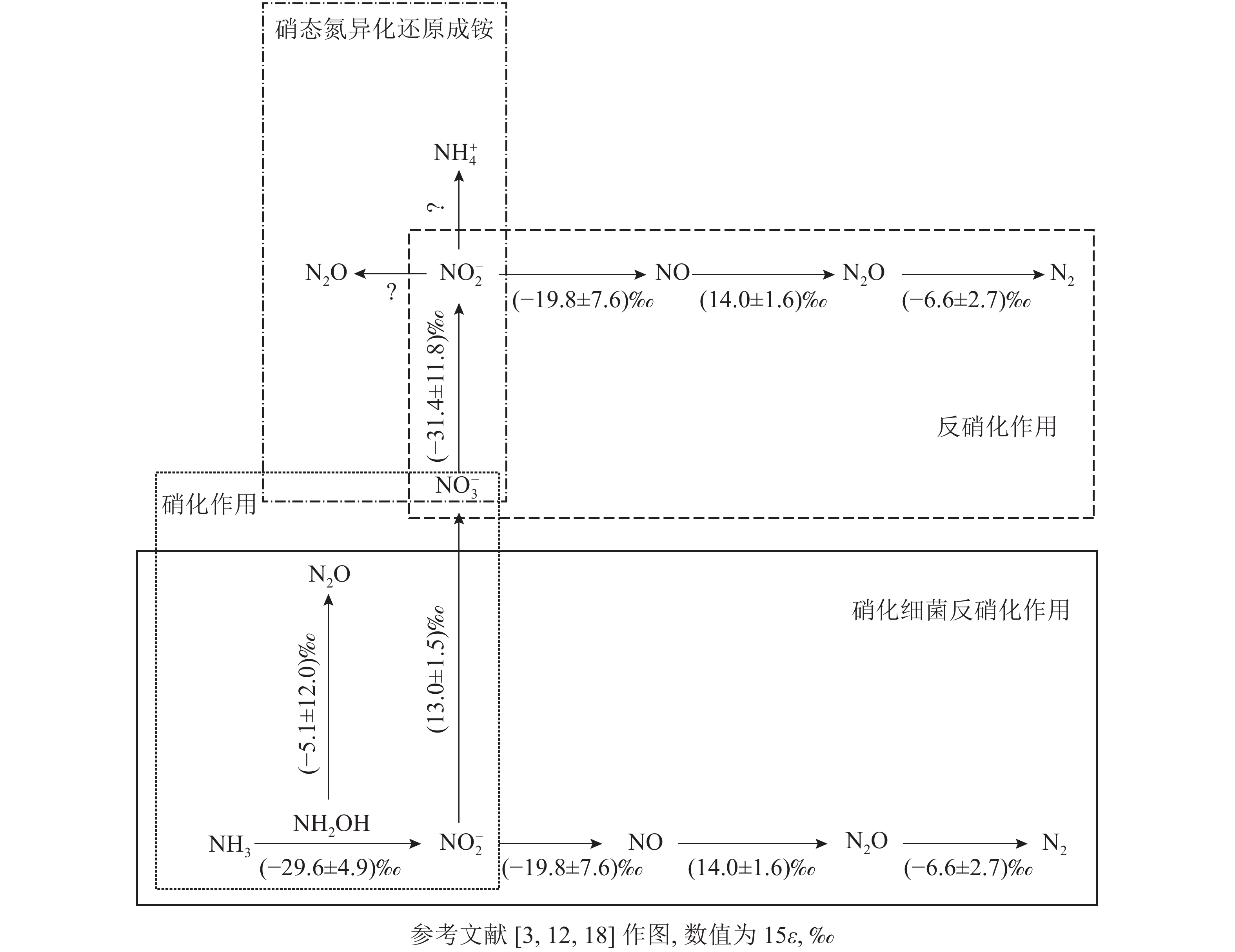
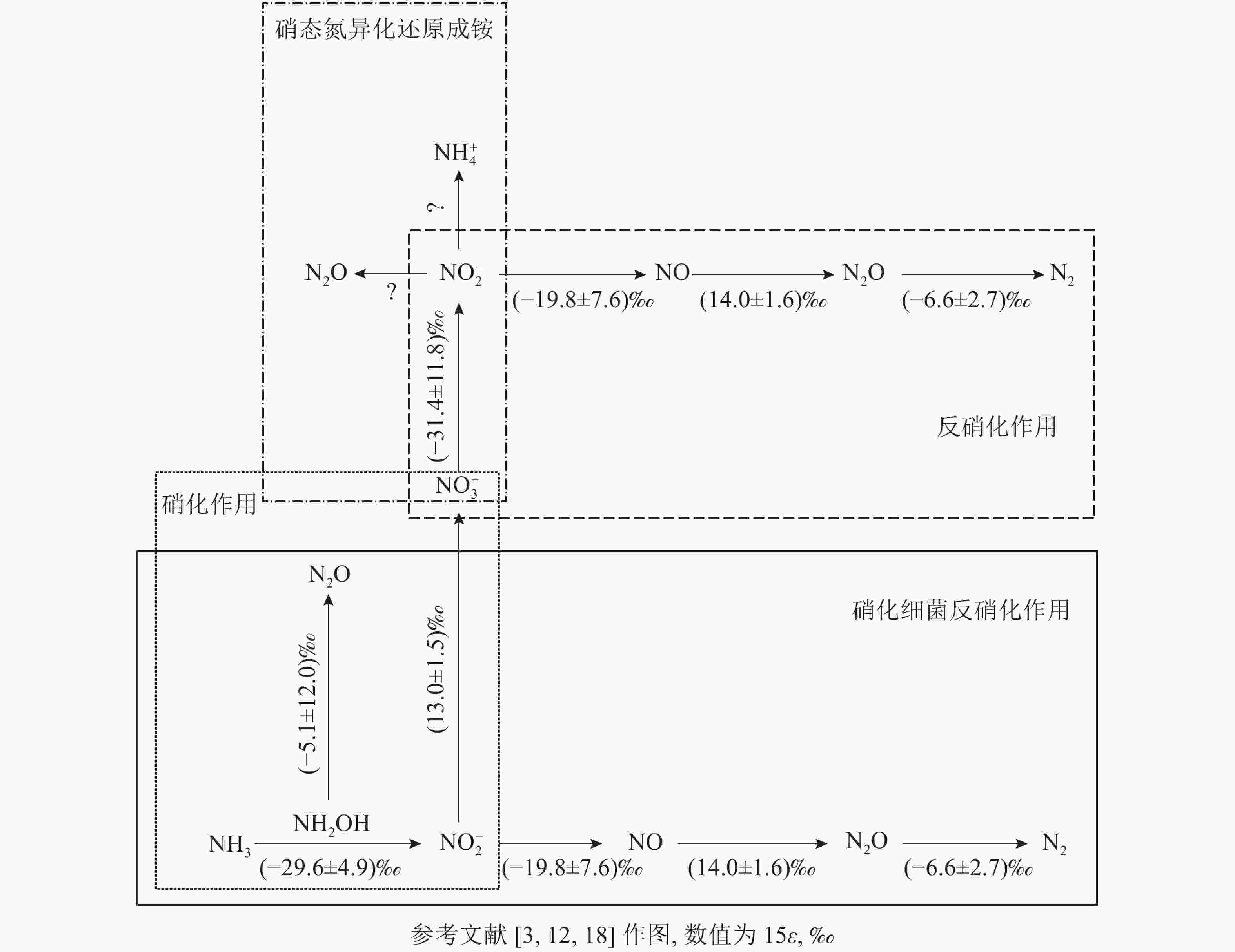
微生物参与的N2O转化过程可分为4个经典过程,分别为硝化作用 (自养/异养硝化作用和硝化细菌反硝化作用)、反硝化作用 (真菌反硝化作用和硝态氮异化还原成铵)、硝化反硝化耦合作用和共反硝化作用。借助分子技术已基本确定控制硝化作用微生物过程的酶(编码基因),但仍需结合稳定同位素技术来分析具体的代谢过程[11]。氮同位素分馏作用是指随着生物化学反应的进行,反应底物和产物δ15N丰度发生差异的现象,这是15N稳定同位素自然丰度技术应用的理论基础[12]。本研究针对微生物驱动的硝化作用和反硝化作用的氮同位素分馏效应进行了梳理。
-
土壤硝化作用(nitrification)可分为自养硝化和异养硝化[13]。当前,自养硝化过程中的稳定同位素分馏得到了广泛的研究。自养硝化作用是指NH4 +或氨气(NH3)经亚硝酸根离子(NO2 −)氧化为NO3 −的过程,包括羟胺氧化和硝化细菌反硝化2个部分。通过纯培养实验进行同位素检测,获得NH3或NH4 +向羟胺(NH2OH)转化以及NH2OH向NO2 −转化2个过程的同位素效应平均值,约(−29.6±4.9)‰。这2个过程主要涉及亚硝化单胞菌,其中欧洲亚硝化单胞菌Nitrosomonas europaea的氮同位素效应为 −38.2‰~−24.0‰;而其他亚硝化单胞菌,包括亚硝化单胞菌N. eutropha、N. marina C-113a、Nitrosospira tenuis,其氮同位素效应范围更大,为−38.2‰~−14.2‰[14-15]。仅CASCIOTTI[16]对NO2 −向 NO3 −的转化过程做了同位素效应研究,同位素效应均值为(13.0±1.5)‰。在自养硝化过程中,N2O主要通过羟胺或NO2 −的化学分解产生[17],同位素效应值也会随反应底物的不同而发生变化。NH2OH到N2O过程在纯培养实验中的同位素效应值为−26.3‰~5.7‰[18],但后来有研究发现:将NO2 −和NH2OH以1∶1混合作为反应底物,其同位素效应值变弱,为–17.8‰~0.8‰,比NH4 +到N2O过程的表现得更弱,表明这些氧化过程会使同位素效应值减弱。仅以NH2OH作为底物来估算反应的同位素效应值,得出的结果为(−5.1±12.0)‰。
硝化细菌反硝化(nitrifier denitrification)是一种由氨氧化细菌(AOB)驱动的,但又有别于自养的氮转化过程[10],该过程中,NH4 +或NH3氧化产生的NO2 −被同一类氨氧化细菌进一步还原为N2O和氮气(N2)[10]。该步骤涉及的酶和编码基因与硝化作用和反硝化作用中参与相应步骤的酶和基因相同,因此该过程的同位素效应值可参考硝化作用和反硝化作用的相应过程。目前,关于硝化细菌反硝化过程能否还原N2O形成N2还存在争议。有研究认为:AOB中并未发现编码N2O还原酶同源物质的基因[19],这表明硝化细菌反硝化过程可能不是N2O的还原过程;然而,BEYER等[20]认为:尽管传统AOB中没有发现N2O还原酶,但它存在类似N2O还原酶的电子铜蛋白亚硝基(nitrosocyanin),这可能与N2O还原成N2有关,但其具体作用机制也还有待研究证实。
-
土壤反硝化作用(denitrification)一般是指反硝化细菌在厌氧或微氧条件下将NO3 −、NO2 −或者N2O作为呼吸过程的末端电子受体,并将其还原为NO2 −、NO、N2O或者是N2。N2O是反硝化过程的中间产物[4],甚至某些条件下是反硝化的最终产物[21-22]。反硝化过程的N2O还原成N2是N2O消耗的关键过程,在调节自然生态系统N2O排放中扮演着重要角色[23]。在土壤培养实验中,测得NO3 − 向NO2 −转化过程中的同位素效应值为−52.8‰~−10.0‰,平均值约(−31.4±11.8)‰[24]。NO2 −向NO转化的同位素效应较弱,并且该过程以细菌和真菌参与的反硝化作用为主,同位素效应平均值约(−19.8±7.6)‰。NO向N2O转化的同位素效应值为(14.0±1.6)‰[25]。OSTROM等[26]纯培养实验的研究结果表明:N2O还原为N2过程的同位素效应平均值为(−4.1±6.8)‰,但该过程同时存在正反2种同位素效应。在不考虑反向同位素效应时,其同位素值为−9.1‰~−2.5‰,平均值为(−6.6±2.7)‰[18, 27]。
除细菌外,某些真菌(如尖孢镰刀菌Fusarium oxysporum)也可以通过反硝化过程产生N2O,称为真菌反硝化[28-29]。但真菌一般缺乏编码N2O还原酶的基因,因而N2O是真菌反硝化的最终产物[28]。真菌反硝化作用对N2O排放总量的贡献尚未完全阐明[30],此过程的同位素分馏效应也有待进一步研究。
硝态氮异化还原成铵态氮的过程(DNRA)是指在厌氧环境、高pH以及大量的易氧化态有机物存在下,微生物以NO3 −或NO2 −作为电子受体,还原成可利用性NH4 +,将活性氨滞留在生态系统中的过程。NO3 −还原成NO2 −后,NO2 −主要还原成NH4 +并且伴随NO2 −的短暂积累和N2O的产生[31]。其中N2O是该过程中NO2 −还原的副产物[32-33]。实际上,自然环境中DNRA过程对N2O排放的贡献研究仍然较少。有一些研究认为:DNRA过程在N2O产生和排放中所占的比例较小,甚至可被忽略[34]。NO3 −还原为 NO2 −过程的同位素效应值也为(–31.4±11.8)‰[24]。但目前亚硝酸盐还原为铵盐这一关键反应的同位素效应值尚不清楚。此外,一些DNRA过程细菌,如Wolinella succinogenes和Anaeromyxo bacterdehalogenans也具有编码N2O还原酶的基因,这可能对N2O还原具有潜在贡献,但其确切过程还需进一步证实[33,35]。
-
土壤产生N2O是复杂的微生物过程,研究多个影响因子协同作用对N2O排放量变化的影响,有助于了解N2O排放的内在机制[5, 36]。在N2O转化过程中,不同微生物同位素分馏的差异构成了同位素手段分析微生物过程的基础[10]。在纯培养实验中(如真菌纯培养实验),同位素效应的可变性可能是因不同的微生物菌株或多种微生物导致,也可以由特定微生物的同位素效应来解释,例如,脱氮副球菌的值在–28.6‰~–10.0‰之间变化很大[18]。不同菌株之间的部分变异性可能来自不同的酶结构[15],但单个微生物体测定值出现的巨大变化表明特定的实验条件(如扩散底物的可用性、中间产物的积累等)对特定的同位素效应起着重要作用[18]。
同位素分馏效应主要受到反应平衡条件以及反应速率的影响(图1)。而环境因素的改变(如土壤温度、水分等)会影响反应速率,并进一步对微生物的生理过程、群落组成和反应活性产生影响,进而影响N2O的产生和排放过程及同位素分馏效应。关于实验的环境条件(如温度、湿度、底物浓度等)与同位素效应之间的关系,有少部分学者开展了相应的研究。MARIOTTI等[37-38]研究了同位素效应对温度的依赖性,他们在土壤培养实验中观测到30 ℃下同位素分馏效应(−24.6‰)明显弱于10 ℃下的值(−31.2‰)。较高反应温度下反应速率较快,导致生成物转化效率高并使得同位素分馏效应较小[39];而在低温下的反应进程缓慢,通过扩散传递的底物相对较高,因此表现出更强的酶同位素效应。MATHIEU等[40]还通过在底物中添加NO3 −来研究同位素组成的影响。随着底物消耗量的增加,培养实验中也观察到了同位素效应的降低[41-42]。当同位素效应转移到扩散的较弱同位素效应时,会导致同位素效应减少[37]。然而,在不同湿度和温度条件下使用山地和湿地进行的土壤培养实验结果相互矛盾[39],反应速率与同位素效应差异之间没有显著关系。其中,各处理间的反硝化速率相差1个数量级以上,但测定的同位素效应相差不到9‰。以上研究说明:同位素分馏效应(尤其是野外环境)的评估依然具有较大不确定性,除反应速率之外可能还存在其他制约因子,比如功能微生物群落构成、微生物活性等。
-
稳定同位素自然丰度技术是指利用N2O的15N和18O的自然丰度变异来研究N2O的产生、排放过程[43]。N2O自然丰度作为N2O源和汇的一种更综合的示踪剂,已广泛用于土壤N2O源解析[44-45] (图2)。然而,由于δ15Nbulk和δ18O往往依赖于底物的同位素组成,且各个过程中同位素分馏效应存在不确定性,很难通过δ15Nbulk和δ18O的变异准确解释N2O排放来源[5, 45]。
同位素异位体法是在稳定同位素自然丰度技术上发展起来的,但不同于稳定同位素自然丰度技术,同位素异位体法(δ15NSP)[46]并不受到底物同位素丰度影响,在N2O扩散过程中相对稳定[47-48],其仅受N2O形成的机制和途径[49]以及N2O还原过程的影响。此项优点为高精度N2O溯源提供基础,且不干扰系统、适用于田间试验,此外还提供了估算DNRA过程产生N2O的潜力[5]。N2O分子为不对称的直线型结构,其分子式为N=N=O,中间和边缘不同的氮原子被分别称为α原子和β原子[50]。N2O的各产生途径因受不同的微生物或酶调控,造成N−O键的断裂位置不同,故生成的N2O的SP值也有所差异,这被认为是运用同位素异位体法溯源N2O产生途径的理论基础[45]。同位素异位体法N2O分子中SP值可以判别两部分N2O产生过程,一部分为硝化细菌硝化作用和真菌反硝化作用产生的N2O过程(N2ON),另一部分为硝化细菌反硝化作用和反硝化作用产生的N2O过程(N2OD)。同位素异位体法已经开始被用来区分土壤中N2O各产生过程的微生物来源[7-9],表现出良好的应用前景。
然而,仅使用δ15NSP进行源解析也会面临一些问题:①N2O还原过程中SP值的增加,即N2O还原过程中的同位素分馏效应。②真菌反硝化作用和硝化作用的SP值重叠。③不同培养物和反应过程中SP的变异性。此外,正如MOHN等[51]所证明的,由于不同的分析技术和缺乏可用的N2O同位素标准物质,可能导致不同实验室间SP值测定结果有较大差异。虽然国际上已发布2个N2O同位素标准样品USGS51和USGS52[52],但有关野外同位素值的测定仍有待确定。为了解决上述问题,很多学者在不断探索。问题①可以在模型中解决,因为有研究表明:在N2O还原为N2的情况下,SP的同位素效应稳定范围为−8‰~−2‰[47, 53-54]。但问题②尚未解决。有研究[25, 55-56]表明:SP在反应过程中是稳定的,但鉴于P450nor真菌一氧化氮还原酶还原氧化氮(NO)过程中SP从15‰增加到29‰,SP值的稳定性可能需要进一步证实,这也是问题③有待解答的问题,不同培养物中不同的酶和N2O之间存在的特定偶联机制。
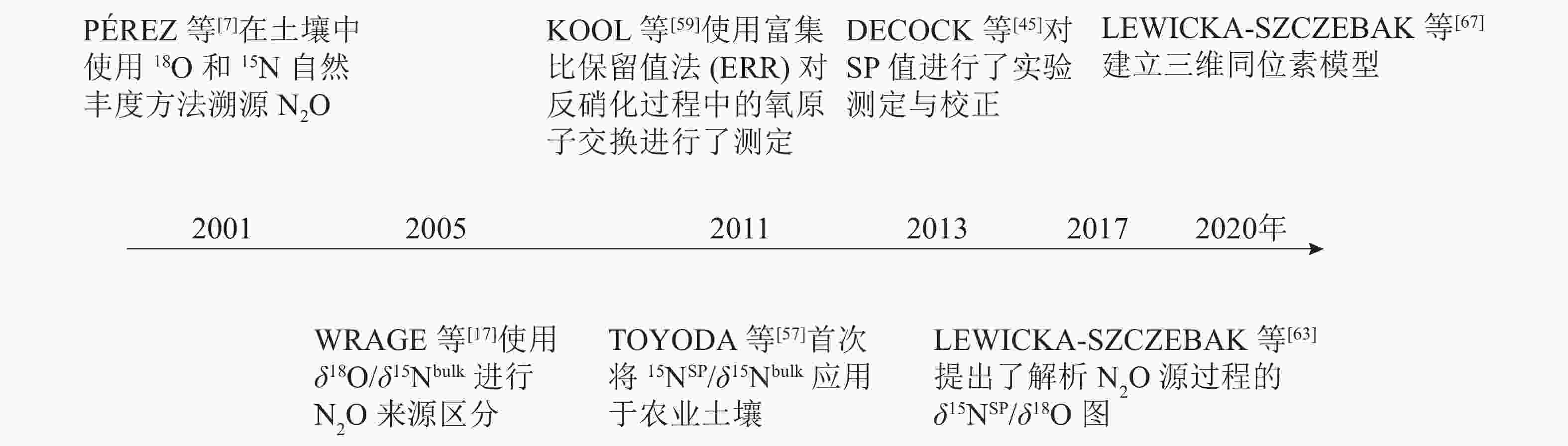
为了更准确地测定N2O还原程度和微生物贡献,实际应用中通常采用双同位素图谱法[28, 57-58],即使用δ18O/δ15Nbulk、δ15NSP/δ15Nbulk或δ15NSP/δ18O,即用2个同位素特征值来评估N2O不同产生途径的贡献率和N2O的还原程度。WRAGE等[17]使用了δ18O/δ15Nbulk进行硝化、硝化细菌反硝化及反硝化过程产生N2O的来源区分。但在实验环境中水的18O与土壤中O2以及NO3 −存在发生氧交换的风险,导致结果分析不准确。H2O中氧原子会与NO2 −和NO3 −中氧原子发生氧交换,且它们对N2O中氧原子的贡献与微生物种类有关。因此,需要考虑自然条件下氧交换对δ18O值的影响。KOOL等[59]使用富集比保留值法(ERR)对反硝化过程中的氧原子交换进行了测定,尽管有待改进,但这种方法能够区分硝化、硝化细菌反硝化、硝化反硝化耦合作用等N2O生产过程,并应用于一系列土壤。TOYODA等[57]首次将δ15NSP/δ15Nbulk应用于农业土壤。之后,有研究[59-62]利用这种关系来确定N2O混合比例和N2O还原程度,发现δ18O也可以很好地示踪N2O的产生过程,这得益于细菌反硝化期间高的氧原子(O)交换量,从而使该途径的δ18O值非常稳定。基于这一发现,LEWICKA-SZCZEBAK等[63]提出了解析N2O源过程的δ15NSP/δ18O图,可用于校正反硝化作用中N2O还原的影响和定量微生物作用贡献,最近已经广为应用[64-66]。δ15NSP/δ15Nbulk和δ15NSP/δ18O已联合应用于现场研究[65],并在计算N2O还原程度和真菌、细菌反硝化值之间显示出较好的一致性。但利用双同位素图谱法在精确量化N2O产生的路径比例和N2O还原程度方面仍存在许多限制,比如单一产生路径的同位素特征值范围广、不同产生路径的同位素特征值范围的重叠、反应底物同位素组成的变化,以及与N2O还原相关的分馏因子的可变性等。因此基于双同位素图谱法所提供的定量结果的准确性仍有待探究。
最近,LEWICKA-SZCZEBAK等[67]在双同位素图谱法的基础上,把3个同位素特征值(δ15Nbulk、δ18O、δ15NSP)结合起来计算N2O不同产生途径的贡献率和N2O的还原程度。提出了一种基于三维同位素的复杂模型,并对其结果进行了验证。这是首次在实验室和田间条件下对N2O同位素自然丰度法溯源N2O进行系统地验证研究。然而将N2O同位素特征值完全整合到模型中,需要建立一个包含同位素效应及其不确定性的综合数据库[18]。
-
未来关于稳定同位素技术在土壤N2O溯源中的研究需注意以下几个方面:①同位素自然丰度法在N2O溯源应用中还有很大的提升空间。ZHANG等[68]指出:需要进一步探究同位素特征值稳定性来提高对N2O微生物过程的识别。把同位素特征值(δ15Nbulk、δ15NSP、δ18O)与底物同位素(如δ15
${\rm{N}}_{{\rm{NO}}_3^-} $ 、δ15${\rm{N}}_{{\rm{NH}}_4^+} $ 、δ18${\rm{O}}_{{\rm{NO}}_3^-} $ 、δ18${\rm{O}}_{{\rm{H}}_2{\rm{O}}} $ )相结合,分析反应底物在N2O生成过程中的分馏效应可以提高源解析的准确性[69]。把N2O的δ15Nbulk、δ18O、δ15NSP同位素特征值结合起来有益于识别未知源[70],但在不同的pH、氧化还原状况、基质以及非生物N2O产生途径的条件下测得不同的δ15NSP,说明同位素效应及其变异性是制约精确度的关键,其中微生物过程及其导致的同位素分馏效应是N2O溯源的重要切入点。此外,目前对于18O富集系数ε18O的研究还相对较少,且一些研究认为:不同过程产生N2O的δ18O无明显区别[39],这表明对δ18O的特征值研究有助于准确解析N2O的来源。并且未来还需对N2O产生过程中每一步的δ15NSP都进行测定,探究环境因素与产生N2O具体的生化过程速率之间的关系(包括共反硝化),在排除共反硝化的影响下,对真菌反硝化的δ15NSP进行改善[56, 71]。②除了N2O转化过程中同位素分馏效应会导致误差外,N2O同位素的分析和测定也需要标定和校准[72-73]。OSTROM等[52]提出2种N2O气体标准物质USGS51和USGS52用于实验室标定和校准。未来仍需要多个实验室间进行更多平行比较并制定统一的校准方案,如对团簇同位素的精确测定有助于质谱值重叠样品的校准。③新方法的探索。稳定同位素技术与分子技术结合[74]。很多研究都提出应该把δ15N与qPCR、基因拷贝数的测定等分子技术相结合来对N2O生物过程进行区分,并且可以结合微生物生态学分析和量化N2O∶N2的产生和不同微生物N2O来源之间的分配[75-79]。另外,团簇同位素特征值的方法也开始流行。SP值不能用于区分硝化和真菌反硝化产生N2O的过程,但是可以使用团簇同位素特征值的方法,通过具体产生途径中特定的酶来判断[80],但如何准确判断特定的酶仍需要进一步的探索。
Stable isotope natural abundance techniques in the studies on nitrous oxide production and emission processes: a review
-
摘要: 氧化亚氮(N2O)是主要的温室气体之一,并且对平流层臭氧层分解起到重要作用。土壤中N2O的产生和排放过程复杂多样,对其进行精准溯源与过程区分有助于制定减排策略。稳定同位素自然丰度技术利用N2O的同位素值δ15Nbulk(N2O中15N在整体水平上的同位素特征值)、δ18O(N2O中18O在整体水平上的同位素特征值)以及δ15Nsp(N2O分子内15N的位点特异性同位素值),可以示踪N2O来源、指示N2O产生的微生物作用途径,在N2O转化过程溯源中已取得重要进展。而同位素分馏效应是稳定同位素自然丰度技术应用的理论基础,其中微生物过程及其导致的同位素分馏是需要重点关注的问题。本研究概述了同位素分馏效应在N2O的产生、排放过程中的研究进展及其主要影响因素,梳理了同位素特征值δ15Nbulk、δ18O和δ15Nsp在分析N2O来源的研究进展,并且提出了影响准确区分过程的因素。因素包括单一产生路径的同位素特征值范围广、不同产生路径的同位素特征值范围的重叠、反应底物同位素组成的变化以及与N2O还原相关的分馏因子的可变性等问题。明确了今后需加强δ15Nsp等N2O同位素特征值分馏效应的测定,利用组合同位素特征值及先进手段进行全面的N2O溯源研究。图2参80Abstract: Nitrous oxide (N2O) is one of the potent greenhouse gases and also plays an important role in ozone layer decomposition. N2O production and emission processes in soil are complexed. Therefore, accurate source partitioning will help to constrain emission budgets. The application of stable isotope natural abundance technique have stimulated significant progress in N2O source partitioning and promoted identification in various N2O microbial production processes, which make use of various N2O isotope signatures δ15Nbulk(the average of15N), δ18O(the average of 18O) and δ15Nsp(site preference of 15N in different positions of N2O molecule). However, some factors also add uncertainties to N2O source partitioning, such as the range of isotope signatures, changes of isotope composition, and various fractionation factors associated with N2O reduction. It is also noteworthy that microbial processes and related isotopic effects are critical. In this review, the isotopic effects during N2O production and reduction and related factors are summarized; advances in approaches for N2O source-partitioning are concluded, including isotope natural abundance and isotopomer methods. The review focused on the progress of isotopic signatures δ15Nbulk, δ18O and δ15Nsp value in constraing N2O sources. In the future, the measurement of isotope fractionation, a combination of isotope signatures and advanced methodologies are advised for better studying N2O sources and pathways. [Ch, 2 fig. 80 ref.]
-
-
[1] BUTTERBACH-BAHL K, BAGGS E M, DANNENMANN M, et al. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? [J]. Philosl Transac Royal Soc B Biol Sci, 2013, 368(1621): 1 − 13. [2] DUAN H, YE L, ERLER D, et al. Quantifying nitrous oxide production pathways in wastewater treatment systems using isotope technology: a critical review [J]. Water Res, 2017, 122: 96 − 113. [3] BAGGS E M. A review of stable isotope techniques for N2O source partitioning in soils: recent progress, remaining challenges and future considerations [J]. Rap Commun Mass Spectrom, 2010, 22(11): 1664 − 1672. [4] 林伟, 李昱佳, 王宇, 等. 基于双同位素图谱评估肥料类型对滴灌白菜地N2O来源的影响[J]. 农业工程学报, 2020, 36(23): 109 − 116. LIN Wei, LI Yujia, WANG Yu, et al. Evaluation of N2O sources from Chinese cabbage fields affected by fertilizer types under drip irrigation based on dual isotopocule plot method [J]. Transac Chin Soc Agric Eng, 2020, 36(23): 109 − 116. [5] LEWICKA-SZCZEBAK D, GIESEMANN A, WELL R, et al. Quantifying N2O reduction to N2 based on N2O isotopocules-validation with independent methods (helium incubation and 15N gas flux method) [J]. Biogeosciences, 2017, 14(3): 711 − 732. [6] 林伟, 房福力, 张薇, 等. 稳定同位素技术在土壤N2O溯源研究中的应用[J]. 应用生态学报, 2017, 28(7): 2344 − 2352. LIN Wei, FANG Fuli, ZHANG Wei, et al. A review on development of stable isotope technique in the studies of N2O formation mechanism [J]. Chin J Appl Ecol, 2017, 28(7): 2344 − 2352. [7] PEREZ T, TRUMBORE S E, TYLER S C, et al. Identifying the agricultural imprint on the global N2O budget using stable isotopes[J]. J Geophys Res, 2001, 106: 9869. doi: 10.1029/2000JD900809. [8] YAMULKI S, TOYODA S, YOSHIDA N, et al. Diurnal fluxes and the isotopomer ratios of N2O in a temperate grassland following urine amendment [J]. Rapid Commun Mass Spectrum, 2001, 15: 1263 − 1269. [9] BOL R, TOYODA S, YAMULKI S, et al. Dual isotope and isotopomer ratios of N2O emitted from a temperate grassland soil after fertiliser application [J]. Rapid Commun Mass Spectrum, 2010, 17(22): 2550 − 2556. [10] HATTORI S, PALMA Y N, ITOH Y, et al. Isotopic evidence for seasonality of microbial internal nitrogen cycles in a temperate forested catchment with heavy snowfall [J]. Sci Total Environ, 2019, 690(10): 290 − 299. [11] LIN Wei, LI Yuzhong, LI Yujia, et al. Advances in the mechanism of microbe-driven nitrogen cycling [J]. J Plant Nutr Fert, 2020, 26(6): 1146 − 1155. [12] 方运霆, 刘冬伟, 朱飞飞, 等. 氮稳定同位素技术在陆地生态系统氮循环研究中的应用[J]. 植物生态学报, 2020, 26(6): 1146 − 1155. FANG Yunting, LIU Dongwei, ZHU Feifei, et al. Applications of nitrogen stable isotope techniques in the study of nitrogen cycling in terrestrial ecosystems [J]. Chin J Plant Ecol, 2020, 26(6): 1146 − 1155. [13] BOER W D, KOWALCHUK G A. Nitrification in acid soils: microorganisms and mechanisms [J]. Soil Biol Biochem, 2001, 33(7/8): 853 − 866. [14] ARP D J, STEIN L Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria [J]. Crit Rev Biochem Mol Biol, 2003, 38(6): 47 − 495. [15] KOBAYASHI K, MAKABE A, YANO M, et al. Dual nitrogen and oxygen isotope fractionation during anaerobic ammonium oxidation by anammox bacteria [J]. ISME J, 2019, 13(10): 2426 − 2436. [16] CASCIOTTI K L. Inverse kinetic isotope fractionation during bacterial nitrite oxidation [J]. Geochim Cosmochim Acta, 2009, 73(7): 2061 − 2076. [17] WRAGE N, GROENIGEN J W V, OENEMA O, et al. A novel dual-isotope labelling method for distinguishing between soil sources of N2O [J]. Rap Commun Mass Spectrom, 2005, 19(22): 3298 − 3306. [18] DENK T, MOHN J, DECOCK C, et al. The nitrogen cycle: A review of isotope effects and isotope modeling approaches [J]. Soil Biol Biochem, 2017, 105: 121 − 137. [19] SHAW L J, NICOL G W, SMITH Z, et al. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway [J]. Environ Microbiol, 2006, 8(2): 214 − 222. [20] BEYER S, GILCH S, MEYER O, et al. Transcription of genes coding for metabolic key functions in Nitrosomonas europaea during aerobic and anaerobic growth [J]. J Mol Microbiol Biotechnol, 2009, 16(3/4): 187 − 197. [21] ZHANG Jinbo, CAI Zuocong, CHENG Yi, et al. Denitrification and total nitrogen gas production from forest soils of eastern China [J]. Soil Biol Biochem, 2009, 41(12): 2551 − 2557. [22] BRAKER G, CONRAD R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? [J]. Adv Appl Microbiol, 2011, 75: 33 − 70. [23] CHAPUIS-LARDY L, WRAGE N, METAY A, et al. Soils, a sink for N2O? a review [J]. Glob Change Biol, 2010, 13(1): 1 − 17. [24] PEREZ T, GARCIA-MONTIEL D, TRUMBORE S, et al. Nitrous oxide nitrification and denitrification N-15 enrichment factors from Amazon forest soils [J]. Ecol Appl, 2006, 16(6): 2153 − 2167. [25] YANG Hui, GANDHI H, OSTROM N E, et al. Isotopic fractionation by a fungal P450 nitric oxide reductase during the production of N2O [J]. Environ Sci Technol, 2014, 48(18): 10707 − 10715. [26] OSTROM N E, PITT A, SUTKA R, et al. Isotopologue effects during N2O reduction in soils and in pure cultures of denitrifiers[J]. J Geophys Res Biogeosci, 2007, 112(G2). doi: 10.1029/2006JG000287. [27] LEWICKA-SZCZEBAK D, WELL R, BOL R, et al. Isotope fractionation factors controlling isotopocule signatures of soil-emitted N2O produced by denitrification processes of various rates [J]. Rapid Commun Mass Spectrom, 2015, 29(3): 269 − 282. [28] SUTKA R L, ADAMS G C, OSTROM N E, et al. Isotopologue fractionation during N2O production by fungal denitrification [J]. Rapid Commun Mass Spectrom, 2008, 22(24): 3989 − 3996. [29] THAMDRUP B, DALSGAARD T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments [J]. Appl Environ Microbiol, 2002, 68(3): 131 − 1318. [30] MAEDA K, SPOR A, EDEL-HERMANN V, et al. N2O production, a widespread trait in fungi[J]. Sci Rep, 2015, 5: 7. doi: 10.1038/srep09697. [31] SMITH M S. Dissimilatory reduction of NO2 to NH4 + and N2O by a soil Citrobacter sp. [J]. Appl Environ Microbiol, 1982, 43(4): 854 − 860. [32] CORKER H, POOLE R K. Nitric oxide formation by Escherichia coli dependence on nitrite reductase, the NO-sensing regulator Fnr, and flavohemoglobin Hmp [J]. J Biol Chem, 2003, 278(34): 31584 − 31592. [33] SANFORD R A, WAGNER D D, WU Q, et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils [J]. Proc Nat Acad Sci USA, 2012, 109(48): 19709 − 19714. [34] MURRAY N J, PHINN S R, DEWITT M, et al. The global distribution and trajectory of tidal flats [J]. Nature, 2019, 565(7738): 222 − 225. [35] SIMON J, EINSLE O, KRONECK P M H, et al. The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase [J]. FEBS Lett, 2004, 569(1/3): 7 − 12. [36] LARSEN K S, ANDRESEN L C, BEIER C, et al. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: synthesizing results of the CLIMAITE project after two years of treatments [J]. Glob Change Biol, 2011, 17(5): 1884 − 1899. [37] MARIOTTI A, GERMON J C, LECLERC A. Nitrogen isotope fractionation associated with the NO2-N2O step of denitrification in soils [J]. Can J Soil Sci, 1982, 62(2): 227 − 241. [38] MARIOTTI A, GERMON J C, HUBERT P, et al. Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes [J]. Plant Soil, 1981, 62(3): 413 − 430. [39] SNIDER D M, SCHIFF S L, SPOELSTRA J. 15N/14N and 18O/16O stable isotope ratios of nitrous oxide produced during denitrification in temperate forest soils [J]. Geochim Cosmochim Acta, 2009, 73(4): 877 − 888. [40] MATHIEU O, LÉVÊQUE J, HÉNAULT C, et al. Influence of 15N enrichment on the net isotopic fractionation factor during the reduction of nitrate to nitrous oxide in soil [J]. Rapid Commun Mass Spectrom, 2010, 21(8): 1447 − 1451. [41] BRITISH INFECTION A. The epidemiology, prevention, investigation and treatment of Lyme borreliosis in United Kingdom patients: a position statement by the British Infection Association [J]. J Infect, 2011, 62(5): 329 − 338. [42] TOYODA S, MUTOBE H, YAMAGISHI H, et al. Fractionation of N2O isotopomers during production by denitrifier [J]. Soil Biol Biochem, 2005, 37(8): 1535 − 1545. [43] TOYODA S, IWAI H, KOBA K, et al. Isotopomeric analysis of N2O dissolved in a river in the Tokyo metropolitan area [J]. Rapid Commun Mass Spectrom, 2010, 23(6): 809 − 821. [44] KOBA K, OSAKA K, TOBARI Y, et al. Biogeochemistry of nitrous oxide in groundwater in a forested ecosystem elucidated by nitrous oxide isotopomer measurements [J]. Geochim Cosmochim Acta, 2009, 73(11): 3115 − 3133. [45] DECOCK C, SIX J. How reliable is the intramolecular distribution of N-15 in N2O to source partition N2O emitted from soil? [J]. Soil Biol Biochem, 2013, 65: 114 − 127. [46] YU Longfei, HARRIS E, LEWICKA-SZCZEBAK D, et al. What can we learn from N2O isotope data? analytics, processes and modelling[J]. Rapid Commun Mass Spectrom, 2020, 34(20): e8858. doi: 10.1002/rcm.8858. [47] LEWICKA-SZCZEBAK, WELL, KOSTER, et al. Experimental determinations of isotopic fractionation factors associated with N2O production and reduction during denitrification in soils [J]. Geochim Cosmochim Acta, 2014, 134: 55 − 73. [48] WELL R, FLESSA H. Isotope fractionation factors of N2O diffusion [J]. Rapid Commun Mass Spectrom, 2010, 22(17): 2621 − 2628. [49] SUTKA R L, OSTROM N E, OSTROM P H, et al. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances [J]. Appl Environ Microbiol, 2006, 72(1): 638 − 644. [50] TOYODA S, YOSHIDA N. Determination of nitrogen isotopomers of nitrous oxide on a modified isotope ratio mass spectrometer-Analytical Chemistry (ACS Publications) [J]. Anal Chem, 1999, 71(20): 4711 − 4718. [51] MOHN J, WOLF B, TOYODA S, et al. Interlaboratory assessment of nitrous oxide isotopomer analysis by isotope ratio mass spectrometry and laser spectroscopy: current status and perspectives [J]. Rapid Commun Mass Spectrom, 2014, 28(18): 1995 − 2007. [52] OSTROM N E, OSTROM P H. Mining the isotopic complexity of nitrous oxide: a review of challenges and opportunities [J]. Biogeochemistry, 2017, 132(3): 359 − 372. [53] JINUNTUYA-NORTMAN M, SUTKA R L, OSTROM P H, et al. Isotopologue fractionation during microbial reduction of N2O within soil mesocosms as a function of water-filled pore space [J]. Soil Biol Biochem, 2008, 40(9): 2273 − 2280. [54] WELL R, FLESSA H. Isotopologue enrichment factors of N(2)O reduction in soils [J]. Rapid Commun Mass Spectrom, 2010, 23(18): 2996 − 3002. [55] HEIL J, WOLF B, BRUGGEMANN N, et al. Site-specific N-15 isotopic signatures of abiotically produced N2O [J]. Geochim Cosmochim Acta, 2014, 139: 72 − 82. [56] ALDOSSARI N, ISHII S. Fungal denitrification revisited-recent advancements and future opportunities[J]. Soil Biol Biochem, 2021, 157: 108250. doi: 10.1016/j.soilbio.2021.108250. [57] TOYODA S, YANO M, NISHIMURA S-I, et al. Characterization and production and consumption processes of N2O emitted from temperate agricultural soils determined via isotopomer ratio analysis[J]. Glob Biogeochem Cycles, 2011, 25(2): GB2008. doi: 10.1029/2009GB003769. [58] YANO M, TOYODA S, TOKIDA T, et al. Isotopomer analysis of production, consumption and soil-to-atmosphere emission processes of N2O at the beginning of paddy field irrigation [J]. Soil Biol Biochem, 2014, 70: 66 − 78. [59] KOOL D, van GROENIGEN J W, WRAGE-MÖNNIG N. Source determination of nitrous oxide based on nitrogen and oxygen isotope tracing [J]. Methods Enzymol, 2011, 496: 139 − 160. [60] KATO T, TOYODA S, YOSHIDA N, et al. Isotopomer and isotopologue signatures of N2O produced in alpine ecosystems on the Qinghai-Tibetan Plateau [J]. Rapid Commun Mass Spectrom, 2013, 27(13): 1517 − 1526. [61] WOLF B, MERBOLD L, DECOCK C, et al. First on-line isotopic characterization of N2O emitted from intensively managed grassland [J]. Biogeosci Discuss, 2015, 12(2): 1573 − 1611. [62] ZOU Yun, HIRONO Y, YANAI Y, et al. Isotopomer analysis of nitrous oxide accumulated in soil cultivated with tea (Camellia sinensis) in Shizuoka, central Japan [J]. Soil Biol Biochem, 2014, 77: 276 − 291. [63] LEWICKA-SZCZEBAK D, AUGUSTIN J, GIESEMANN A, et al. Quantifying N2O reduction to N2 based on N2O isotopocules: validation with independent methods (helium incubation and 15N gas flux method) [J]. Biogeosciences, 2017, 14: 71 − 732. [64] BUCHEN C, LEWICKA-SZCZEBAK D, FLESSA H, et al. Estimating N2O processes during grassland renewal and grassland conversion to maize cropping using N2O isotopocules [J]. Rapid Commun Mass Spectrom, 2018, 32(13): 1053 − 1067. [65] IBRAIM E, WOLF B, HARRIS E, et al. Attribution of N2O sources in a grassland soil with laser spectroscopy based isotopocule analysis [J]. Biogeosciences, 2019, 16(16): 3247 − 3266. [66] WU Di, WELL R, CÁRDENAS L M, et al. Quantifying N2O reduction to N2 during denitrification in soils via isotopic mapping approach: model evaluation and uncertainty analysis[J]. Environ Res, 2019, 179: 108806. doi: 10.1016/j.envres.2019.108806. [67] LEWICKA-SZCZEBAK D, LEWICKI M P, WELL R. N2O isotope approaches for source partitioning of N2O production and estimation of N2O reduction: validation with the N-15 gas-flux method in laboratory and field studies [J]. Biogeosciences, 2020, 17(22): 5513 − 5537. [68] ZHANG Wei, LI Yuzhong, XU Chuunying, et al. Isotope signatures of N2O emitted from vegetable soil: ammonia oxidation drives N2O production in NH4 +-fertilized soil of north China[J]. Sci Rep, 2016, 6: 29257. doi: 10.1038/srep29257. [69] ROHE L, WELL R, LEWICKA-SZCZEBAK D. Use of oxygen isotopes to differentiate between nitrous oxide produced by fungi or bacteria during denitrification [J]. Rapid Commun Mass Spectrom, 2017, 31(16): 1297 − 1312. [70] ROHE L, ANDERSON T H, FLESSA H, et al. Comparing modified substrate induced respiration with selective inhibition (SIRIN) and N2O isotope approaches to estimate fungal contribution to denitrification in three arable soils under anoxic conditions[J]. Biogeosci Discuss, 2020. doi: 10.5194/bg-2020-285. [71] ALDOSSARI N, ISHII S. Fungal denitrification revisited-recent advancements and future opportunities[J]. Soil Biol Biochem, 2021, 157: 108250. doi: 10.1016/j.soilbio.2021.108250. [72] TOYODA S, YOSHIDA N, KOBA K. Isotopocule analysis of biologically produced nitrous oxide in various environments [J]. Mass Spectrom Rev, 2017, 36: 135 − 160. [73] BOHLKE J K, MROCZKOWSKI S J, COPLEN T B. Oxygen isotopes in nitrate: new reference materials for: 180:170:160 mesurements and observations on nitrate-water equilibration [J]. Rapid Commun Mass Spectrom, 2003, 17(16): 1835 − 1846. [74] CHALK P M, INACIO C T, CHEN D L. An overview of contemporary advances in the usage of N-15 natural abundance (delta N-15) as a tracer of agro-ecosystem N cycle processes that impact the environment[J]. Agric Ecosyst Environ, 2019, 283: 106570. doi: 10.1016/j.agee.2019.106570. [75] OSTROM N, OSTROM P. The Isotopomers of Nitrous Oxide: Analytical Considerations and Application to Resolution of Microbial Production Pathways[M]. Heidelberg: Springer, 2012. [76] RICHARDSON D, FELGATE H, WATMOUGH N, et al. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle: could enzymic regulation hold the key? [J]. Trends Biotechnol, 2009, 27(7): 388 − 397. [77] SUN Lifei, SANG Changpeng, WANG Chao, et al. N2O production in the organic and mineral horizons of soil had different responses to increasing temperature [J]. J Soils Sediment, 2019, 19(10): 3499 − 3511. [78] DESLOOVER J, VLAEMINCK S E, CLAUWAERT P, et al. Strategies to mitigate N2O emissions from biological nitrogen removal systems [J]. Curr Opin Biotechnol, 2012, 23(3): 474 − 482. [79] 朱莹, 田浩, 王芸, 等. 环境因子对土壤微生物产生和还原N2O过程的影响[J]. 基因组学与应用生物学, 2017, 36(12): 5191 − 5198. ZHU Ying, TIAN Hao, WANG Yun, et al. The effects of environmental factors on N2O production and reduction process of soil microorganisms [J]. Genom Appl Biol, 2017, 36(12): 5191 − 5198. [80] YEUNG L Y. Combinatorial effects on clumped isotopes and their significance in biogeochemistry [J]. Geochim Cosmochim Acta, 2016, 172: 22 − 38. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20210458






 下载:
下载: