-
换锦花Lycoris sprengeri为石蒜属Lycoris球根花卉,春初出叶,叶片为带状,宽约1.0 cm,长约30.0 cm,叶顶钝圆;秋初开花,花茎高约60.0 cm,伞形花序4~6朵,6片倒披针形花瓣,长约4.5 cm,宽约1.0 cm;花多淡紫红色,花被顶端蓝色,花色花型丰富,是石蒜属特殊的复色花卉[1]。
目前,换锦花的花色性状改良主要以传统的种间杂交和选择育种为主,分子标记育种为辅的育种模式。徐炳声等[2]利用杂交授粉技术,以换锦花为母本,中国石蒜L. chinensis为父本,选育出粉白色的秀丽石蒜L.× elegans;张定成等[3]在安徽和淮南发现三倍体和二倍体野生换锦花资源,表明换锦花可能是由其他石蒜属植物杂交而来。然而换锦花生长周期长,在自然状态下授粉率低且结实少,种子萌发率低,制约了换锦花传统育种研究。为了克服传统杂交育种的缺陷,不少研究者利用现代分子生物学技术探索换锦花花色分子育种性状改良的有效手段[4−6]。花色苷生物合成途径是类黄酮生物合成的一个分支途径,主要由结构基因和调控基因共同调控,花色苷积累受光照、温度和水分等多种因素的影响,植物受到强光、低温、氮亏缺等逆境协迫时会大量合成花色苷以增强自身抗性[7],花色苷的生物合成也受到植物体自身生长发育过程的影响[8]。大量研究表明:R2R3-MYB 是花色苷生物合成的重要调控因子,常与bHLH和WD40形成MBW复合体结合到结构基因启动子序列上共同调控葡萄Vitis vinifera、风信子Hyacinthus orientalis、苹果Malus pumila花色素苷的生物合成[9]。许振渊等[10]和侯朔等[11]克隆了换锦花R2R3-MYB转录因子LsMYB4和LsMYB5基因,周洋丽等[12]通过病毒介导的基因沉默(VIGS)技术研究发现LsMYB4和LsMYB5是换锦花花青素合成的抑制性转录因子。周洋丽[13]和薛惠敏等[14]成功克隆了换锦花LsANS、LsF3'H、LsUFGT1和LsUFGT2基因启动子序列,为研究换锦花花色形成的调控机制和遗传改良提供基础。为进一步探讨换锦花花色形成的调控网络,本研究根据换锦花花瓣(红色部分和蓝色部分)转录组信息筛选到与换锦花花色形成相关的差异R2R3-MYB转录因子LsMYB7并进行生物信息学分析,通过病毒介导的基因沉默(VIGS)技术研究该基因调控花色苷积累的相关功能,结果可为通过基因工程手段改良换锦花的花色奠定理论基础。
-
2019年8—9月于浙江农林大学石蒜属植物种质资源圃采集换锦花花瓣,取换锦花小花苞(2.0~3.5 cm)、大花苞(4.5~6.0 cm)、盛花期和败花期4个不同花发育时期(图1A)和不同花色无性系H1、H2、H3、H4和H5盛花期花瓣(图1B),液氮速冻后于−80 ℃冰箱保存备用。
-
采用RNAiso plus法提取换锦花花瓣总RNA[15],参照PrimeScript 1st Strand cDNA Synthesis Kit试剂盒(Takara)方法合成cDNA 第1链。
-
根据转录组测序信息设计LsMYB7基因 cDNA序列特异引物LsMYB7-F:CAAGCAGTGGTCTCAACA和LsMYB7-R:AGAACAGCACTACTAAAGGT,参照Premix PrimeSTAR HS的PCR扩增体系扩增目的基因cDNA序列,PCR扩增反应程序为94 ℃变性30 s;58 ℃ 退火30 s;72 ℃延伸90 s,32个循环,72 ℃延伸10 min,质量浓度为1.0%琼脂糖凝胶电泳检测PCR扩增产物,于凝胶成像系统(Bio-RAD)观察拍照;采用Hingene琼脂糖凝胶回收试剂盒(杭州麦克德勒科技有限公司)回收目的DNA,于−20 ℃保存备用。
-
参照pEASY-Blunt Zero Cloning Kit说明书将目的基因LsMYB7序列连接到pEASY-Blunt载体,转化大肠埃希菌Escherichia coli DH5α感受态细胞;在含有氨苄青霉素(Amp)、异丙基-β-D-硫代半乳糖苷(IPTG)和5-溴-4-氯-3-吲哚 β-D-半乳糖苷(X-Gal)的LB培养基(Luria-Bertani medium)上培养过夜,挑取阳性克隆于LB液体(含50 mg·L−1 Amp)培养基中,37 ℃振荡培养,PCR检测呈阳性的菌液送浙江有康生物科技有限公司测序;采用质粒DNA提取试剂盒(杭州创试生物科技有限公司)提取目的序列重组质粒DNA,于−20 ℃保存。
-
采用Primer 5.0设计LsMYB7基因和花色形成相关基因(LsCHS、LsF3H、LsANS、LsUFGT1和LsUFGT2)的RT-qPCR引物(表1),使用PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒(Takara)方法合成cDNA 第1链。参照SYBR® Premix Ex TaqTMⅡ(Takara)方法,于CFX96TM荧光定量 PCR 仪(BIO-RAD)进行RT-qPCR验证。RT-qPCR体系(20.0 uL):cDNA(<100 ng) 1.6 uL,正反引物各0.8 uL,TB Green Premix Ex Taq Ⅱ 10.0 uL,RNase Free ddH2O 6.8 uL。RT-qPCR程序为95 ℃ 30 s,95 ℃ 3 s,60 ℃ 30 s,循环40次。以LsGADPH为内参基因[15],2−ΔΔCt方法计算实时荧光各基因的相对表达量,重复3次。
表 1 RT-qPCR所用引物
Table 1. Primers for fluorescence RT-qPCR
引物 正向(5′→3′) 反向(5′→3′) LsGADPH AGGGTTTGATGACCACCGTGCA ACAGCCTTGGCAGCTCCAGTAC LsMYB7 GCGCGGAGTTCTTGGCTCTGAT TCTGGCACCGTTCTCATCACGC LsCHS CAAGACATGGTGGTGGTCGAGGTC CGAGGAGTTTGGTGAGCTGGTAGTC LsF3H AACCGAGGACGCAACGGAATGC ACCATCTTCATCGCAGCCACCA LsANS CGTGCCAGGTCTCCAGGTCTTCTA TCGAGAGTGTCACCGACGTGAACTA LsUFGT1 GGTGGTGAAGGATGAGGAAGGTAGG GTTGAACCGCTCGAACCGCAATC LsUFGT2
LsF3'HGCGTAGCCTTCTCCTTCCTCACCT
TTGTACAGCCATGCACAGAATCCGCCATGAATCGCTTCACCTCCTC
GCAACCAAGGCAAGAAATCA说明:引物参考文献[16]。 -
参照刘跃平等[17]方法提取并用高效液相色谱(HPLC)测定换锦花花瓣花色苷质量浓度。精密称量换锦花花瓣干粉0.040 0 g,加入2 mL体积分数为1%甲醇/HCl提取溶液,振荡混匀;20 ℃超声提取30 min;12 000 r·min−1,离心15 min,取上清液;0.45 μm滤膜过滤;梯度洗脱:流动相为甲醇(A)-体积分数为1%的甲酸水(B),0~20 min(A体积分数从5%升至60%),20~25 min(A体积分数从60%升至100%),25~30 min(A体积分数保持100%),流速1 mL·min−1,检测波长280 nm,柱温30 ℃,进样量10 uL;以矢车菊素-3-O-葡萄糖苷、天竺葵素-3-O-葡萄糖苷、飞燕草素-3-O-葡萄糖苷等3个花色苷为标准品。每样3个生物学重复。
-
采用XbaⅠ和BamHⅠ分步酶切法酶切亚细胞定位载体pAN580,再采用ClonExpress Ⅱ One Step Cloning Kit (杭州霆喜生物科技有限公司)将LsMYB7基因序列连接到pAN580上,并转化大肠埃希菌DH5α感受态细胞,在含有50 g·L−1Amp的LB培养基上进行筛选,挑选阳性克隆进行菌液PCR检测,成功构建pAN580-LsMYB7亚细胞定位载体,采用无内毒素质粒提取试剂盒(AxyPrep)提取pAN580-LsMYB7重组载体质粒DNA;聚乙二醇法(PEG 4000)转化烟草Nicotiana tabacum原生质体,用激光共聚焦荧光显微镜观察拍照。
-
采用双酶切法构建VIGS沉默载体pTRV2-LsMYB7:利用Primer 5.0设计长为400 bp的LsMYB7基因cDNA序列插入片段引物pTRV-LsMYB7-F: TACCGAATTCTCTAGAGAGGACAACATGCTACAATCCC和pTRV-LsMYB7-R:GCTCGGTACCGGATCCGCTCGAATCGCTAACATCCG;用限制性内切酶XbaI和BamHI分别双酶切VIGS载体(pTRV2)与LsMYB7基因的插入序列,T4 DNA连接酶连接载体与目的序列,转化大肠埃希菌DH5α,并在含有50 g·L−1 卡那霉素(Kan)的LB固体培养基筛选,提取pTRV2-LsMYB7重组质粒DNA。
-
采用微量注射法转化换锦花花苞[12],将重组质粒pTRV2-LsMYB7、pTRV2和pTRV1质粒DNA转化根癌农杆菌Agrobacterium tumefaciens GV3101,于YEP液体培养基(50 mg·L−1 Rif和50 mg·L−1 Kan)中,28 ℃,220 r·min−1振荡培养12~14 h;4 000 r·min−1, 离心10 min,去上清液;加入侵染液(10 mmol·L−1 MES + 200 μmol·L−1 AS + 10 mol·L−1 MgCl2,pH 5.6±0.03)重悬后的菌液D(600)=0.8~1.0;将pTRV1与pTRV2空载体、pTRV2-LsMYB7农杆菌液按照体积比1∶1混合均匀,室温黑暗静置4~6 h;使用1 mL注射器,吸取1 mL混合农杆菌菌液。选取3~4 d的换锦花花苞,在花苞基部缓慢注射混合根癌农杆菌菌液,充分侵染换锦花花苞,侵染5 d后采集花瓣用于LsMYB7和花色形成相关结构基因的表达分析(见1.2.4和表1)。
-
采用Excel 2020 统计和整理数据,采用SPSS 20.0 对数据进行差异显著性分析,采用Graphpad 8.0作图。
-
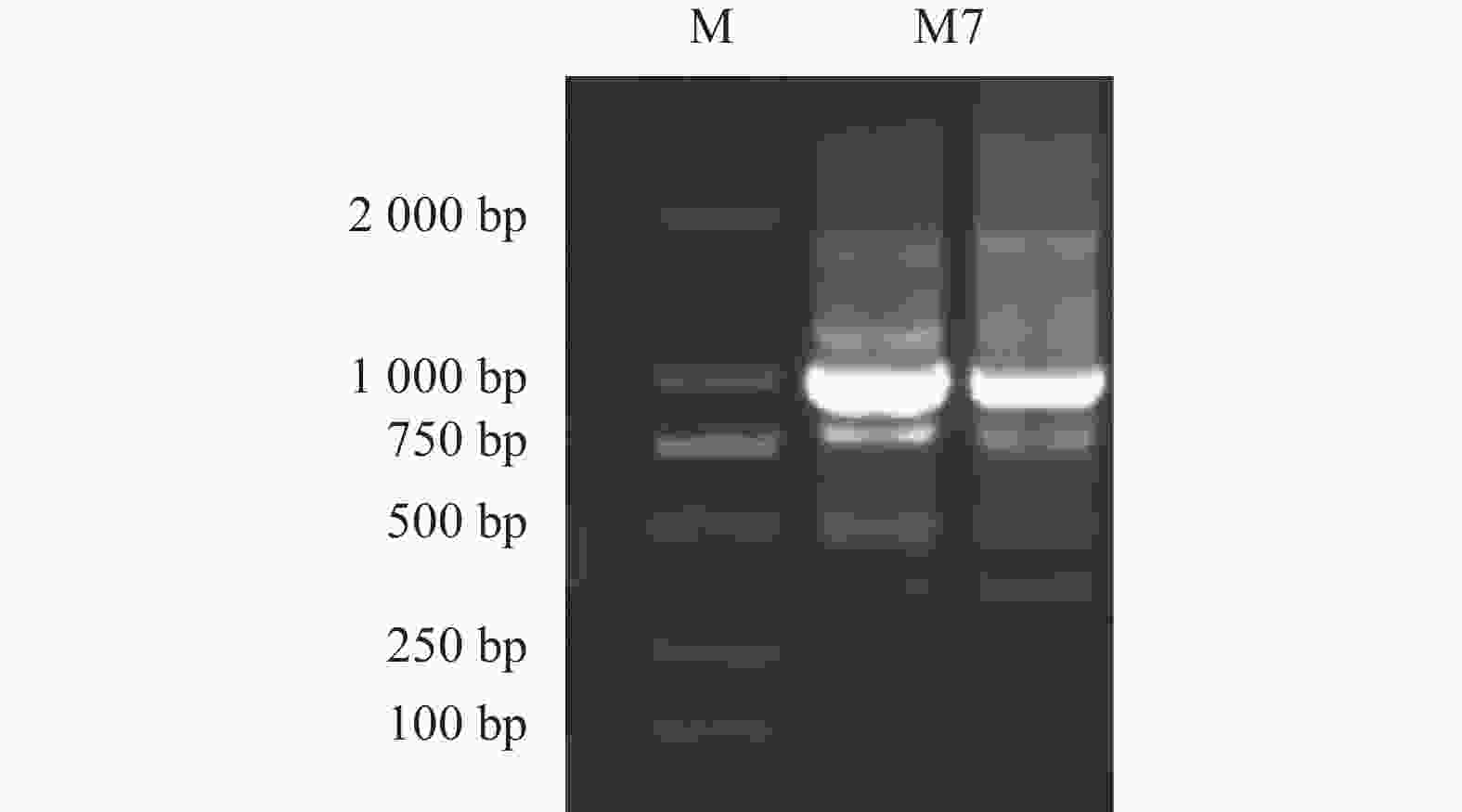
以换锦花花瓣的cDNA为模板,采用RT-qPCR技术扩增获得长度为951 bp 的LsMYB7 cDNA序列(图2)。该cDNA序列含有1个825 bp的开放阅读框(ORF),编码274个氨基酸,分子量为6.84 kD,蛋白分子式为C2402H3981N825O982S258,理论等电点(pI)为5.04,不稳定系数为43.43,总体亲水性(GRAVY)为0.936。
-
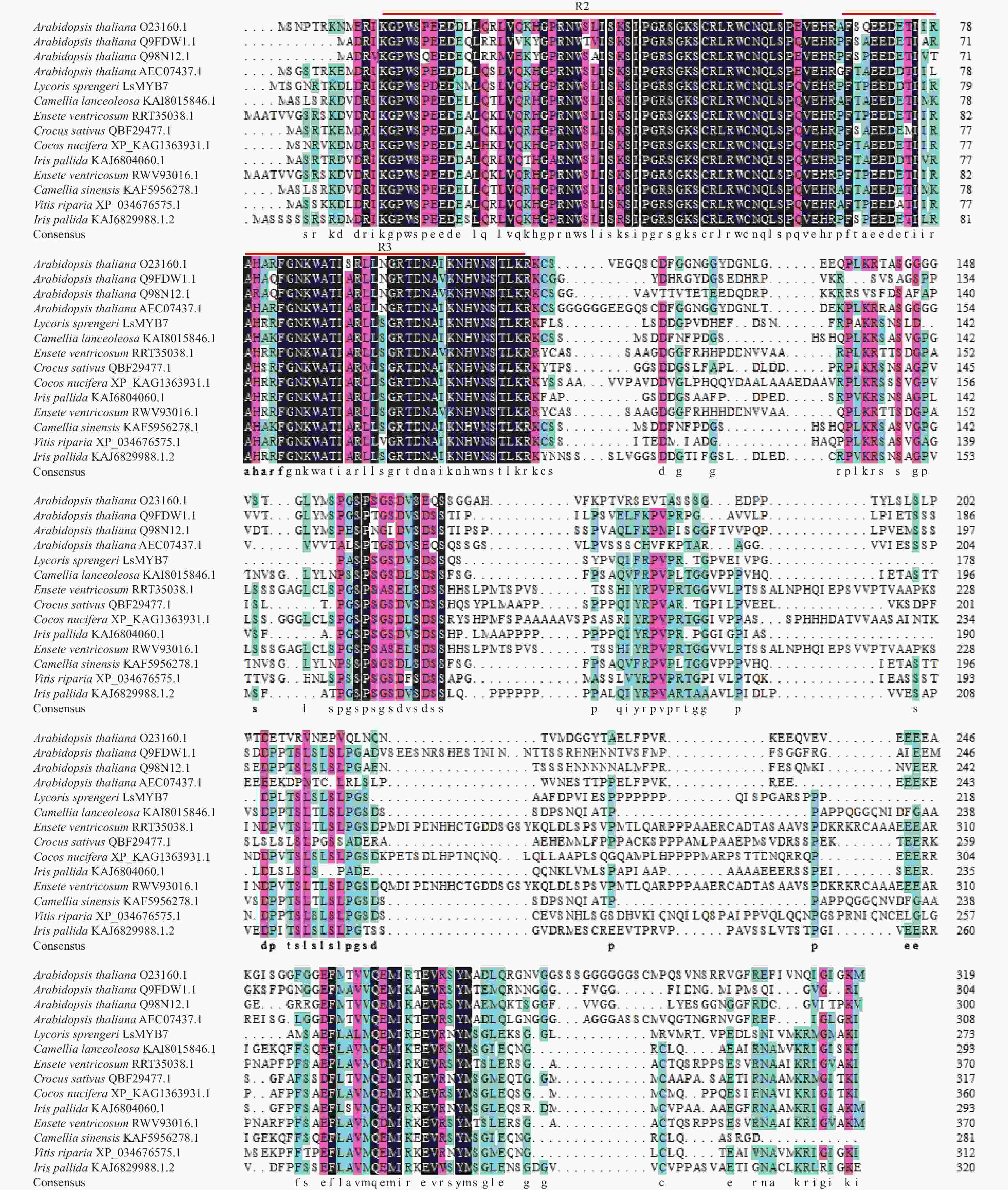
通过与美国国家生物技术信息中心(NCBI)搜索的其他植物MYB氨基酸同源序列进行比对,发现换锦花LsMYB7蛋白含有2个R2R3-MYB典型的SANT结构域R2和R3[18](图3),属于R2R3-MYB类转录因子。与茶Camellia sinensis KAF5956278.1、狭叶油茶Camellia lanceoleosa KAI8015846.1、粗柄象腿蕉Ensete ventricosum RRT35038.1/RWV93016.1、椰子Cocos nucifera XP_KAG1363931.1、番红花Crocus sativus QBF29477.1、香根鸢尾Iris pallida KAJ6804060.1/KAJ6829988.1、河岸葡萄Vitis riparia XP_034676575.1和拟南芥Arabidopsis thaliana等其他植物的同源性为44.52%~60.57%,其中,与茶KAF5956278.1的同源性最高达60.57%,其次是香根鸢尾 KAJ6804060.1/KAJ6829988.1(59.32%),与拟南芥AtMYB44(Q9FDW1.1)、AtMYB70(AEC07437.1)、AtMYB73(O23160.1)和AtMYB77(Q98N12.1)的同源性为44.52%~47.44%,且同源部分均集中在N端的R2R3 DNA结合结构域,而C端的同源性较低。
-
从NCBI数据库下载拟南芥(AtMYB 16条)、换锦花(LsMYB 2条)和石蒜L. radiata (LrMYB 2条) R2R3-MYB氨基酸序列进行比对并构建系统进化树(图4)。参考拟南芥MYB基因家族的分类方法[19],LsMYB7与拟南芥R2R3-MYB S22亚家族的AtMYB44、AtMYB70、AtMYB73和AtMYB77 聚为一类,由于拟南芥S22亚家族参与调控拟南芥的生长和发育过程,响应高盐、干旱低温等非生物胁迫反应,同一亚族基因的功能相似,结合花色基因表达分析,推测LsMYB7可能通过响应干旱和高温等调控换锦花花瓣花色苷的形成。
-
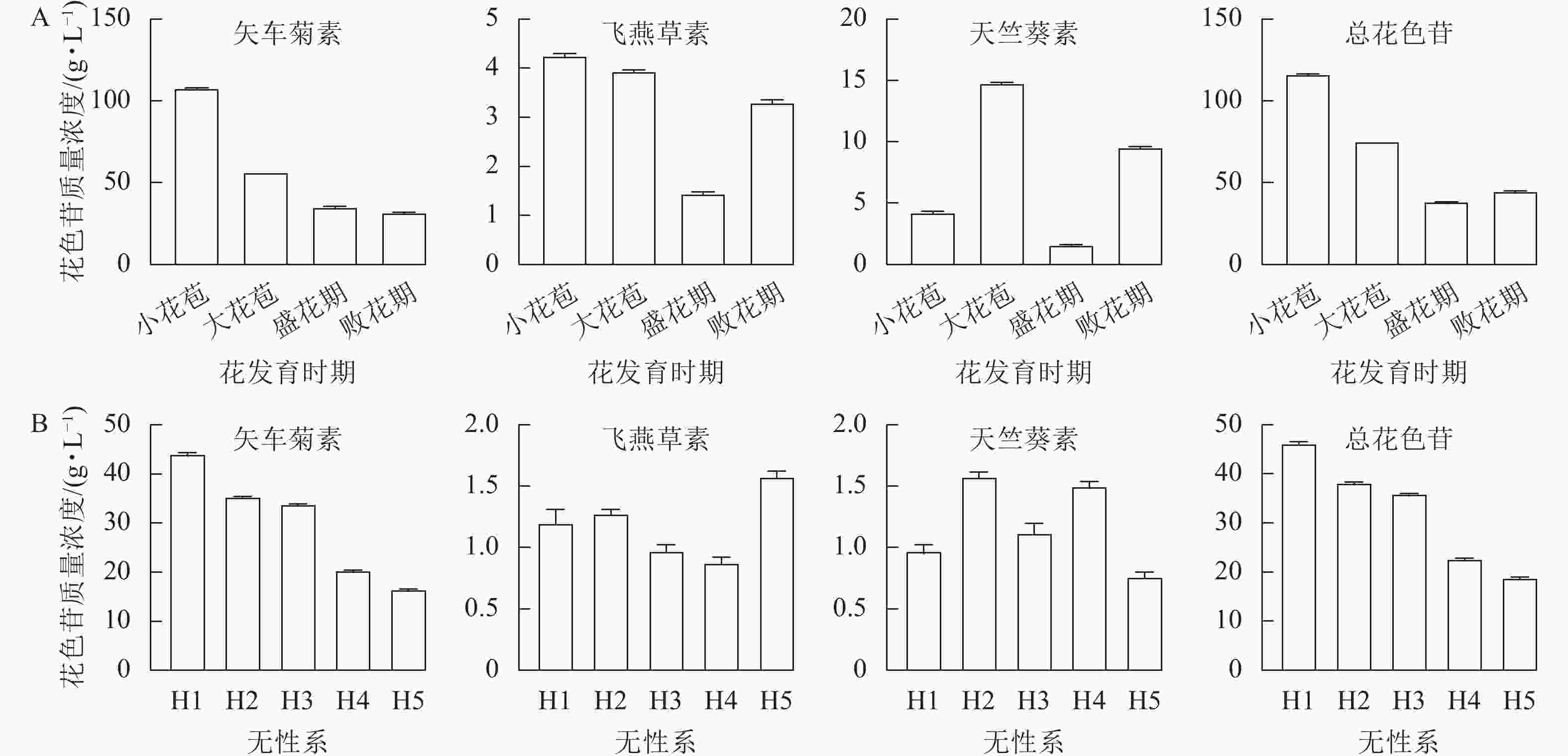
利用HPLC分别测定换锦花4个花发育时期和5个不同花色无性系(H1、H2、H3、H4和H5)盛花期花瓣的花色苷质量浓度(图5A和5B),结果表明:换锦花花瓣花色苷的主要成分为矢车菊素,含有少量的天竺葵素和飞燕草素,说明花色苷的种类和质量浓度决定换锦花花色的多样性。随着换锦花花器官的生长发育,花瓣花色苷总质量浓度和矢车菊素质量浓度呈逐渐下降的趋势,小花苞时期矢车菊素质量浓度大约是败花期的3倍,说明换锦花花瓣花色苷的积累可能主要在花瓣发育的早期完成(图1A和5A);在浅色换锦花无性系H4和H5中,矢车菊素质量浓度明显低于深色换锦花无性系H1、H2和H3(图1B和5B)。因此,从换锦花不同花发育时期和不同花色无性系花瓣颜色和花色苷质量浓度来看,矢车菊素对换锦花花瓣的颜色影响最大,矢车菊素质量浓度越高,换锦花花瓣的颜色就越深。
-
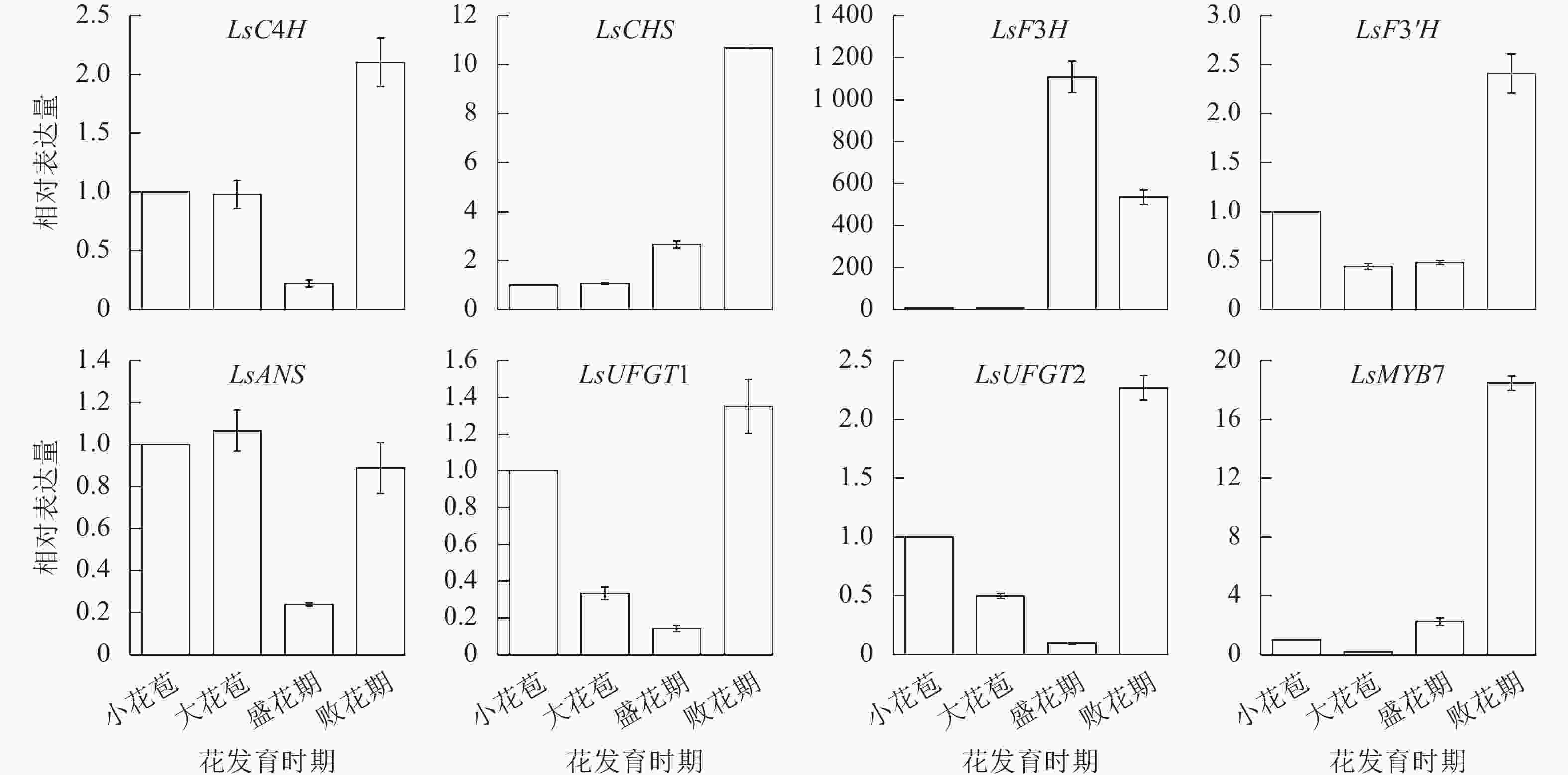
利用RT-qPCR对LsMYB7和换锦花花色形成相关基因(LsC4H、LsCHS、LsF3H、LsF3'H、LsANS和LsUFGT1、LsUFGT2)在换锦花不同发育时期花瓣中的表达进行分析,结果(图6)表明:LsMYB7与LsCHS和LsF3'H基因的表达随着换锦花花苞发育呈逐渐上升趋势,LsC4H、LsCHS、LsUFGT1、LsUFGT2、LsF3'H和LsMYB7在败花期大量表达,LsF3H则在花苞发育前期几乎无表达,盛花期开始大量表达,而在败花期表达量开始下降;LsANS、LsUFGTs等花色苷合成的后期基因在盛花期的表达最低。其中LsCHS和LsF3'H基因的表达与花色苷总质量浓度和矢车菊素质量浓度正好相反(图5A)。
-
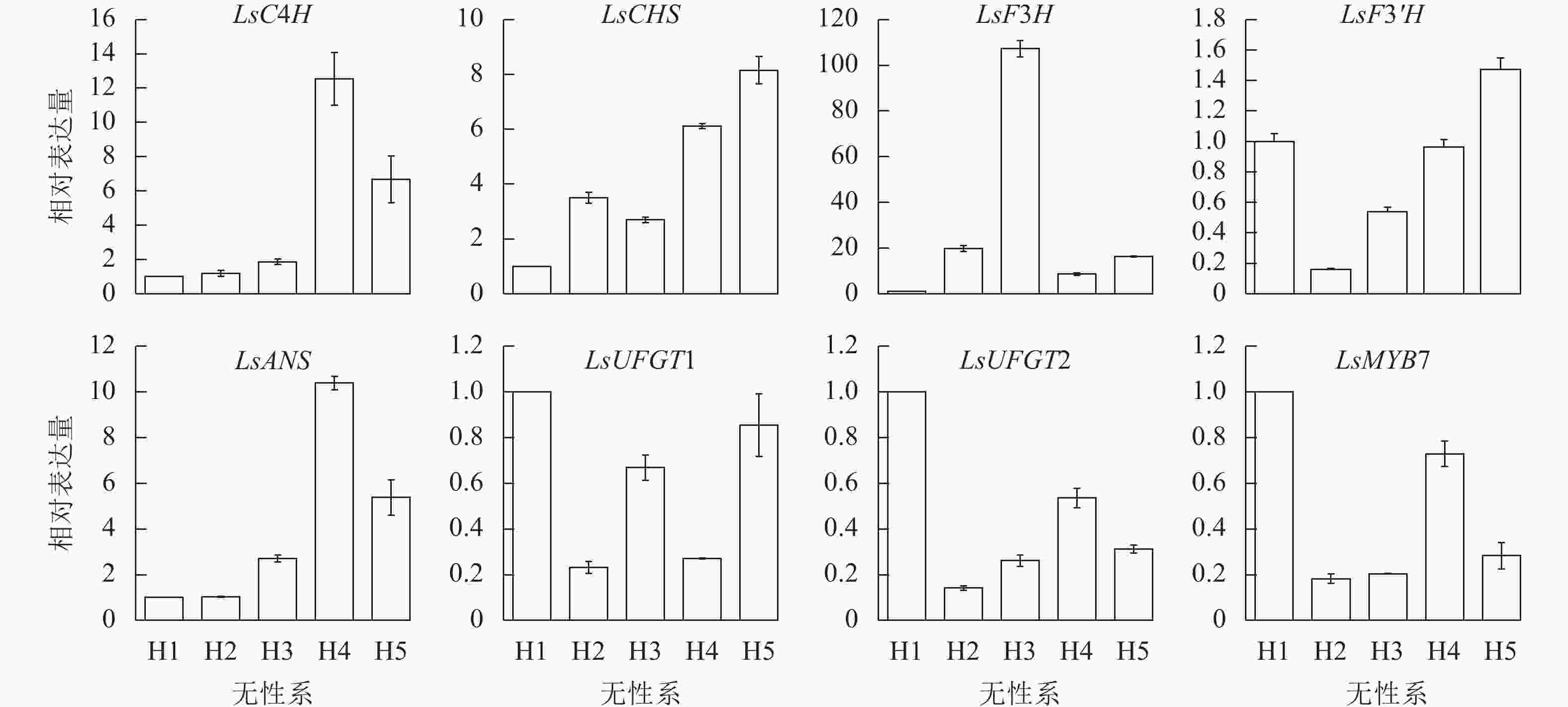
LsMYB7和换锦花花色形成关键基因LsC4H、LsCHS、LsF3H、LsF3'H、LsANS、LsUFGT1和LsUFGT2在换锦花不同花色无性系中的表达差异显著(图7)。2个花色苷合成的前期基因LsC4H和LsCHS及后期基因LsANS在淡色的无性系换锦花花瓣中表达量高。LsF3H在蓝色为主的H3无性系中表达量达到最高。LsMYB7在H1和H4中表达量比较高。LsMYB7基因的表达与LsCHS和LsF3'H基因的表达正好相反,而与不同花色无性系花色苷总质量浓度和矢车菊素质量浓度基本一致,这一结果与不同花发育时期的基因表达结果一致。因此,LsMYB7可能对LsCHS和LsF3'H基因的表达有一定的调控作用。
-
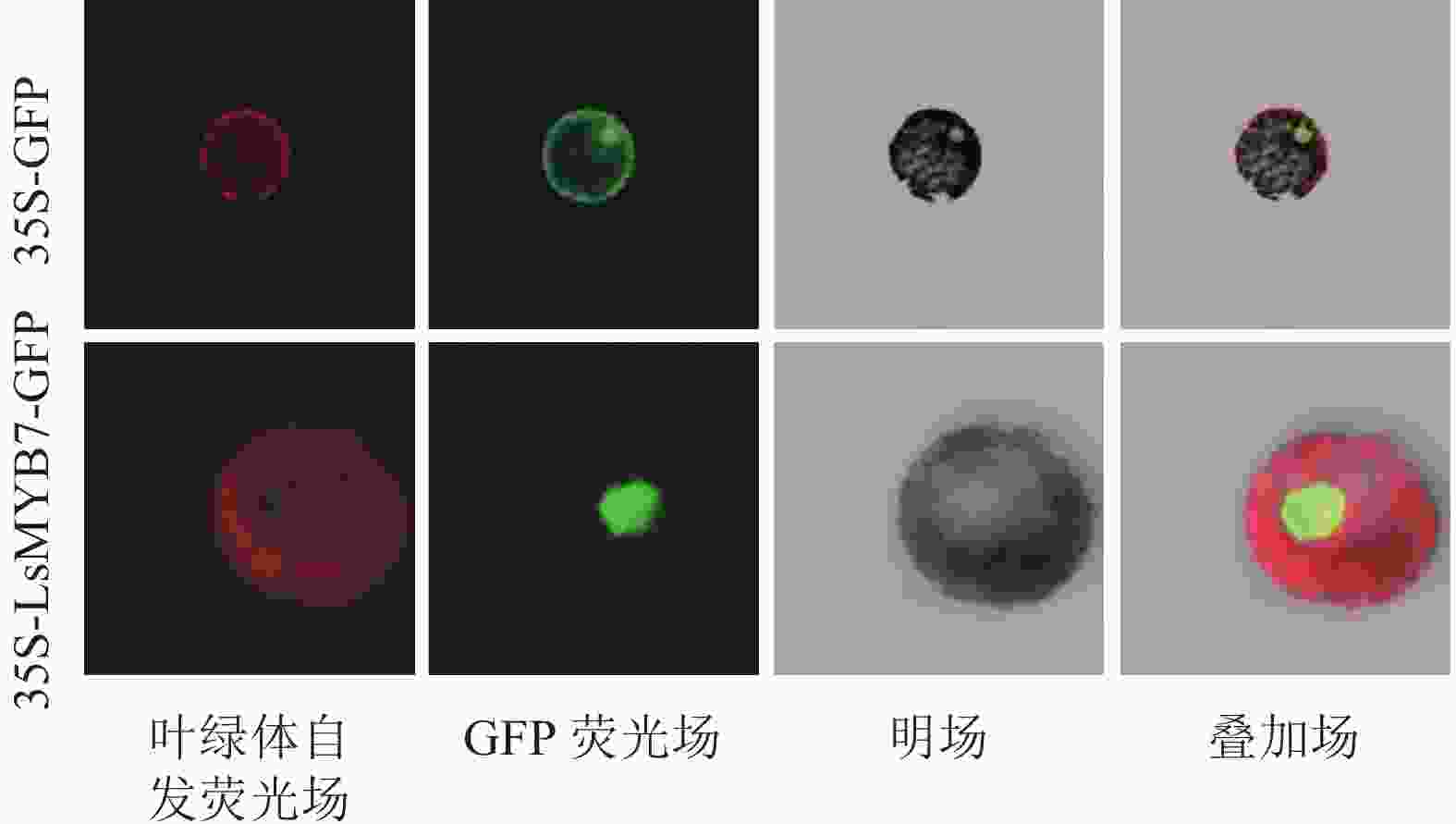
通过双酶切法构建亚细胞定位载体pAN580-LsMYB7,转化烟草原生质体,于激光共聚焦荧光显微镜观察,LsMYB7荧光信号定位在细胞核中(图8),说明LsMYB7基因为转录因子基因,在细胞核中起转录调控的作用。
-
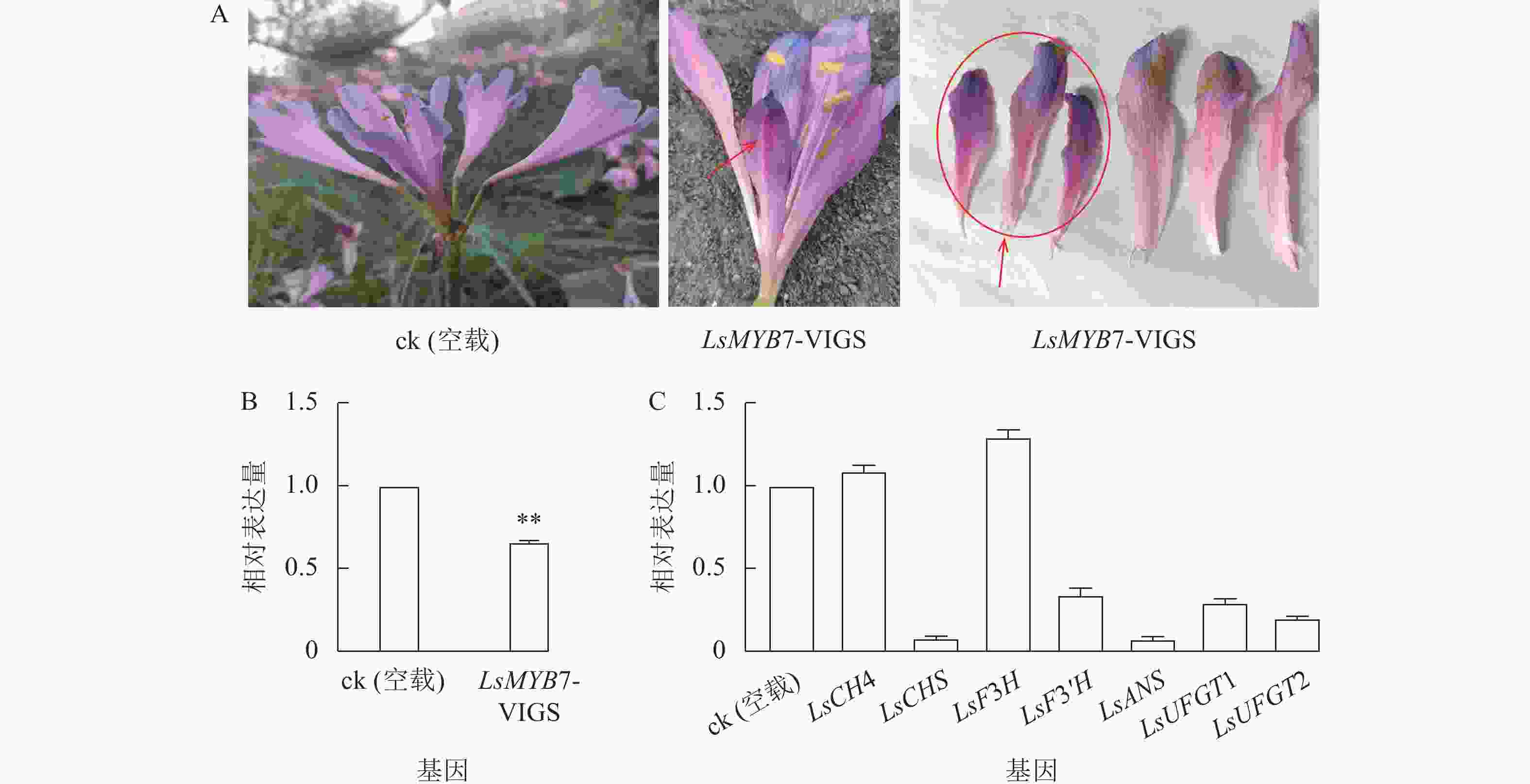
构建LsMYB7的VIGS基因沉默载体pTRV2-LsMYB7,通过微量注射法转化换锦花花苞,LsMYB7 基因沉默后,换锦花同朵花中一半花瓣明显变短,且颜色变深(图9A);对LsMYB7和花色苷形成相关基因在换锦花花瓣中的表达进行分析(图9B和C),与ck (空载)相比,LsMYB7基因换锦花花瓣中的表达量明显下降,同时,花色苷形成相关基因LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2的表达量极显著下降(P<0.01),而LsF3H基因的表达量反而上升,LsC4H基因表达变化不大,说明LsMYB7转录因子可能参与调控花瓣的生长发育,且对LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2的表达起正调控作用,而对LsF3H基因的表达起负调控作用。
-
植物R2R3-MYB转录因子广泛参与调控次生代谢、细胞形态发生、激素刺激、环境胁迫应答、分生组织形成和细胞周期等过程[20]。根据C-末端的不同,拟南芥R2R3-MYB基因家族可分成22个不同的亚族,其中,拟南芥S4、S5、S6和S7亚族基因参与花青素和类黄酮类化合物生物合成途径的结构基因的转录调控[18, 21−22],换锦花LsMYB4、LsMYB5和拟南芥S4亚族基因聚为一类。通过花青素形成相关基因表达和VIGS基因沉默技术研究表明,LsMYB4和LsMYB5对花青素生物合成基因的表达有负调控的作用[10−12]。
S22亚族基因AtMYB44、AtMYB77、AtMYB73和AtMYB70主要参与拟南芥响应高盐、干旱、低温等非生物胁迫反应,其中,AtMYB73基因启动子中含有ABA响应元件ABRE及干旱胁迫和热胁迫顺式作用元件。AtMYB73突变体atmyb73在干旱胁迫处理下,ABA 下游基因ABI2、ABI5 的表达水平均较野生型明显增强。外源 ABA 处理野生型和突变体种子、幼苗,获得了与干旱胁迫处理类似的结果[23−24]。本研究结果表明:LsMYB7蛋白含有2个R2R3-MYB典型的SANT结构域R2和R3,属R2R3-MYB类转录因子,且R2和R3的保守结构域与其他植物高度同源,系统进化树分析结果显示LsMYB7和S22亚族基因AtMYB44、AtMYB77、AtMYB73和AtMYB70聚为一类,推测LsMYB7可能与S22亚族基因有相似的功能。
本研究中,LsMYB7基因的表达与换锦花花色苷形成相关基因LsCHS、Ls4CL2和LsUFGT2的表达趋势一致,推测LsMYB7可能参与换锦花花瓣花色苷的生物合成。而LsMYB7属于拟南芥S22亚族,未见该亚族基因AtMYB44、AtMYB77、AtMYB73和AtMYB70参与花色苷的生物合成的转录调控。已有报道认为AtMYB73/44能够参与调控拟南芥对干旱胁迫的响应[25−26],而干旱胁迫可诱导植物细胞合成和积累花色苷。花色苷的光化学性质、亚细胞积累位点及在植物器官、组织中的空间分布决定了花色苷能强化植物的耐旱性,其中,花色苷提高植物细胞在干旱胁迫下的抗氧化能力可能是花色苷强化植物耐旱性的主要原因[27]。有研究表明:干旱胁迫可激活紫麦Triticum aestioum ‘Guizi 1’、甘薯Ipomoes batats等植物花色苷合成相关基因表达,通过提高花青素含量抵御干旱胁迫[28−29]。本研究中LsMYB7 基因沉默后,花色苷形成相关基因LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2的表达明显下降,说明LsMYB7可能直接或间接调控花色苷的积累,而LsMYB7与S22亚族基因聚为一类,由于换锦花开花季为夏末秋初的8—9月,此时多为高温和干旱季节,因此推测LsMYB7可能通过对干旱胁迫响应来调控花色苷形成相关基因的表达,大量积累花色苷。这一研究结果与云南文山辣椒Capsicum annuum、番茄Lycopersicon esculentum的研究[30−31]相似,因此,植物可以通过干旱胁迫激活与花色苷合成相关基因的表达水平促进花色苷积累,进而提高抗旱能力。
-
本研究通过RT-qPCR获得长为951 bp 的LsMYB7 cDNA序列,开放阅读框(ORF) 825 bp,编码274个氨基酸,LsMYB7为R2R3-MYB转录因子家族,定位于细胞核,与其他植物R2R3-MYB有较高的同源性。系统进化分析表明LsMYB7和参与调控干旱等非生物胁迫响应的S22亚族基因聚为一类;LsMYB7基因主要在败花期和花色苷含量较高的H1无性系中表达,与花色苷合成相关基因的表达趋势一致;LsMYB7基因沉默后,换锦花部分花瓣明显变短,颜色变深,LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2等花色苷形成相关基因的表达显著下调,因此,LsMYB7参与调控换锦花花瓣的生长发育,且通过对LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2花色苷形成相关基因的表达调控花色苷的积累。此外,LsMYB7可能通过响应干旱胁迫激活花青素合成相关基因表达促进花色苷积累,进而提高换锦花的抗旱能力,LsMYB7调控花色苷积累抵御干旱的机制需要进一步研究。
Cloning and function analysis of LsMYB7 gene in Lycoris sprengeri
-
摘要:
目的 研究转录因子LsMYB7基因对换锦花Lycoris sprengeri花色苷积累的调控作用。 方法 采用实时荧光定量PCR(RT-qPCR)方法从换锦花花瓣中克隆获得花色苷形成相关R2R3-MYB转录因子LsMYB7基因,并进行生物信息学分析,再通过病毒介导的基因沉默(VIGS)技术研究LsMYB7基因对花色苷积累的调控作用。 结果 克隆到1条长951 bp的 LsMYB7基因cDNA序列,开放阅读框(ORF)为825 bp,编码274个氨基酸,LsMYB7蛋白含有2个R2和R3结构域,属R2R3-MYB转录因子家族;系统进化分析表明LsMYB7与拟南芥Arabidopsis thaliana S22亚族基因聚为一类;LsMYB7亚细胞定位于细胞核,在不同花发育阶段和不同花色无性系中,LsMYB7基因表达与花色苷合成相关基因的表达趋势一致,主要在败花期和花色苷含量较高的H1无性系中表达;LsMYB7基因沉默后,换锦花花瓣明显变短,颜色变深,且LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2等花色苷形成相关基因的表达显著下调。 结论 LsMYB7属R2R3-MYB转录因子家族S22亚族,通过正向调控LsCHS、LsF3'H、LsANS、LsUFGT1和LsUFGT2花色苷生物合成相关基因的表达促进花色苷积累。图9表1参31 -
关键词:
- 换锦花 /
- R2R3-MYB转录因子 /
- 花色苷积累 /
- 病毒介导的基因沉默 /
- 调控作用
Abstract:Objective The purpose is to study the regulatory effect of LsMYB7 gene on the accumulation of anthocyanins in Lycoris sprengeri. Method R2R3-MYB transcription factor LsMYB7 was screened from petals of L. sprengeri. The expression of LsMYB7 and structure genes related to anthocyanin biosynthesis was analyzed by real-time quantitative PCR technology (RT-qPCR). The function of LsMYB7 on anthocyanin accumulation was studied by virus-induced gene silencing (VIGS) technology. Result A 951 bp LsMYB7 gene cDNA sequence was cloned from petals in L. srprengei, containing 825 bp open reading frame (ORF) which encoded 274 amino acids. LsMYB7 protein contained two R2 and R3 domain, and belonged to R2R3-MYB transcription factor family. Phylogenetic analysis showed that LsMYB7 was clustered one group with Arabidopsis thaliana S22 subfamily genes, involved in regulating responses to abiotic stress such as drought and high temperature. LsMYB7 was localized in the nucleus. The expression trend of LsMYB7 gene was consistent with genes related to anthocyanin biosynthesis in different flower development stages and different flower color clones, and mainly expressed in petals of H1 clones with high anthocyanin content and last flowering period. The petals of L. sprengeri became significantly shorter and darker in color, and the expression of genes related to anthocyanin biosynthesis, such as LsCHS, LsF3'H, LsANS, LsUFGT1, and LsUFGT2, was significantly downregulated after LsMYB7 gene being silenced. Conclusion LsMYB7 belongs to S22 subfamily of the R2R3-MYB transcription factor family, and gets involved in regulating the expression of genes (like LsCHS, LsF3'H, LsANS, LsUFGT1 and LsUFGT2) related to anthocyanin biosynthesis and promoting anthocyanin accumulation. [Ch, 9 fig. 1 tab. 31 ref.] -
表 1 RT-qPCR所用引物
Table 1. Primers for fluorescence RT-qPCR
引物 正向(5′→3′) 反向(5′→3′) LsGADPH AGGGTTTGATGACCACCGTGCA ACAGCCTTGGCAGCTCCAGTAC LsMYB7 GCGCGGAGTTCTTGGCTCTGAT TCTGGCACCGTTCTCATCACGC LsCHS CAAGACATGGTGGTGGTCGAGGTC CGAGGAGTTTGGTGAGCTGGTAGTC LsF3H AACCGAGGACGCAACGGAATGC ACCATCTTCATCGCAGCCACCA LsANS CGTGCCAGGTCTCCAGGTCTTCTA TCGAGAGTGTCACCGACGTGAACTA LsUFGT1 GGTGGTGAAGGATGAGGAAGGTAGG GTTGAACCGCTCGAACCGCAATC LsUFGT2
LsF3'HGCGTAGCCTTCTCCTTCCTCACCT
TTGTACAGCCATGCACAGAATCCGCCATGAATCGCTTCACCTCCTC
GCAACCAAGGCAAGAAATCA说明:引物参考文献[16]。 -
[1] 钱啸虎, 徐垠, 胡之璧, 等. 中国植物志: 第16卷第1分册[M]. 北京: 科学出版社, 1985: 25. QIAN Xiaohu, XU Yin, HU Zhibi, et al. Flora Reipublicae Popularis Sinicae: Vol. 16(1) [M]. Beijing: Science Press, 1985: 25. [2] 徐炳声, 林巾箴, 俞志洲, 等. 换锦花和中国石蒜的种间杂交[J]. 园艺学报, 1986, 4(4): 283 − 284. XU Bingsheng, LIN Jinzhen, YU Zhizhou, et al. Interspecific hybridization between Lycoris sprengeri and Lycoris chinensis [J]. Acta Horticulturae Sinica, 1986, 4(4): 283 − 284. [3] 张定成, 孙叶根, 郑艳, 等. 三倍体换锦花在安徽发现[J]. 植物分类学报, 1999, 37(1): 36 − 40. ZHANG Dingcheng, SUN Yegen, ZHENG Yan, et al. The discovery of triploid Lycoris sprengeri Comes ex Baker from Anhui, China [J]. Acta Phytotaxonomica Sinica, 1999, 37(1): 36 − 40. [4] ZHANG Fengjiao, ZHUANG Weibin, SHU Xiaochun, et al. Complete chloroplast genome of Lycoris sprengeri (Amaryllidaceae) and genetic comparison [J]. Mitochondrial DNA Part B, 2019, 4(2): 3577 − 3578. [5] YANG Feng, LI Chaohan, DEBATOSH D, et al, Comprehensive transcriptome and metabolic profiling of petal color development in Lycoris sprengeri [J/OL]. Frontiers in Plant Science, 2021, 12: 747131 [2023-06-15]. doi: 10.3389/fpls.2021.747131. [6] 王黎, 周琪, 高燕会. 石蒜属种间杂交种的鉴定和分子身份证构建[J]. 浙江农林大学学报, 2022, 39(3): 562 − 570. WANG Li, ZHOU Qi, GAO Yanhui. Construction of molecular identification card of Lycoris interspecific hybrids [J]. Journal of Zhejiang A&F University, 2022, 39(3): 562 − 570. [7] 洪艳, 武宇薇, 宋想, 等. 光照调控园艺作物花青素苷生物合成的分子机制[J]. 园艺学报, 2021, 48(10): 1983 − 2000. HONG Yan, WU Yuwei, SONG Xiang, et al. Molecular mechanism of light-induced anthocyanin biosynthesis in horticultural crops [J]. Acta Horticulturae Sinica, 2021, 48(10): 1983 − 2000. [8] GU Kaidi, WANG Chukun, HU Dagang, et al. 2019. How do anthocyanins paint our horticultural products? [J]. Scientia Horticulturae, 249: 257−262. [9] 王峰, 王秀杰, 赵胜男, 等. 光对园艺植物花青素生物合成的调控作用[J]. 中国农业科学, 2020, 53(23): 4904 − 4917. WANG Feng, WANG Xiujie, ZHAO Shengnan, et al. Light regulation of anthocyanin biosynthesis in horticultural crops [J]. Scientia Agricultura Sinica, 2020, 53(23): 4904 − 4917. [10] 许振渊, 高燕会, 周芬静, 等. 换锦花LsMYB4基因的克隆与表达分析[J]. 园艺学报, 2014, 41(11): 2281 − 2390. XU Zhenyuan, GAO Yanhui, ZHOU Fenjing, et al. Cloning and expression analysis of LsMYB4 gene in Lycoris sprengeri [J]. Acta Horticulturae Sinica, 2014, 41(11): 2281 − 2390. [11] 侯朔, 高燕会, 童再康. 换锦花LsMYB5基因的克隆与表达分析[J]. 农业生物技术学报, 2019, 27(12): 2164 − 2174. HOU Shuo, GAO Yanhui, TONG Zaikang. Cloning and expression analysis of LsMYB5 gene in Lycoris sprengeri [J]. Journal of Agricultural Biotechnology, 2019, 27(12): 2164 − 2174. [12] 周洋丽, 侯朔, 郑正权, 等. 基于VIGS基因沉默体系的换锦花LsMYBs基因功能研究[J]. 农业生物技术学报, 2020, 28(6): 974 − 983. ZHOU Yangli, HOU Shuo, ZHENG Zhengquan, et al. Study on LsMYBs gene function in Lycoris sprengeri based on VIGS gene silencing system [J]. Journal of Agricultural Biotechnology, 2020, 28(6): 974 − 983. [13] 周洋丽. LsMYB4、LsMYB5 调控的换锦花花瓣呈色的分子机制[D]. 杭州: 浙江农林大学, 2020. ZHOU Yangli. Molecular Mechanism of Petal Color of Lycoris sprengeri Regulated by LsMYB4 and LsMYB5 [D]. Hangzhou: Zhejiang A&F University, 2020. [14] 薛惠敏, 周洋丽, 高燕会. 换锦花花青素合成酶基因(LsANS)的克隆及启动子功能分析[J]. 农业生物技术学报, 2022, 30(8): 1468 − 1479. XUE Huimin, ZHOU Yangli, GAO Yanhui. Cloning and promoter function analysis of the anthocyanins synthase gene (LsANS) in Lycoris sprengeri [J]. Journal of Agricultural Biotechnology, 2022, 30(8): 1468 − 1479. [15] 蒋婷婷, 高燕会, 童再康. 石蒜属植物实时荧光定量PCR内参基因的选择[J]. 园艺学报, 2015, 42(6): 1129 − 1138. JIANG Tingting, GAO Yanhui, TONG Zaikang. Selection of reference genes for quantitative real-time PCR in Lycoris [J]. Acta Horticulturae Sinica, 2015, 42(6): 1129 − 1138. [16] 郑正权. 换锦花复色花形成LsMYBs和LsbHLHs基因的筛选和功能初步研究[D]. 杭州: 浙江农林大学, 2021. ZHENG Zhengquan. Screening and Functional Analysis of Lsmybs and Lsbhlhs Genes Involved in the Formation of Polychromatic Flowers in Lycoris sprengeri [D]. Hangzhou: Zhejiang A&F University, 2021. [17] 刘跃平, 周洋丽, 高燕会. 换锦花花色苷成分及其稳定性[J]. 浙江农林大学学报, 2021, 38(3): 587 − 596. LIU Yueping, ZHOU Yangli, GAO Yanhui. Effects of physical and chemical factors on anthocyanin stability in Lycoris sprengeri [J]. Journal of Zhejiang A&F University, 2021, 38(3): 587 − 596. [18] STRACKE R, WERBER M, WEISSHAAR B. The R2R3-MYB gene family in Arabidopsis thaliana [J]. Current Opinion in Plant Biology, 2001, 4(5): 447 − 456. [19] DUBOS C, STRACKE R, GROTEWOLD E, et al. MYB transcription factors in Arabidopsis [J]. Trends in Plant Science, 2010, 15(10): 573 − 581. [20] 王霜, 雒晓鹏, 姚英俊, 等. 苦荞R2R3-MYB转录因子调控原花青素生物合成的研究[J]. 西北植物学报, 2019, 39(11): 1911 − 1918. WANG Shuang, LUO Xiaopeng, YAO Yingjun, et al. Characterization of an R2R3-MYB transcription factor involved in the synthesis of proanthocyanidins from Tartary buckwheat [J]. Acta Botanica Boreali-Occidentalia Sinica, 2019, 39(11): 1911 − 1918. [21] 王桂青, 姚红, 吴嘉诚, 等. 中国水仙NtMYB7基因的克隆及功能初步研究[J]. 西北植物学报, 2018, 38(8): 1401 − 1410. WANG Guiqing, YAO Hong, WU Jiacheng, et al. Cloning and functional characterization of NtMYB7 gene in Narcissus tazetta var. chinensis [J]. Acta Botanica Boreali-Occidentalia Sinica, 2018, 38(8): 1401 − 1410. [22] 吴嘉诚, 王桂青, Muhammad, 等. 中国水仙R2R3-MYB基因NtMYB5的克隆和功能研究[J]. 园艺学报, 2018, 45(7): 1327 − 1337. WU Jiacheng, WANG Guiqing, Muhammad, et al. Cloning and functional analysis of R2R3-MYB gene NtMYB5 in Narcissus tazetta var. chinensis [J]. Acta Horticulturae Sinica, 2018, 45(7): 1327 − 1337. [23] 樊锦涛. 拟南芥AtMYB73响应干旱机制初探[D]. 保定: 河北农业大学, 2015. FAN Jintao. Preliminary Exploration for Mechanism of Arabidopsis thaliana AtMYB73 under Drought Stress[D]. Baoding: Agricultural University of Hebei, 2015. [24] 樊锦涛, 蒋琛茜, 邢继红, 等. 拟南芥R2R3-MYB家族第22亚族的结构与功能[J]. 遗传, 2014, 36(10): 985 − 994. FAN Jintao, JIANG Chenxi, XING Jihong, et al. Structure and function of the 22nd subfamily in Arabidopsis R2R3-MYB family [J]. Hereditas, 2014, 36(10): 985 − 994. [25] JUNG C, SEO J S, HAN S W, et al. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis [J]. Plant Physiology, 2008, 146(2): 623 − 635. [26] SEKI M, NARUSAKA M, ISHIDA J, et al. Monitoring the expression proles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray [J]. The Plant Journal, 2002, 31(3): 279 − 292. [27] TANG Xiaohua, ZHAO Changling, WEN Guosong, et al. Physiological mechanism for anthocyanins to strengthen the drought tolerance of plants [J]. Agricultural Science &Technology, 2014, 15(11): 1935 − 1941. [28] LI Xiaolan, LÜ Xiang, WANG Xiaohong, et al. Biotic and abiotic stress-responsive genes are stimulated to resist drought stress in purple wheat [J]. Journal of Integrative Agriculture, 2020, 19(1): 33 − 50. [29] 陈晓丽, 李红兵, 孙振玫, 等. 过表达IbMYB1基因甘薯增强了对土壤干旱胁迫的抗性[J]. 植物生理学报, 2015, 51(9): 1440 − 1446. CHEN Xiaoli, LI Hongbing, SUN Zhenmei, et al. Overexpression of IbMYB1 gene enhanced tolerance to soil drought stress in sweet potato [J]. Plant Physiology Journal, 2015, 51(9): 1440 − 1446. [30] LI Yun, MENG Fanlai, ZHAO Changling, et al. Responses of the anthocyanin and osmolyte contents of the Capsicum annuum cultivars planted in Wenshan prefecture of Yunnan Province to the drought stress simulated by PEG-6000 [J]. Agricultural Science &Technology, 2016, 17(6): 1295 − 1300, 1335. [31] 王鸿雪, 刘天宇, 庄维兵, 等. 花青素苷在植物逆境响应中的功能研究进展[J]. 农业生物技术学报, 2020, 28(1): 174 − 183. WANG Hongxue, LIU Tianyu, ZHUANG Weibing, et al. Research advances in the function of anthocyanin in plant stress response [J]. Journal of Agricultural Biotechnology, 2020, 28(1): 174 − 183. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20230368







 下载:
下载: