-
叶片色素质量分数与其光合能力和营养状况有较好的相关性,通常是植物环境胁迫的良好指示器[1],叶片光谱反射率测定是一种快捷无损估测叶片色素质量分数的方法[2]。研究表明,近红外波段反射率(>750 nm)与700 nm和550 nm的比值(R750/R700,R750/R550)与植物叶片中叶绿素a和叶绿素a+b质量分数具有良好的相关性;绿度归一化光谱指数(gNDVI)对叶绿素a的质量分数变化非常敏感[3]。修正型光谱指数(mSR705)和叶片衰老指数(PSRI)能够很好地反应叶片结构和色素质量分数[4]。北美短叶松 Pinus banksiana高光谱观测显示,光谱指数R750/R710对叶绿素敏感,可用于估测总叶绿素质量分数[5]。当植物在受到胁迫时,叶片色素质量分数降低,色素质量分数与光谱反射率有显著的相关性[6]。可见光部分光谱反射率的变化主要是由叶片的叶绿素质量分数对胁迫异常敏感所致,且对胁迫敏感的波段范围主要集中在550 nm附近的绿光反射峰及700 nm附近的红边附近[7]。温度水分双重胁迫综合作用于光谱反射率的光化学反射指数(PRI),叶片总叶绿素质量分数与红边位置(λred)显著相关[8]。温度胁迫使叶片叶绿素降解,近红外区域光谱反射率随温度胁迫程度的加剧而降低,λred向短波方向移动[9]。这些研究表明:植物胁迫程度与其叶片中色素质量分数及光谱特征均有紧密的联系。毛竹 Phyllostachys edulis林是中国南方重要的森林资源,具有较高的社会、经济和生态效益,在竹产业中具有极其重要的地位。研究显示:绿峰(Rg),红谷(Rr),λred,红边振幅(Dλred)等可作为毛竹林光谱识别的主要参数[10]。毛竹叶片绿光区的光谱反射率随叶绿素a、叶绿素b和类胡萝卜素质量分数增加而逐渐降低,且色素与红边参数有极显著的相关性[11]。低温胁迫是毛竹生长过程中最为常见的胁迫类型,然而,在低温胁迫下,毛竹叶片光合色素质量分数与反射光谱及相关参数的相关性研究未见报道。因此,本研究以3年生毛竹实生苗为研究对象,综合分析低温胁迫条件下叶片色素质量分数与反射光谱的相互关系,以探究毛竹低温胁迫响应机制。
HTML
-
供试材料为毛竹 3年生实生苗,2012年4月中旬将其移入花盆(盆径25 cm)中缓苗。6月中旬选取株高1.0 m,生长良好,无病虫害的毛竹3株,放入人工气候箱中,相对湿度为(60 ± 3)%,光照条件为光照12 h/黑暗12 h,光照强度为1 000 μmol·m-2·s-1,适应培养2 d后进行温度胁迫。处理温度设有25 ℃,20 ℃,15 ℃,10 ℃和5 ℃等5个梯度,从25 ℃依次降低,以2 ℃·h-1过渡到下一温度,在各温度中处理24 h,选取毛竹植株中部当年生枝条顶端向下第3~4片完整的功能叶片,测定各项指标。
-
称取毛竹叶片0.2 g,剪碎后置于带盖的试管中,加5.0 mL 体积分数为80%的丙酮,室温下遮光萃取至叶片完全变白后,分别在470,645和663 nm处测定其D(λ)值,按Lichtenthaler[12]的方法计算叶绿素a ,叶绿素b 和类胡萝卜素质量分数。在各温度中处理毛竹3株,取样3次·株-1。
-
采用光谱分析仪(UniSpec-SC,美国产)测定毛竹叶片在310~1 130 nm处的反射光谱数据,采样间隔1 nm,分辨率1 nm。测定时间为上午10: 00-12: 00,测定毛竹3株·次-1,选取叶子6片·株-1,取其平均值作为该温度的光谱反射率。测量过程中及时进行标准白板校正,用Multispec 5.1数据处理软件读取反射光谱原始数据。
-
光谱数据微分处理:将毛竹叶片反射光谱通过下列公式进行一阶微分处理得到微分光谱。
其中:λi为波段i处的波长值;Rλi为波长λi处的光谱反射率值;Δλ为波长λ(i-1)到λi的差值,由光谱采样间隔而定。“三边”参数计算方法:分别在490~530 nm,560~640 nm和680~750 nm范围内确定蓝边、黄边和红边位置、幅值和面积。λred为红光范围内一阶导数光谱最大值所对应的波长,Dλred为一阶导数光谱的最大值,红边面积(Sred)为一阶导数光谱线所包围的面积。黄边(黄边位置λyellow,黄边幅值Dλyellow,黄边面积Syellow)和蓝边(蓝边位置λblue,蓝边幅值Dλblue,蓝边面积Sblue)参数与红边参数意义类似。根据叶片特征,筛选出较能反应叶片特征结构的光谱参数[2, 13-22]及相关公式见表 1。
反射光谱参数 定义 叶绿素光谱指数 光谱反射指数 R750 / R705 改良红边比 (R750 - R445) / (R705 + R445) 色素比值指数 R800 / R680, R800 / R635 反射光谱比分析 R675/ R700, R650 / R700 归一化植被指数 (R803 - R680) / (R800 + R680) 红边归一化指数 (R750 - R705) / (R750 + R705) 色素归一化指数 (R800 R675) / (R800+ R675), (R800 - R650) / (R800 + R650) 类胡萝卜素光谱指数 反射光谱比率分析 R760 / R500 色素比值指数 R800 / R500 植物衰老反射指数 (R680 - R500) / R750 改良的类胡萝卜素指数 R780 [(1/R510) — (1/R550)] 光化学反射指数 (R531 - R570) / (R531 + R570) 花青素光谱指数 红绿比指数 Sum (R600 / Sum (R500 ~ R599) Table 1. Reflectance spectrum parameters
-
采用SPSS 13.0进行数据处理和统计分析,用OriginPro 8.0软件对光谱信息进行去噪处理,提取出与叶绿素有关的特征波段,并进行绘图。
1.1. 材料处理
1.2. 研究方法
1.2.1. 叶绿素和类胡萝卜素质量分数测定
1.2.2. 光谱数据采集
1.2.3. 光谱分析方法
1.3. 数据处理
-
叶绿素质量分数是表征毛竹叶片光合作用状况的主要指标。在温度胁迫下,毛竹叶片叶绿素a,叶绿素b及类胡萝卜素质量分数变化基本一致,均随着温度降低而逐渐降低(表 2)。当温度降到15 ℃时,叶绿素a和类胡萝卜素有极显著的降低(P<0.01),分别比25 ℃时降低了4.2%和10.9%;当温度达到5 ℃时,叶绿素a和类胡萝卜素质量分数比对照25 ℃时分别降低了6.9%和23.1%;与叶绿素a和类胡萝卜素相比,叶绿素b变化趋势不明显。
温度/℃ 叶片色素质量分数/(mg·g-1) 叶绿素a 叶绿素b 叶绿素a+b 类胡萝卜素 叶绿素a/b 25(ck) 2.22±0.01aA 0.86±.02aA 3.09±.03aA 0.08±0.01aA 2.57±0.03aA 20 2.22±0.01aAB 0.86±0.01aA 3.08±.03abA 0.08±0.01aA 2.56±0.02aA 15 2.12±0.01bBC 0.83±0.01aA 2.97±.02cA 0.07±0.01bAB 2.55±0.01aA 10 2.12±0.01bBC 0.85±.02aA 2.99±0.01bcA 0.06±0.01bB 2.50±0.07aA 5 2.07±0.01bC 0.85±.02aA 2.97±0.01cA 0.06±0.01cC 2.49 ±0.06aA 说明:小写字母不同表示0.05水平上的差异性(P<0.05),大写字母不同表示0.01水平上的差异性(P<0.01)。 Table 2. Effects of low temperature stress on pigments content in Phyllostachys edulis leaves
-
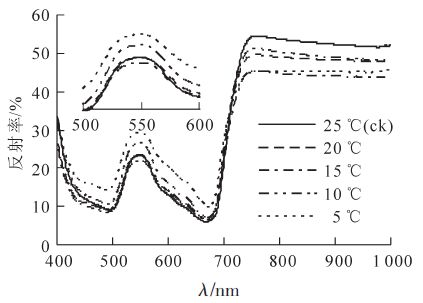
低温胁迫显著影响毛竹叶片的光谱反射特征,在不同波段区域光谱响应不同(图 1)。在680 nm附近的红光区域和490 nm的蓝光区域有吸收低谷,而在550 nm处有反射峰,在近红外区域,由于没有叶片色素的吸收,反射率增加,使得780~1 000 nm波段出现较高的反射平台[23]。随着温度的降低,反射光谱在蓝光区(525~605 nm)存在明显差异,其次是黄光区(605~655 nm)和近红外区(750~1 000 nm)。毛竹叶片的反射率在460~680 nm波段随温度降低而逐渐升高,10 ℃时出现显著增加,在波长550 nm处,当温度降低到5 ℃时,反射率比对照25 ℃提高了24.5%(P<0.01);在750~1 000 nm波段,反射率随温度降低而降低。
-
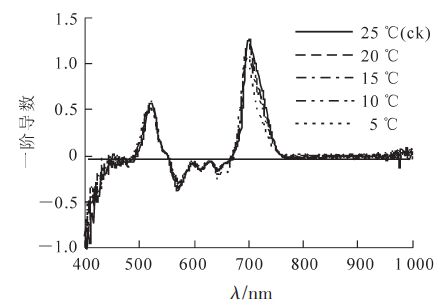
在分析光谱数据过程中,对原始光谱数据进行微分变换,以减少背景噪声的影响,从而提高参数的监测效果。将低温胁迫后毛竹叶片的反射光谱数据进行一阶导数处理,结果如图 2所示。不同温度处理的毛竹叶片在680~760 nm波段内都只有1个峰值。随着温度的降低,λred逐渐向短波方向移动(表 3)。Sred随之减小,15 ℃时差异显著。Sblue随着温度降低呈现出逐渐增加的趋势,而λblue则逐渐减小;λyellow随温度降低而减小,而Syellow和Dλyellow则随着温度的降低呈现先升高后降低的趋势。无论是蓝边还是黄边,最明显的变化都出现在5℃时(表 3)。

Figure 2. First derivative of reflectance spectra in Ph. edulis leaves under low temperature stresss
参数 不同温度下的参数值 25(ck) 20 15 10 5℃ λblue 520.50 ± 1.50 aA 519.70 ± 0.01 aA 520.10 ± 1.50 aA 519.60 ± 1.29 aA 518.70 ± 0.43 aA λblue 0.58 ± 0.04 aA 0.55 ± 0.04 aA 0.51 ± 0.07 aA 0.61 ± 0.12 aA 0.53 ± 0.11 aA Sblue 11.90 ± 1.20 aA 12.10 ± 1.40 aA 12.60 ± 1.90 aA 13.70 ± 1.20 aA 13.50 ± 2.20 aA λyellow 569.20 ± 0.10 bAB 568.50 ± 0.50 bB 571.50 ± 0.20 aA 569.20 ± 1.10 bAB 568.10 ± 0.01 bB Dyellow -0.38 ± 0.01 bA -0.32 ± 0.02 abA -0.29 ± 0.04 aA -0.37 ± 0.04 abA -0.31 ± 0.05 aA Syellow 13.84 ± 0.87 aA 12.82 ± 1.03 aA 12.01 ± 1.79 aA 14.61 ± 2.72 aA 13.47 ± 2.12 aA λred 700.20 ± 1.20 aA 699.50 ± 0.80 abA 699.00 ± 1.40 abA 697.70 ± 1.40 abA 696.50 ± 0.10 bA Dλred 1.27 ± 0.02 aA 1.19 ± 0.05 aA 1.09 ± 0.17 aA 1.14 ± 0.14 aA 1.08 ± 0.16 aA Sred 47.66 ± 1.80 aA 42.02 ± 1.53 abAB 40.31 ± 1.72 abAB 39.52 ± 0.95 bcAB 34.71 ± 2.91 cB 说明:小写字母不同表示0.05水平上的差异性(P<0.05),大写字母不同表示0.01水平上的差异性(P<0.01)。 Table 3. Effects of low temperature stress on "three edge" parameters in Ph. edulis leaves
-
光谱参数是绿色植物的光谱反射特征,是反映植物生长状况的最常用光谱变量。本试验中筛选出的叶绿素、类胡萝卜素和花青素的相关光谱参数,均随着温度降低有显著地增加或降低,且大部分参数的变化幅度达到了极显著(P<0.01)水平。表 4可知:光谱反射指数、改良红边比、色素比值指数a、色素比值指数b、归一化植被指数、红边归一化指数、色素归一化指数a、色素归一化指数等叶绿素相关参数,及反射光谱比分析、色素比值指数、改良的类胡萝卜素指数、光化学反射指数等类胡萝卜素相关参数随温度的降低而降低;反射光谱比率分析a、红绿比指数和反射光谱比率分析b随着温度降低而升高;除反射光谱比率分析a外,各光谱参数变化均达极显著(P<0.01)水平。
参数 不同温度下的参数值 25(ck) 20 15 10 5℃ 光谱反射指数a 1.982 ± 0.169 aA 1.778 ± 0.172 abAB 1.744 ± 0.151 abAB 1.805 ± 0.374 abAB 1.476 ± 0.134 bB 改良红边比b 0.626 ± 0.013 aA 0.638 ± 0.013 aA 0.619 ± 0.058 aA 0.590 ± 0.092 aA 0.466 ± 0.027 bB 色素比值指数a 7.984 ± 0.817 aA 6.545 ± 0.745 aAB 6.395 ± 1.156 aAB 7.268 ± 2.477 aAB 4.365 ± 0.584 bB 色素比值指数b 5.828 ± 0.829 aA 4.870 ± 0.782 aAB 4.589 ± 0.858 abAB 4.914 ± 2.205 aAB 2.883 ± 0.427 bB 反射光谱比分析a 0.291 ± 0.009 bA 0.305 ± 0.032 bA 0.311 ± 0.034 bA 0.294 ± 0.066 bA 0.369 ± 0.018 aA 反射光谱比分析b 0.338 ± 0.011 bB 0.352 ± 0.039 bB 0.368 ± 0.033 bB 0.373 ± 0.084 bB 0.482 ± 0.016 aA 归一化植被指数 0.776 ± 0.020 aA 0.733 ± 0.029 aAB 0.724 ± 0.049 aA 0.739 ± 0.086 aAB 0.624 ± 0.039 bB 红边归一化指数 0.328 ± 0.037 aA 0.278 ± 0.044 abAB 0.269 ± 0.041 abAB 0.278 ± 0.090 abAB 0.190 ± 0.044 bB 色素归一化指数a 0.792 ± 0.017 aA 0.754 ± 0.027 aA 0.743 ± 0.047 aAB 0.758 ± 0.083 aA 0.647 ± 0.031 bB 色素归一化指数b 0.762 ± 0.024 aA 0.720 ± 0.044 aA 0.703 ± 0.053 aA 0.700 ± 0.109 aA 0.561 ± 0.047 bB 反射光谱比率分析 5.869 ± 0.539 aA 5.298 ± 0.619 aA 5.138 ± 0.899 aA 5.052 ± 1.708 aA 2.970 ± 0.229 bB 色素比值指数 5.779 ± 0.547 aA 5.225 ± 0.591 aA 5.064 ± 0.888 aA 4.964 ± 1.689 aA 2.976 ± 0.264 bB 植物衰老反射指数 -0.047 ± 0.004 bA -0.038 ± 0.007 bA -0.041 ± 0.011 abA -0.070 ± 0.029 bAB -0.107 ± 0.025 cB 改良的类胡萝卜素指数 2.478 ± 0.358 aA 2.055 ± 0.326 bAB 1.956 ± 0.449 bAB 2.002 ± 0.993 aAB 0.898 ± 0.113 bB 光化学反射指数 0.060 ± 0.004 aA 0.052 ± 0.012 abAB 0.050 ± 0.006 abAB 0.056 ± 0.011 aAB 0.040 ± 0.006 bB 红绿比指数 0.524 ± 0.004 bB 0.565 ± 0.031 bAB 0.567 ± 0.030 bAB 0.536 ± 0.049 bB 0.616 ± 0.010 aA 说明:小写字母不同表示0.05水平上的差异性(P<0.05),大写字母不同表示0.01水平上的差异性(P<0.01)。 Table 4. Effects of low temperature stress on reflectance spectrum parameters in Phyllostachys edulis leaves
-
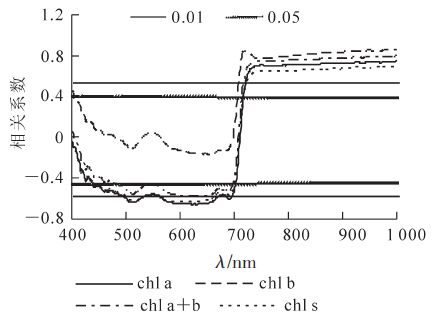
对低温胁迫后毛竹叶色素质量分数与光谱反射率进行相关分析表明(图 3),波长小于710 nm,毛竹叶叶绿素a,叶绿素a+b和类胡萝卜素质量分数与反射率呈负相关,且于452~700 nm的反射率达显著(P<0.05)相关,在620 nm处相关系数最大。光谱反射率与除叶绿素b外的各色素质量分数均显著相关,叶绿素a与光谱反射率的相关系数较类胡萝卜素要高;波长大于710 nm,叶绿素a,叶绿素b,叶绿素a+b和类胡萝卜素质量分数与光谱反射率呈正相关,且均达到极显著(P<0.01)水平。
-
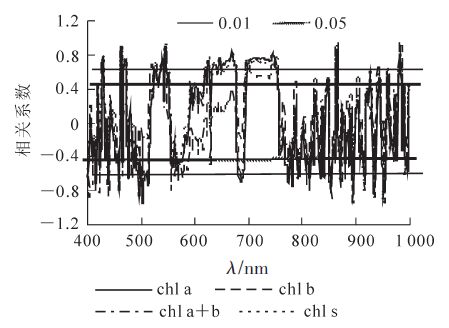
毛竹叶片色素质量分数与一阶微分光谱之间的相关系数图表明(图 4),叶绿素a,叶绿素a+b和类胡萝卜素质量分数与一阶微分光谱在450~462,480~516,521~549,554~587,589~676,695~756,757~820 nm处均达极显著相关。其中,在450~462,480~516,554~587和757~820 nm等4个波段达极显著负相关,480~516 nm波段相关系数的绝对值最大,513 nm处为-0.973;而在其他几个显著相关波段呈极显著正相关,其中695~756 nm相关系数最大,745 nm处为-0.822,属于红边范围。可见蓝光和红光区域是毛竹叶片色素微分光谱的敏感区域。
-
相关分析表明:毛竹叶片绝大多数参数都与叶绿素a,叶绿素a+b及类胡萝卜素质量分数之间存在极显著(P<0.01)的相关性(表 5)。叶绿素a,叶绿素a+b及类胡萝卜素质量分数与光谱反射指数、改良红边比、色素比值指数b、色素归一化指数b、归一化植被指数、红边归一化指数、反射光谱比分析、色数比值指数、改良的类胡萝卜素指数、光化学反射指数、λred,Dλred和Sred等光谱参数极显著正相关,其中与光化学反射指数相关系数最小,与Sred的相关系数最大。叶绿素a,叶绿素a+b及类胡萝卜素质量分数与反射光谱比分析a、反射光谱比分析b、红绿比指数呈负相关,其中与红绿比指数相关系数最小,与反射光谱比分析b的相关系数最大。叶绿素b与除Dλred,Sred以外的各参数均未达到显著相关,与前面叶绿素b质量分数和反射率及微分光谱相关性未达显著水平相一致。
光谱参数 叶绿素a 叶绿素b 叶 绿 素 a+b 类胡萝卜素 光谱反射指数 0.709** 0.447 0.682 ** 0.656 ** 改良红边比 0.677** 0.235 0.614 ** 0.653 ** 色素比值指数a 0.608** 0.395 0.587* 0.545* 色素比值指数b 0.717** 0.433 0.686 ** 0.666 ** 反射光谱比a -0.568** -0.304 -0.536* -0.510* 反射光谱比b -0.699** -0.316 -0.649 ** -0.663 ** 归一化植被指数 0.669** 0.368 0.633 ** 0.618 ** 红边归一化指数 0.724** 0.423 0.690 ** 0.676 ** 色素归一化指数a 0.673** 0.369 0.637 ** 0.623 ** 色素归一化指数b 0.724** 0.360 0.678 ** 0.685 ** 反射光谱比率分析 0.696** 0.321 0.647 ** 0.656 ** 色素比值指数 0.702** 0.325 0.653 ** 0.662 ** 植物衰老反射指数 0.663** 0.139 0.548* 0.659 ** 改良的类胡萝卜素指数 0.706** 0.369 0.665 ** 0.661 ** 光化学反射指数 0.633** 0.472 0.623 ** 0.567* 红绿比指数 -0.538** -0.381 -0.525* -0.468 λred 0.851** 0.397 0.792 ** 0.843 ** Dλred 0.652** 0740** 0.690 ** 0.580* Sred 0.881** 0.675** 0.870 ** 0.845 ** 说明:*表示差异显著(P<0.05),**表示差异极显著(P<0.01)。 Table 5. Correlation between reflectance spectrum parameters and pigment contents in Phyllostachys edulis leaves
2.1. 低温胁迫对毛竹叶片光合色素质量分数的影响
2.2. 低温胁迫对毛竹叶片反射光谱的影响
2.2.1. 低温胁迫对毛竹叶片光谱反射率的影响
2.2.2. 低温胁迫对毛竹叶片反射光谱“三边”参数的影响
2.2.3. 低温胁迫对毛竹叶片反射光谱参数的影响
2.3. 低温胁迫下毛竹叶片色素质量分数与反射光谱特征的相关性
2.3.1. 色素质量分数与光谱反射率的相关性
2.3.2. 色素质量分数与微分光谱的相关性
2.3.3. 色素质量分数与光谱特征参数的相关性
-
毛竹叶片叶绿素a和类胡萝卜素质量分数随温度降低而降低,是由于植物色素的合成过程,绝大部分都要有酶的参与,低温降低了叶绿体中一系列合成酶的活性[24];再者,低温胁迫引起叶片叶绿体结构变形、膜系统受到破坏,体内活性氧积累过多,从而加速了色素的降解[25],因此低温胁迫下的色素质量分数的降低,不仅是合成受阻,而且存在色素分解的加剧。植物色素是植物体内主要的吸收光能物质,其质量分数往往是植物环境胁迫、光合能力等生理状态的良好指示剂[26],因而常用于监测植物长势、估算植被光合效率和生物量[27]。毛竹叶片叶绿素b变化不明显,这与前人的研究结果有所不同,可能是低温处理时间太短,叶绿素b对低温胁迫不敏感,也可能与毛竹苗生长阶段和叶片发育状况有关。有待进一步研究探讨。
低温胁迫下毛竹叶片光谱显著差异主要在可见光区,且随温度降低而升高。植物绿光区(525~605 nm)及红光到近红外波段(680~750 nm)的反射率突变区域,包含着丰富的信息[28]。研究显示温度胁迫后的水稻Oryza sativa在近红外区反射率随温度胁迫程度的加剧而降低,可见光区与之相反[9],这与毛竹叶片研究一致。毛竹叶片光谱参数光谱反射指数、改良红边比、色素比值指数b、归一化植被指数、红边归一化指数、色素比值指数a、色素归一化指数b、反射光谱比率分析、色数比值指数、改良的类胡萝卜素指数、光化学反射指数和Sred等,在低温胁迫下变化尤为明显(表 4),其中反射光谱比分析[29]、色素比值指数[15]、色素比值指数[15]、光谱反射指数[13]和Sred[30]等表现光谱特征优于其他参数,可作为监测依据。
毛竹叶片25 ℃时红光吸收较多。随着温度降低,叶结构的破坏和叶绿素减少,导致红边区域吸收能力降低,反射增加,红边向短波方向移动,Sred也随之减小。叶绿素的吸收光谱区主要是在550~750 nm波段,生长旺盛时红光吸收较多[31-32],红光区光谱特征,可指示叶绿素质量分数的变化情况[33-34]。可见光和近红外区域是色素感受胁迫的敏感区域,色素质量分数与一阶微分光谱在红边(695~754 nm)内相关性最高[35]。毛竹叶片绿光区光谱反射率与色素质量分数(除叶绿素b外)呈显著负相关,红边(680~780 nm)内一阶微分值,与叶片色素(除叶绿素b外)质量分数达极显著相关,与前人有相似的结果。红光波段和绿光波段在评价毛竹长势上是十分有用的。
光谱参数由光谱反射曲线衍生而来,在表现植物生长状况上更为具体和更具指向性,光谱反射指数、改良红边比、色数比值指数b、归一化植被指数、红边归一化指数、色素比值指数a、色素归一化指数b、反射光谱比率分析、色数比值指数、光化学反射指数及Sred等毛竹光谱参数与色素质量分数(除叶绿素b外)表现显著相关性。研究显示植物叶片色素质量分数与光谱参数也有着紧密的关系[33, 36],光谱参数、改良的类胡萝卜素指数、归一化植被指数及色素归一化指数a、色素归一化指数b可用来分析叶绿素a、叶绿素b和叶绿素a+b质量分数,反射光谱比分析、光化学反射指数、改良的类胡萝卜素指数及红绿比指数可用来监测类胡萝卜素和花青素质量分数[37]。λred,Sred,红边归一化指数,红边斜率(Sred)[38],修正的简单化值植被指数(mSR705)和修正的归一化差值植被指数(mND705)[4]等几个反射光谱特征参数均与叶绿素质量分数存在指数函数关系。红边参数λred,Dλred,Sred由于充分发挥了微分技术的优势,对植株生化组分的估测具有较高的能力[28, 39],毛竹叶片红边参数与色素质量分数极显著的相关性也充分印证了这一点,其数值大小受叶绿素及其叶片结构的影响,对胁迫最为敏感[40],采用光谱参数法估测毛竹叶片色素质量分数是切实可行的。
低温胁迫下毛竹叶片色素和光谱特征均有显著差异,且叶片色素质量分数与反射光谱及各参数有极显著的相关性,可通过叶片光谱特征以及反射光谱比分析、色素归一化指数、色数比值指数、红边归一化指数和Sred 等光谱参数,方便快捷地反映毛竹生长状况。









 DownLoad:
DownLoad: